Abstract
Trauma injuries often cause peripheral nerve damage and disability. A goal in neural tissue engineering is to develop synthetic nerve conduits for peripheral nerve regeneration having therapeutic efficacy comparable to that of autografts. Nanofibrous conduits with aligned nanofibers have been shown to promote nerve regeneration, but current fabrication methods rely on rolling a fibrous sheet into the shape of a conduit, which results in a graft with inconsistent size and a discontinuous joint or seam. In addition, the long-term effects of nanofibrous nerve conduits, in comparison with autografts, are still unknown. Here we developed a novel one-step electrospinning process and, for the first time, fabricated a seamless bi-layer nanofibrous nerve conduit: the luminal layer having longitudinally aligned nanofibers to promote nerve regeneration, and the outer layer having randomly organized nanofibers for mechanical support. Long-term in vivo studies demonstrated that bi-layer aligned nanofibrous nerve conduits were superior to random nanofibrous conduits and had comparable therapeutic effects to autografts for nerve regeneration. In summary, we showed that the engineered nanostructure had a significant impact on neural tissue regeneration in situ. The results from this study will also lead to the scalable fabrication of engineered nanofibrous nerve conduits with designed nanostructure. This technology platform can be combined with drug delivery and cell therapies for tissue engineering.
Introduction
Peripheral nerve damage is common after traumatic injuries. The most severe form of damage is a complete nerve transection, which results in loss of sensory and motor function at the nerve target site. The current gold standard of treatment for a transected nerve is bridging the injury gap with a nerve autologous graft (autograft) harvested from another site in the body.1 The nerve autograft has the advantages of serving as a physical guide for regenerating nerve fibers.2,3 Disadvantages of this technique include nerve size mismatches, additional surgery, and the loss of function and morbidity at the donor site. Further, in many instances there is no suitable nerve autograft available, especially for nerve transection with a large gap.
In the past three decades, nerve conduits made of synthetic polymers or native matrices have been developed as alternatives to autografts. Nondegradable nerve conduits, including silicon and poly (2-hydroxyethyl methacrylate-co-methyl methacrylate), may eventually lead to chronic inflammation, foreign body reactions, and tube collapse that induces nerve compression.4,5 Biodegradable materials provide an ideal alternative to current nerve injury therapy.6 Biodegradable polymers such as poly (glycolic acid),7,8 poly-caprolactone,9 or poly (L-lactic acid),10 as well as natural biomaterials such as collagen,11 chitosan,12,13 or keratin,14 have been used as nerve conduit materials. These biodegradable and biocompatible conduits could provide a contained semi-permeable environment for nerve regeneration. However, none of these nerve conduits has achieved therapeutic effects comparable to autografts.
It is generally recognized that cues from physical and biochemical guidance can promote nerve growth.15 In early studies, micropatterned channels and extracellular matrices were used to guide axon growth in specific directions.16,17 Recently, we and others have shown that electrospun aligned nanofibers can promote axon growth and Schwann cell maturation in vitro18–20 and enhance nerve regeneration in vivo.21–23 However, the fabrication of seamless nerve conduits (as opposed to a sheet rolled into a cylindrical shape) with highly aligned nanofibers and clinically relevant mechanical properties has not been realized. Further, the in vivo effects of aligned nanofibers on nerve regeneration are inconclusive,21 and the long-term (>6 months) effects of aligned nanofibers on nerve regeneration have not been investigated.
We hypothesized that a seamless nerve conduit with aligned nanofibers on the luminal surface provide a well-controlled microenvironment to promote nerve regeneration. In this study, we developed a single-step electrospinning process to fabricate seamless nanofibrous nerve conduits with a fully integrated bi-layered structure: the luminal surface has longitudinally aligned nanofibers for nerve guidance and the outer layer has randomly oriented nanofibers for structural support. The study demonstrates, for the first time, the ability to directly electrospin conduit structures with longitudinally aligned nanofibers. Unlike previous attempts, wherein aligned sheets were inserted within conduits or aligned sheets were rolled into conduits21, the seamless nerve conduit in this study avoids tedious, unreliable manufacturing processes and the potential risks of sub-optimal nerve growth, discontinuities, and structural weaknesses associated with having seams within conduits walls.
To demonstrate clinically relevant durability for supporting nerve growth, we evaluated the ability of the nerve conduit to maintain structural and mechanical integrity under simulated physiological conditions using an in vitro hydrolytic degradation model. We demonstrated biological performance of the nanofibrous nerve conduits by systematically comparing the long-term (up to 12 months) efficacy of nerve conduits with autografts in a rat sciatic nerve transection model. Histomorphometry and electrophysiology analysis showed that bi-layer aligned nanofibrous nerve conduits had therapeutic efficacy comparable to autografts. In addition, we demonstrated superior nerve regeneration and muscle function recovery with aligned nanofibrous nerve conduits compared to random nanofibrous nerve conduits.
