Abstract
Anti–N-methyl-d-aspartate (NMDA) receptor encephalitis represents a new category of immune-mediated neurologic disorders. Viral encephalitis is often the presumptive diagnosis because of the acute neurologic changes, cerebrospinal fluid lymphocytic pleocytosis, and occasional hyperthermia. We report here the case of a previously healthy 7-year-old boy with new-onset aggressive behavior, seizure activity, and orofacial dyskinesias with cerebrospinal fluid and serum that tested positive for anti-NMDA receptors.
Keywords: anti–NMDA-receptor encephalitis, paraneoplastic syndrome, encephalitis
Anti–N-methyl-d-aspartate (NMDA) receptor encephalitis is a disorder of antibodies to the limbic system that largely affects children. It is often dismissed initially as a psychiatric syndrome. When symptoms persist and cerebrospinal fluid (CSF) is obtained, a viral encephalitis is frequently diagnosed. Because this is a potentially treatable disorder and occasionally the first indication of an underlying malignancy, prompt recognition and treatment of this disorder is critical.
CASE REPORT
A previously healthy 7-year-old boy presented to Johns Hopkins Hospital emergency department with a 7-day history of violent outbursts, inappropriate speech, and complex partial seizures. He had no significant past medical history, was growing and developing appropriately, and did not receive any medications. Forty-eight hours before any behavioral changes he complained of headaches, myalgias, and fatigue and remained home from school. When he returned to school, his teacher reported an episode of staring aimlessly during which he was unresponsive and was clenching his left hand. Over the next several days he made statements of unclear significance such as “I am having a heart attack” and had bizarre and inappropriate smiling. He became increasingly violent and physically assaulted his mother on several occasions. He was able to recall his aggressive behavior and displayed remorse. He also had intermittent orofacial dyskinesias and bizarre involuntary movements.
On presentation to the hospital, he was extremely agitated and attacked several employees. These outbursts were controlled temporarily with haloperidol, propofol, and benzodiazepines. His temperature was 37.4°C, and he was normotensive. His Glascow Coma Score was 13. He was alert and oriented to person but not to place or time. He was able to localize movement in response to painful stimulation. His speech was occasionally slurred and sometimes incomprehensible. He had conjugate gaze and no nuchal rigidity. His lungs were clear, and no cardiac murmurs were appreciated. His abdomen was soft without hepatosplenomegaly. No skin rashes were appreciated. Cranial nerves II through XII were normal. His fundoscopic examination was unremarkable. Muscle tone and bulk were normal; there were no fasciculations or involuntary movements. He was not cooperative with formal muscle-power testing, but he appeared to have mild distal left-arm weakness. Sensation and reflexes were intact.
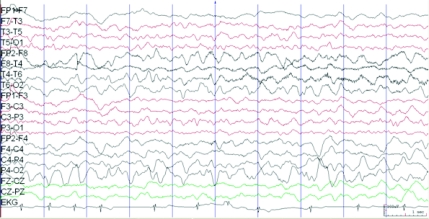
Acyclovir therapy was initiated empirically. CSF was significant for 14 white blood cells per μL, all of which were lymphocytes, 2 red blood cells, and normal CSF glucose and protein levels. Gram-staining and a CSF bacterial culture did not reveal any organisms. His peripheral white blood cell count was 12 800/μL. Results of polymerase chain reaction and serology testing for common neurotropic viruses (including herpes simplex virus, lymphocytic choriomeningitis virus, enterovirus, and West Nile virus) were negative in blood and CSF. Results of fluid attenuation inversion recovery and diffusion-weighted brain MRI were unremarkable. No abnormal gadolinium enhancement was seen. Scalp electroencephalogram (EEG) monitoring revealed focal right-sided slow activity (Fig 1); subsequent EEG 2 days later revealed right posterior sharp waves indicative of a seizure focus.
FIGURE 1.
EEG (longitudinal bipolar montage) that shows medium- to high-voltage sharply contoured semirhythmical slow activity over the right temporofrontal region. The patient had a secondarily generalized seizure that originated over this region 2 minutes after this EEG segment.
Results of testing for antistreptolysin O antibodies, Bartonella henselae serology, thyroid function, heavy metals, antinuclear antibodies, and inborn errors of metabolism were unremarkable. On hospital day (HD) 9, the patient had complex partial seizures involving the left side of his body. These seizures were eventually controlled with phenytoin, levetiracetam, and valproic acid. Over the next several days he had increasingly poor cognitive function, and his Glascow Coma Score declined to 8. He demonstrated autonomic instability with episodes of bradycardia and labile blood pressure. Pulse steroids were administered beginning on HD 12 for 5 days with a taper, but there was no immediate effect.
On HD 22, results of serum (from a sample obtained on HD 8) serology testing for anti–NMDA receptor antibodies were positive. Repeat CSF analysis revealed the presence of oligoclonal bands and anti-NMDA receptor antibodies. Results of screening for an underlying malignancy with MRI of the brain, abdomen, and pelvis were unremarkable. Plasmapharesis was initiated on HD 25; 7 total treatments were performed on alternating days followed by intravenous γ globulin (2 g/kg). A slight clinical improvement was observed after treatment. The child's abnormal movements became less apparent, and by week 9 of his illness, he could execute simple commands. Slight impulsive behavior and memory deficit have persisted, and he still exhibited poor attention and planning 6 months after clinical presentation.