Materials and Methods
Bi-layer nerve conduit fabrication
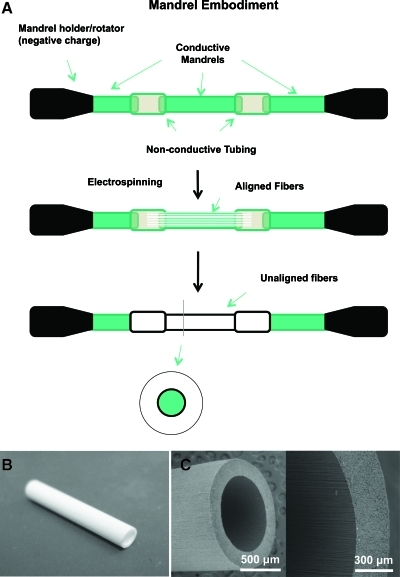
Nonwoven aligned nanofibrous nerve conduits composed of poly (L-lactide-co-caprolactone) (70:30; Purac Biomaterials), poly(propylene glycol) (Acros Organics), and sodium acetate (Sigma) were fabricated using a customized electrospinning process. The aligned nanofibrous nerve conduits comprised a luminal region of longitudinally aligned nanofibers and an outer region of randomly oriented nanofibers. PLCL, PPG, and sodium acetate were dissolved in a volatile organic solvent, hexafluoroisopropanol (Matrix Scientific). The electrospinning apparatus consisted of a syringe pump capable of delivering the polymer solution to the tip of a needle secured onto a mechanized platform suspended over a 1.6-mm-outer-diameter rotating mandrel collector assembly. The needle platform was charged by a positive-polarity power supply and the mandrel assembly was charged by a negative-polarity power supply. Longitudinal fiber alignment was achieved through the design of the rotating mandrel collector assembly. By interspersing electrically insulating polymer sections between electrically conducting stainless steel sections, the mandrel assembly biased deposition of electrospun fibers with orientation parallel to the long axis of the mandrel. After deposition of longitudinally aligned electrospun fibers, subsequent fiber deposition on the mandrel was essentially randomly oriented. The mandrel assembly was rotated around its long axis and the needle was traversed between the ends of the assembly to ensure even fiber deposition (Fig. 1A).
FIG. 1.
(A) Schematic illustration of our approach to fabricate nanofibrous nerve conduits. (B) A nanofibrous nerve conduit (1.5 cm length, 1.6 mm ID). (C) Scanning electron microscopy image of a nerve conduit showing aligned fibers in luminal layer and random fibers in outer layer. Color images available online at www.liebertonline.com/tec
Upon completion of electrospinning, the nerve conduits were air-dried on the steel mandrel for two nights to remove residual hexafluoroisopropanol. The nerve conduits were then rinsed in deionized water and cut to appropriate length. Random nanofibrous nerve conduits were fabricated using the same polymer solution but with a uniform stainless steel mandrel as the collector assembly. All nerve conduits were sterilized with ethylene oxide gas before characterization and in vivo implantation studies.
Alignment characterization
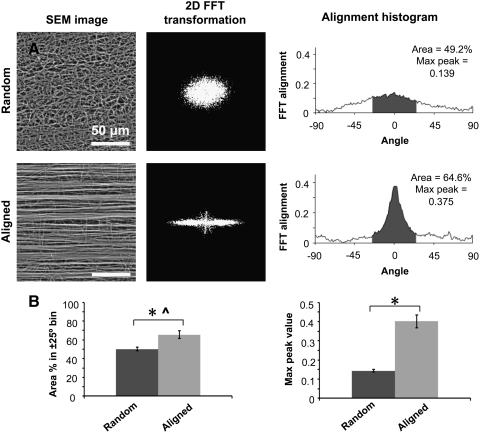
High and low magnification microscopic images of the electrospun nanofibrous nerve conduits were captured using a Hitachi TM-1000 scanning electron microscope (SEM; Hitachi U.S.A.). To analyze fiber alignment, a modification of previously published Fast Fourier Transform (FFT) techniques was used.24 Briefly, SEM images of the nerve conduit surfaces were converted to gray scale, resized, and masked with a circular pattern using Adobe Photoshop (Adobe). The images were then imported into ImageJ software and transformed using the two-dimensional FFT function. This function converted the image into a spatial distribution corresponding to the changes in pixel intensity across the sample. Transformed images were rotated by 90° to match the alignment of the original images. Each transformed image was then analyzed in a circular coordinate system using the oval-profile ImageJ plugin (courtesy of Bill O'Connell, http://rsbweb.nih.gov/ij/plugins/oval-profile.html). The pixel intensities along each radian in the transformed image were summed and plotted as an intensity-distribution histogram. For consistency of analysis, the pixel intensity data in each histogram were normalized by the lowest value and then reduced by 1, such that the lowest value was always re-designated as 0. The angle of maximum pixel intensity was also re-designated as 0° in the graphs, and data were shown for only 180° since the original images were largely symmetrical. For each random or aligned nerve conduit sample, an FFT alignment histogram was generated from an SEM image (1000×magnification) taken from the luminal surface of the sample. Relative extent of alignment was determined by examining the max peak value of the FFT alignment curve of each sample as well as the area under the histogram within 25° of the peak angle.
Tensile testing and in vitro degradation
Ultimate tensile load of the aligned nerve conduits was determined after in vitro degradation using a Chatillon TCD225 Series Digital Force Tester equipped with a 100 N load cell and a pair of Universal Wedge Grips (GF-9 series, rated to 2.5 kN) (Ametek, Inc.). Nerve conduit samples were first subjected to in vitro degradation by incubating in Sorensen's buffer (pH 7.4) in a temperature-controlled oven at 37°C for 0-, 4-, and 8-week time points. Just before mechanical testing, samples were allowed to equilibrate at room temperature in Sorensen's buffer for 10 min. Samples were then secured in the Chatillon grips at a gauge length of 2.5 cm and subjected to tensile load along their longitudinal axes at a rate of 100 mm/min until failure.
In vivo implantation
All animal study procedures were approved by the Institutional Review Board Service and Institutional Animal Care and Use Committee at the University of California, Berkeley. Adult female Lewis rats (250±30 g) were anesthetized with 1.5% isoflurane in 70% N2O/30% O2. Body temperature was maintained at 37.0°C±0.5°C during surgery. Briefly, the rat was set in the left recumbent position, and right gluteal and posterior thigh incisions were made to expose the right sciatic nerve deep to femoris muscle. Under a surgical microscope, 1 cm of the sciatic nerve was excised to create a nerve lesion gap. For nerve conduit groups, both nerve ends were inserted 1 mm into the 1.2 cm tube lumen to create 1 cm nerve lesion gap and sutured in place with two 10–0 nylon monofilament sutures. For the autograft group, the nerve defect was sutured with a 10-mm reversed nerve segment instead of the nerve conduit. Previous study has shown that the critical gap length, characterized by outgrowth of axons that reconnected across the gap, is 9.7±1.8 mm.25 The overlying muscle layers were approximated with interrupted 4–0 nylon sutures and stainless steel wound clips were used to close the skin wound.
The animals were divided into experimental groups according to the composition of the implanted nerve conduits as follows: random nanofibrous nerve conduits (n=12), aligned nanofibrous nerve conduits (n=12), and autograft (a reversed nerve segment) (n=12). In every group, half of the animals were sacrificed 2 months postsurgery, and the other half were maintained for 12 months postsurgery.
Electrophysiology
Electrophysiology analysis was performed at the 2-month and 12-month postsurgery time points of all experimental groups (n=6). The animals were put in a lateral position and body temperature was maintained at 37°C on a thermostatic pad. The right sciatic nerve was re-exposed through the thigh muscle incision. Bipolar hooked platinum simulating electrodes were placed under the sciatic nerve 5-mm proximal to the graft suturing point. The stimulating electrodes were connected to a pulse generator (SYS-A310; World Precision Instruments Inc.) and delivered electrical signals to the nerve. To record the evoked compound muscle action potential (CMAP) signals, a sharp tungsten needle was inserted percutaneously into the midpoint of the right gastrocnemius muscle. A second tungsten needle probe was positioned subcutaneously over the gastrocnemius muscle. The opposite pole was also grounded to an Ag/AgCl2 electrode placed in a superficial muscle layer near the skin. Signals generated at the tungsten probe were fed to an AC amplifier (DAM-80, WPI) and amplified 10,000 times. The signals were recorded by 4-Channel Data Acquisition System (Lab-Trax-4, WPI) and displayed on a computer monitor. Nerve simulation was elicited using a stimulus fourfold stronger than the original threshold, below which there was no action potential. The amplitude and latency of the action potential waveform were determined to assess the recovery of injured sciatic nerve. Conduction velocities were calculated from derived latencies and the measured distance between the stimulus and recording probes.
Histological evaluation
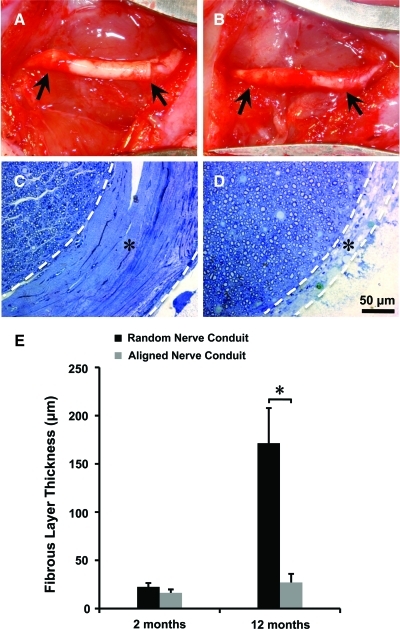
At the end of the electrophysiological tests, the animals were sacrificed and sciatic nerve and conduit samples were explanted (Fig. 5A, B). The tissue samples were fixed with 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1M Sodium Cacodylate (pH 7.2), and postfixed with 1% osmium tetroxide (Electron Miscroscopy Sciences (EMS)) solution. The fixed samples were trimmed, dehydrated stepwise in increasing concentrations of acetone, and embedded in EMbed 812 resin (EMS). Ultra-thin transverse sections were obtained at two sites of a specimen, the midpoint of the graft (5–7 mm from the proximal end of the graft) and the distal nerve tissue (3 mm distal to the coaptation suture site). The samples were sectioned by using a Leica Ultracut E microtome (Leica Microsystems) at 800-nm thickness, and stained with 1% toluidine blue.
FIG. 5.