DISCUSSION
NMDA receptors are glutamate receptors with roles in synaptic transmission as well as neuropsychiatric disease. Anti-NMDA receptor encephalitis is a disorder mediated by antibodies against the NR1 subunit of the NMDA receptor.1,2 Although the NR1/NR2 heterodimers are present throughout the nervous system, they are predominantly expressed in the neocortex and hippocampus. The manifestations that result from anti-NMDA receptor antibodies have been attributed to the decreased surface density and synaptic localization of NMDA receptors, and there is a concomitant decrease in NMDA receptor-mediated synaptic currents.3
Anti-NMDA receptor encephalitis was originally described as a paraneoplastic syndrome associated with ovarian teratomas containing neural tissue with antibodies cross-reacting to the NMDA receptor. Although teratomas of the ovaries and testicles are identified as the underlying malignancy in 98% of cases in which a tumor is identified, this syndrome has also been reported in association with neuroblastomas and small-cell lung cancer.4–6 Approximately 50% of adult patients and nearly 70% of pediatric patients who present with this condition have no identifiable tumor.1,5,7 The pathogenesis of the immune response, particularly in patients without a malignancy, is unknown. Although the incidence of this condition is not known, the disorder may be more frequent than previously thought; 1 tertiary care pediatric series reported 8 new pediatric diagnoses over an 8-month time period.5 Children as young as 23 months of age have been identified to have serum and CSF antibodies to the NMDA receptor.5
The disorder consists of a defined set of clinical features.1 Prodromal symptoms including fever, headache, upper respiratory symptoms, or gastrointestinal symptoms are noted in approximately half of the patients. These symptoms are followed a few days later by mood and behavioral changes, dyskinesias, and psychiatric symptoms. Children often become increasingly agitated; they sometimes become paranoid or combative and have delusional thoughts and display inappropriate laughing or crying. A decline in the level of consciousness occurs with possible seizure activity and autonomic instability, followed by gradual partial or complete recovery.
In most reports, brain imaging is reported to be normal with slow, disorganized activity on EEG.1 Approximately half of the patients have been reported to have fluid attenuation inversion recovery or T2-weighted signal changes, but there seems to be minimal correlation with patient symptoms.1 There is some evidence of an inflammatory process with CSF pleocytosis and oligoclonal bands in the CSF. Antibody titers seem to correlate with disease severity, and antibody activity results in a reversible decrease in synaptic NMDA receptors.1 On brain-biopsy specimens, however, there is a paucity of inflammatory infiltrates relative to the severity of the neurologic deficits.2
Viral encephalitis is often the presumptive diagnosis, suggested by the acute neurologic change, CSF lymphocytic pleocytosis, and occasional hyperthermia. Because most patients develop a prodromic viral-like illness, a postinfectious immune-mediated etiology has been postulated. The California Encephalitis Project found that 63% of patients with encephalitis had no identifiable etiology, which suggests that a portion may be immune-mediated.8 This hypothesis gained credence by the identification of anti-NMDA receptor antibodies in approximately half of the cases that were initially termed “idiopathic encephalitis with psychiatric manifestations.”5 The absence of viral antigens in the CSF, the benefit of immunotherapy, and the presence of CSF oligoclonal bands are supportive of an immune-mediated phenomenon.
Despite the severity of the disorder, patients often improve with immunotherapy and tumor removal.9 The presence of a tumor seems to be a positive prognostic factor.1,5 Removal of tumors has been demonstrated to reduce ICU length of stay and likelihood of relapse.2 Various therapies have been used for this condition, including corticosteroids, intravenous immunoglobulins, and plasmapharesis.10 Although there have been no rigorous trials of therapeutic agents, the benefit of aggressive immunotherapy is supported by improvements observed in patients whose conditions failed to respond to first-line immunotherapies and subsequently responded to rituximab, cyclophosphamide, or both.1,5,11 It is unclear what role immunotherapy played in our patient's gradual improvement, although it may have hastened improvement.
Recovery is usually protracted over months to years, and amnesia of the entire illness is often reported.1 In 2 large series, 75% of the patients had complete or near-complete recovery.1,5 The remainder of them, however, had severe deficits or died. Relapse may occur with or without a detectable tumor, presumably resulting from the persistence of antibody within the central nervous system.6 On the basis of experience with patients whose initial teratoma or second teratoma was identified many months after the first episode of encephalitis or at relapse, periodic MRI or ultrasound studies for at least 2 years have been suggested by some experts.2 Teratomas of the testis are frequently missed with positron emission tomography.6
CONCLUSIONS
Anti-NMDA receptor encephalitis should be considered in the differential diagnosis of encephalitis when acute behavioral changes, seizures, or dyskinesias present. It is a potentially treatable disorder and can be an early marker of a malignancy. Future research is needed to elucidate triggers of the immune-mediated process and optimal method and duration of therapy.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
Abbreviations:
- CSF
- cerebrospinal fluid
- EEG
- electroencephalogram
- HD
- hospital day
- NMDA
- N-methyl-d-aspartate
REFERENCES
- 1. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan. Neurology. 2008;70(7):504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866–5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lebas A, Husson B, Didelot A, Honnorat J, Tardieu M. Expanding spectrum of encephalitis with NMDA receptor antibodies in young children. J Child Neurol. 2010;25(6):742–745 [DOI] [PubMed] [Google Scholar]
- 5. Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66(1):11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(pt 6):1655–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43(12):1565–1577 [DOI] [PubMed] [Google Scholar]
- 9. Tüzün E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence of antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol (Berl). 2009;118(6):737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schimmel M, Bein CG, Vincent A, Schenk W, Penzien J. Successful treatment of anti-N-methyl-D-aspartate receptor encephalitis presenting with catatonia. Arch Dis Child. 2009;94(4):314–316 [DOI] [PubMed] [Google Scholar]
- 11. Ishiura H, Matsuda S, Higashihara M, et al. Response of anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology. 2008;71(23):1921–1926 [DOI] [PubMed] [Google Scholar]