Photographs showing aligned nanofibrous nerve conduit 2 months (A) and 12 months (B) postsurgery. The arrows indicate two ends of the nerve conduits. Representative micrographs of toluidine-blue staining showing the regenerated nerve and fibrous capsule layer in random (C) and aligned nanofibrous conduit groups (D) at 12 months. White dashed lines show the boundary of the fibrous capsule layer labeled with “*.” Scale bar=50 μm. (E) Quantification of the thickness of the fibrous capsule layer. *Significant difference (p<0.05) using two-tailed unpaired t-test and the data were presented as mean±SD. Color images available online at www.liebertonline.com/tec
Digitized images of the stained tissue cross sections were acquired with a Zeiss Axioscope microscope (Zeiss). A dense fibrous capsule layer was formed between the outer surface of the regenerated nerve tissue and the luminal surface of the nerve conduit. Six pictures from each sample at mid-graft regions were randomly taken and the thickness of the capsule layer was measured and compared between aligned and random nanofibrous nerve conduit groups at different time points. Three to five pictures of high-powered fields (15,244 μm2, at a magnification of 630×) were randomly taken from each sample and myelinated axons with greater than 1-μm2 area were selected for analysis. This was done for 2-month (n=6) and 12-month (n=6) samples to evaluate axonal regeneration. ImageJ software was used to measure the thickness of the capsule layer, myelinated axon area, and myelin sheath thickness at mid-graft regions.
For axon diameter analysis, all axonal shapes were converted into equivalent circles by adjusting the circularity threshold to 0.35 for ImageJ quantification. This method allowed the inclusion of distorted axons in irregular shape. Equivalent diameters were calculated to generate an axon diameter frequency distribution graph.26
To quantify the thickness of myelin sheath, 600 myelinated axons were selected representatively from each picture with high magnification, and the myelin sheath thickness was measured. The frequency distribution of myelin sheath thickness was then generated for each sample group.26
Behavior examination
Animals were allowed to move freely in an open area for examination for 5 minutes. The hindlimb movements and its posture while ambulating were evaluated with subjective scores 0–3: 3 for normal hind limb function, 2 for deformity movement, 1 for rare movement, and 0 for no movement. Nociceptive withdrawal function was evaluated by observing the withdrawal reflex strength of the hind limb in response to pinch. The withdrawal strength of hind limb was scored from 0 (absent) to 3 (normal), with 1 and 2 for considerably and slightly recovery, respectively. A composite score, 0–6 in increasing functional recovery, was derived from the combined scores of these two tests.
Statistical analysis
For FFT alignment data, a two-tailed unpaired t-test was used (proportional data was also arcsine-transformed to account for non-normal distribution). For data requiring comparisons between more than two groups, including the data from mechanical testing, electrophysiology, fibrous capsule layer, and behavior examination, analysis of variance (Statview 5.0) was first used to compare differences between all groups. Mechanical data were further analyzed between individual groups by using the Holm's t-test. Post-hoc testing was performed to analyze the data of the electrophysiology, fibrous tissue layer, and behavior examination by using Fisher's protected least significant difference. A p-value <0.05 was considered statistically significant.
Results
Characterization of nanofiber organization in nerve conduits
Electrospun nanofibrous nerve conduits with an inner diameter of 1.6 mm were produced (Fig. 1A–C). SEM images of the luminal surfaces showed highly aligned nanofibers for aligned nanofibrous nerve conduits and wavy, randomly oriented nanofibers for the random nanofibrous nerve conduits (Fig. 2A). Quantitatively, the aligned nerve conduit luminal surfaces produced FFT pixel intensity distribution curves with a single sharp peak at the expected angle of alignment, whereas the random nanofibrous nerve conduits produced pixel intensity curves with a lower peak and broader distribution of values (Fig. 2A). The average pixel intensity population within the±25° bin of the expected angle of alignment was 65.52%±4.23% for aligned and 50.26%±2.14% for random nanofibrous nerve conduits (n=5). The average peak at the expected angle of alignment was 0.40±0.01 arbitrary units for aligned and 0.14±0.03 arbitrary units for random nanofibrous nerve conduits. The aligned nanofibrous nerve conduits demonstrated a significantly higher peak and population within the±25° bin than random nanofibrous nerve conduits (Fig. 2B).
FIG. 2.
(A) Alignment analysis of fibers on the luminal surface of nerve conduit (random and aligned). 2D FFT images are displayed in black and white for clarity. (B) Fiber alignment analysis data for random and aligned nerve conduits. Comparison of area under the FFT curve in the±25° bin or the max peak value of the FFT curve. *Significant difference (p<0.05) using two-tailed unpaired t-test; ^proportion data were arcsine-transformed for use in t-test analysis (n=5) and presented as mean±standard deviation (SD). FFT, Fast Fourier Transform.
Mechanical testing of nerve conduits
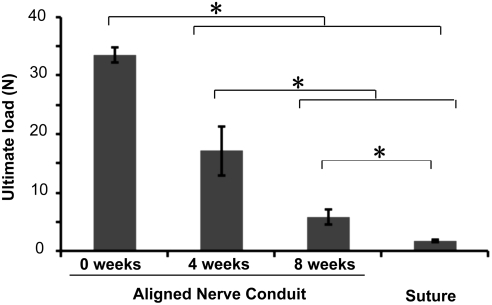
The ultimate tensile load of wetted aligned nanofibrous nerve conduits before in vitro degradation was 33.6±1.3N (Fig. 3). After degradation for 4 or 8 weeks in Sorensen Buffer solution at 37°C, aligned nanofibrous nerve conduits demonstrated ultimate tensile loads of 17.2±4.2N and 5.9±1.3N, respectively (Fig. 3). Comparatively, the average ultimate load for an 8–0 monofilament nylon suture (which is routinely used in clinical applications to approximate nerve ends and to suture nerve stumps within nerve conduits) was 1.8±0.1N (Fig. 3). The aligned nanofibrous nerve conduit had significantly higher ultimate load at all degradation time points than the 8–0 monofilament suture, which was sufficient to maintain the stability of nerve–conduit interface and the integrity of the conduit.
FIG. 3.
The mechanical strength of nerve conduits following in vitro degradation in Sorensen buffer. *Significant difference (p<0.05) using ANOVA followed by Holm's t-test (n=6) and the data were presented as mean±SD. ANOVA, analysis of variance.
Electrophysiology analysis of nerve functional recovery
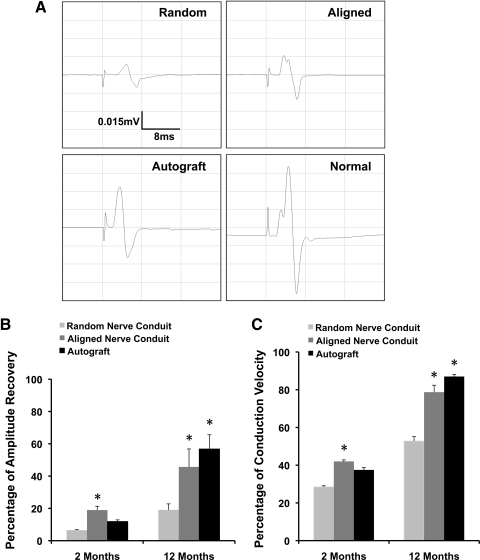
To quantify the functional recovery of regenerated nerves, electrophysiology analysis was performed at 2-month and 12-month time points to assess the responses of the hindlimb gastrocnemius muscle to electrical stimulation at the proximal end of the grafts. The amplitude and conduction velocity of CMAP were measured and calculated as previously described.12,27 CMAP amplitude and conduction velocity from the experimental limb were normalized to values from the animal's contralateral control.
CMAP amplitude of the aligned nanofibrous conduit group (19.0%±2.4%, 45.7%±11.1%) showed significantly better functional recovery than the random nanofibrous conduit group (6.5%±0.3%, 19.1%±3.8%) at 2 and 12 months, respectively (p<0.05). CMAP conduction velocity of aligned nanofibrous nerve conduits (42.0%±3.0%, 78.7%±8.5%) was also significantly higher than random nanofibrous nerve conduits (28.5%±2.2%, 52.8%±8.7%) at 2 and 12 months. There were no statistically significant differences in CMAP amplitude and conduction velocity between the aligned nanofibrous nerve conduit and autograft groups at both the 2-month and 12-month time points. However, at the 2-month time point, autografts (11.9%±1.0%) did not show a significant improvement in CMAP amplitude compared to random nanofibrous conduits.
Temporally, both CMAP amplitude and conduction velocity in the aligned nanofibrous nerve conduit and autograft groups at 12 months showed significant improvement over the respecting 2-month values (Fig. 4). For random nanofibrous nerve conduits, conduction velocity, but not CAMP amplitude, at 12 months showed significant improvement over 2-month values.
FIG. 4.
Summary of electrophysiology data at 2 and 12 months. (A) Representative results recorded at the probe inserted into gastrocnemius muscle of the injury side in the random and aligned nanofibrous nerve conduits, autograft, and normal nerve at 12 months. Percentage of compound muscle action potential amplitude recovery (B) and conduction velocity (C) compared to the animal's contralateral control. *Significant difference (p<0.05) compared to the random nanofibrous nerve conduit groups at 2 months and 12 months using Fisher's protected least significant difference (n=6) and all data were presented as mean±standard error of the mean.
Effects of nanofiber alignment on the formation of a fibrous tissue layer
At 2-month and 12-month of explantation, no significant degradation of nerve conduits was found in both random and aligned groups. As shown in Figure 5, the nerve regenerated in both random (Fig. 5C) and aligned (Fig. 5D) nanofibrous nerve conduit groups at 12 months postsurgery. Microscopic examination of the luminal surface showed that there was a fibrous tissue sheath at the interface of the nerve and the conduit wall in all nanofibrous conduit samples (Fig. 5C, D). In the random nanofibrous nerve conduit group, the fibrous sheath at 12 months was significantly thicker than that at 2 months (Fig. 5E), demonstrating significant growth between 2- and 12-month time points. In contrast, regenerated nerves in the aligned nanofibrous conduit group displayed an epineurial-like fibrous sheath with similar thicknesses at both 2-month and 12-month time points (Fig. 5E), significantly less than that in random nanofibrous conduits at 12 months.
Histomorphometry of regenerated nerve
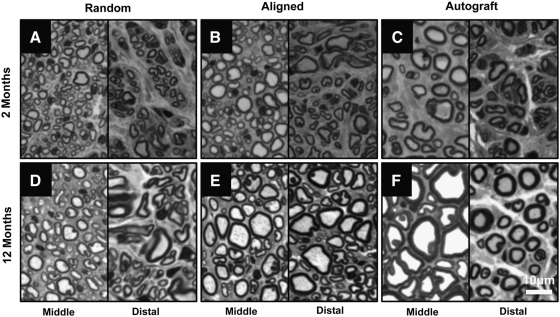
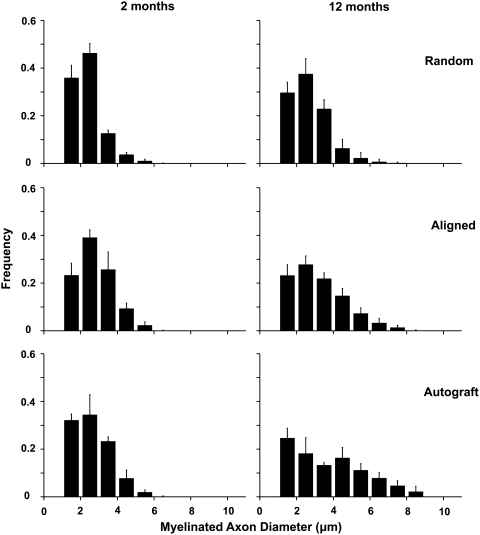
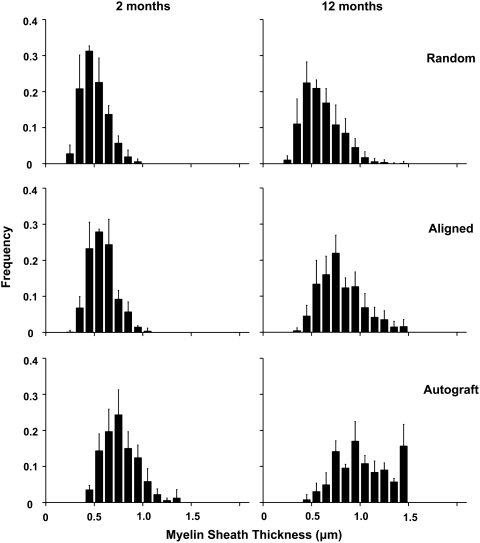
To quantitatively compare the regenerated nerves, histomorphometric analysis of toluidine blue-stained cross sections was performed on 2-month and 12-month samples. Myelinated axons were observed in cross sections from the middle portion of grafts (mid-graft) and distal nerve segments of all samples at the 2-month and 12-month time points: random nanofibrous nerve conduit group (Fig. 6A, D), aligned nanofibrous nerve conduit group (Fig. 6B, E), and autograft group (Fig. 6C, F). Vascularization of the regenerated nerve tissue was also observed and was similar in all samples (data not shown). Quantitative analysis of mid-graft cross sections was performed to measure regenerated axon diameter and myelin sheath thickness. Axon diameter (Fig. 7) and myelin sheath thickness (Fig. 8) were plotted as frequency distribution based on the dimension.
FIG. 6.
Representative micrographs of toluidine-blue-stained cross sections of 2-month (A–C) and 12-month (D–F) samples. (A, D) Radom nanofibrous nerve conduit group; (B, E) aligned nanofibrous nerve conduit group; (C, F) autograft group. Left panels showed the cross sections at the midpoint of representative grafts (∼5 mm from the proximal end of the graft). Right panels show the cross sections at the distal nerve tissue (3 mm distal to the suture site). Scale bar=20 μm.
FIG. 7.
Frequency distribution plot of myelinated axon diameter in aligned and random nanofibrous nerve conduit and autograft groups at 2 and 12 months.
FIG. 8.
Frequency distribution plot of myelin sheath thickness in aligned and random nanofibrous nerve conduit and autograft groups at 2 and 12 months.
At 2 months, both the aligned nanofibrous nerve conduit and autograft groups displayed higher frequencies of large axons (3–6 μm in diameter) than the random nanofibrous nerve conduit group (Fig. 7). Aligned nanofibrous nerve conduit and autograft groups also displayed higher frequencies of thick myelin sheaths (>0.8 μm) than the random nanofibrous nerve conduit group (Fig. 8). Aligned nanofibrous nerve conduit group showed similar axon diameter distribution to autograft group (Fig. 7), but the autograft group had more axons with thicker myelin sheaths.
At the 12-month time point, the axon diameter and myelin sheath thickness of the regenerated nerve tissue were significantly increased compared to those at 2 months in all three groups (Figs. 7 and 8), which indicated the further maturation of the regenerated nerves. The aligned nanofibrous nerve conduit group displayed higher frequencies of large axons (>4 μm in diameter) and thick myelin sheaths (>0.6 μm in thickness) than the random nanofibrous nerve conduit group. The autograft group had the most axons with thicker (>1.2 μm) myelin sheaths.
Behavior analysis of nerve functional recovery
The behavior score of aligned nanofibrous nerve couduit (3.33±0.82, 4.83±0.55) and autograft (3.50±0.84, 4.50±0.75) showed better functional recovery than the random nanofibrous nerve conduit group (2.50±0.55, 3.33±1.03) at 2 months and 12 months, respectively. (p<0.05). There was no significant difference of behavior score between the aligned nanofibrous nerve conduit and autograft at 2-month and 12-month time points. However, at 12-month time point, all experiment groups showed better hindlimb functional improvement over the respective 2-month values (p<0.05). Behavior score was constantly normal for the contralateral control at both timepoints.
Discussion
Due to the difficulty of electrospinning nerve conduits composed of nanofibers aligned along the conduit's long axis, researchers in previous studies21,22 either rolled a film with aligned nanofibers to form a conduit or filled a silicone tube with aligned nanofiber sheets. Here we developed a one-step electrospinning process to fabricate a novel, seamless, tubular nanofibrous nerve conduit composed of two fully integrated layers: a luminal layer with longitudinally aligned nanofibers and an outer layer with randomly organized nanofibers. To our knowledge, this was the first demonstration that a bi-layer tubular device with longitudinally aligned nanofibers can be directly electrospun as a unified, seamless construct.
Unlike previous attempts, the device and process described here are much more amenable and scalable for manufacturing and clinical use. The bi-layer design is likely to provide better suturability and mechanical integrity than a conduit composed entirely of longitudinally aligned nanofibers. Mechanical testing and in vivo results showed that this bi-layer nerve conduit has adequate mechanical strength for suturing and for supporting nerve growth. The in vitro degradation study demonstrated that the nerve guides maintained structural integrity to support nerve growth under physiological conditions for clinically relevant time periods. Direct electrospinning of bi-layer nanofibrous conduits is a fast process that avoids the tedious and unreliable process of rolling and sealing sheets and easily adapts to larger conduit sizes and longer lengths. The seamless construction of the bi-layer nanofibrous conduit also presents a smooth, even luminal surface for nerve growth and poses no risk of mechanical failure or separation at the seam.
We evaluated the nerve regeneration capacity of bi-layer aligned nanofibrous conduits in a rat sciatic nerve transection model with random nanofibrous conduits and autografts as controls. Nerve regeneration and muscle innervation were assessed at 2-month and 12-month time points by using histomorphometry analysis, electrophysiology measurement, and behavior test.
Electrophysiological analysis demonstrated the superior capability of aligned nanofibrous nerve conduits in nerve regeneration when compared to random nanofibrous nerve conduits. Based on the 2-month results, better functional recovery in terms of CMAP amplitude and conduction velocity was observed in the aligned nanofibrous nerve conduit group than in the random nanofibrous nerve conduit group. Interestingly, the advantage of the autograft was not shown in this early recovery period. One explanation is that that autograft may have to remodel its existing cellular and matrix contents (e.g., degradation and reorganization) to allow the ingrowth of regenerating axons. The 2-month electrosphysiology results suggest that aligned nanofibrous conduits were the most efficient in accelerating nerve functional recovery at the early phase. At 12-month time point, both aligned nanofibrous nerve conduit and autograft performed significantly better than random nanofibrous nerve conduit, and there was no statistical difference between aligned nanofibrous nerve conduits and autografts. These results indicate that nanofiber organization had long-term effects on nerve regeneration and that the in vivo performance of aligned nanofibrous nerve conduits is similar to autografts, which is the current gold standard of treatment for peripheral nerve injuries. The results from behavior tests also demonstrated the same trend.
Histological analysis of explanted nerve samples showed myelinated axons, vasculature, and epineurial sheaths in both random and aligned nanofibrous nerve conduits at 2 months and 12 months, similar to that in autografts. Quantitative analysis revealed a higher frequency of large-diameter axons and thick myelin sheaths for the aligned nanofibrous nerve conduit group compared to random nanofibrous nerve conduit group at both time points. Temporal comparison showed obvious shifts toward larger axons and thicker myelin sheath at 12 months for both the aligned nanofibrous nerve conduit and autograft groups. In contrast, the axon diameter and myelin sheath thickness in the random nanofibrous nerve group at 12 months only showed marginal increase. The axon diameter in aligned nanofibrous conduits and autografts had a similar distribution profile. Interestingly, the myelin sheath was generally thicker in autografts than in aligned nanofibrous conduits. It is possible that pre-existing Schwann cells in autografts played an important role in the myelination of regenerating axons, which may explain the difference in myelination between synthetic grafts and autografts.
The difference in the distribution profiles of axon characteristics between aligned and random nanofibrous nerve conduits suggests that longitudinally aligned nanofibers accelerate growth of large myelinated axons, which are morphological characteristics of motor neurons. The presence of larger axons with thicker myelin sheaths may also account for the higher CMAP amplitude and faster conduction velocity measured in the aligned nanofibrous nerve conduit group. A major limitation of nerve repair in humans is the slower growth of motor nerve fibers and relatively poor re-innervation of target muscle compared to sensory nerve fiber growth.1,15 The potential ability of aligned nanofibrous nerve conduits to improve the regeneration of motor nerve fibers and to match the biological performance of autografts merits further study.
Another interesting finding is the difference in the thickness of the fibrous tissue layer on the luminal surface of random and aligned nanofibrous nerve conduits. At 2 months, a thin continuous epineurial-like layer developed at the nerve–conduit interface in both random and aligned nanofibrous nerve conduit groups. At the 12 month time point, a dense connective tissue stroma formed around the regenerated nerve in the random nanofibrous nerve conduit group, whereas a thin tissue layer resembling a normal epineurium was observed in the aligned nanofibrous conduit group. These results suggest that random and aligned nanofibers not only had different effects in the early phase of nerve regeneration, but also exerted long-term effects during the maturation and remodeling of the regenerated nerve. These results may also suggest another advantage of the seamless nanofibrous conduit over nerve conduits with seams or discontinuous luminal surfaces, which may be more susceptible to fibrous capsule formation. The underlying mechanism of fibrous layer formation on the luminal surface of nerve conduits is not clear. Intriguingly, a recent study demonstrated that aligned poly(caprolactone) nanofibers reduced monocyte adhesion but had thicker fibrous capsule on the surface compared to random nanofibers within 4 weeks,28 which could be explained as acute foreign body responses. However, the growth of fibrous layer in our nerve conduits was only found on the luminal surface but not outer surface, and the growth happened between 2 and 12 months. It is likely caused by long-term tissue remodeling instead of acute inflammatory responses. One possible explanation for the differences in epineurial thickness is the faster cell proliferation rate and matrix synthesis rate on random nanofibers. Indeed, we have shown that aligned smooth muscle cells on micropatterned surfaces have lower proliferation rate.29,30 Whether this is the case in nerve conduits awaits further investigation.
Acknowledgments
This project was supported in part by grants from Telemedicine and Advanced Technology Research Center (TATRC) and National Institutes of Health (EB012240 and HL083900). A.W. was supported by a postdoctoral training grant TG2-01164 from California Institute for Regenerative Medicine.
Disclosure Statement
Shyam Patel, Kyle Kurpinski and Song Li have financial interest in NanoNerve Inc. that licensed the technology described here. No other competing financial interests exist.
References
- 1.Belkas J.S. Shoichet M.S. Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 2.Hudson A.R. Morris J. Weddell G. Drury A. Peripheral nerve autografts. J Surg Res. 1972;12:267. doi: 10.1016/0022-4804(72)90021-2. [DOI] [PubMed] [Google Scholar]
- 3.Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol. 1994;23:1. doi: 10.1007/BF01189813. [DOI] [PubMed] [Google Scholar]
- 4.Merle M. Dellon A.L. Campbell J.N. Chang P.S. Complications from silicon-polymer intubulation of nerves. Microsurgery. 1989;10:130. doi: 10.1002/micr.1920100213. [DOI] [PubMed] [Google Scholar]
- 5.Belkas J.S. Munro C.A. Shoichet M.S. Johnston M. Midha R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials. 2005;26:1741. doi: 10.1016/j.biomaterials.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Williams L.R. Longo F.M. Powell H.C. Lundborg G. Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983;218:460. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 7.Mackinnon S.E. Dellon A.L. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85:419. doi: 10.1097/00006534-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Kiyotani T. Teramachi M. Takimoto Y. Nakamura T. Shimizu Y. Endo K. Nerve regeneration across a 25-mm gap bridged by a polyglycolic acid-collagen tube: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 1996;740:66. doi: 10.1016/s0006-8993(96)00848-7. [DOI] [PubMed] [Google Scholar]
- 9.Bender M.D. Bennett J.M. Waddell R.L. Doctor J.S. Marra K.G. Multi-channeled biodegradable polymer/CultiSpher composite nerve guides. Biomaterials. 2004;25:1269. doi: 10.1016/j.biomaterials.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 10.Evans G.R. Brandt K. Katz S. Chauvin P. Otto L. Bogle M., et al. Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23:841. doi: 10.1016/s0142-9612(01)00190-9. [DOI] [PubMed] [Google Scholar]
- 11.Archibald S.J. Shefner J. Krarup C. Madison R.D. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci. 1995;15:4109. doi: 10.1523/JNEUROSCI.15-05-04109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X. Hu W. Cao Y. Yao J. Wu J. Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- 13.Wang A. Ao Q. Cao W. Yu M. He Q. Kong L., et al. Porous chitosan tubular scaffolds with knitted outer wall and controllable inner structure for nerve tissue engineering. J Biomed Mater Res A. 2006;79:36. doi: 10.1002/jbm.a.30683. [DOI] [PubMed] [Google Scholar]
- 14.Sierpinski P. Garrett J. Ma J. Apel P. Klorig D. Smith T., et al. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials. 2008;29:118. doi: 10.1016/j.biomaterials.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt C.E. Leach J.B. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 16.Hirono T. Torimitsu K. Kawana A. Fukuda J. Recognition of artificial microstructures by sensory nerve fibers in culture. Brain Res. 1988;446:189. doi: 10.1016/0006-8993(88)91314-5. [DOI] [PubMed] [Google Scholar]
- 17.Clark P. Britland S. Connolly P. Growth cone guidance and neuron morphology on micropatterned laminin surfaces. J Cell Sci. 1993;105(Pt 1):203. doi: 10.1242/jcs.105.1.203. [DOI] [PubMed] [Google Scholar]
- 18.Yang F. Murugan R. Wang S. Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 19.Patel S. Kurpinski K. Quigley R. Gao H. Hsiao B.S. Poo M.M., et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 20.Chew S.Y. Mi R. Hoke A. Leong K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29:653. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chew S.Y. Mi R. Hoke A. Leong K.W. Aligned protein-polymer composite fibers enhance nerve regeneration: a potential tissue-engineering platform. Adv Funct Mater. 2007;17:1288. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y.T. Haftel V.K. Kumar S. Bellamkonda R.V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel S. Diao E. Li S. Aligned nanofibrous grafts for enhanced nerve regeneration. 6th Combined Meeting of the Orthopaedic Research Societies; Honolulu, Hawaii. 2007. [Google Scholar]
- 24.Ayres C.E. Jha B.S. Meredith H. Bowman J.R. Bowlin G.L. Henderson S.C., et al. Measuring fiber alignment in electrospun scaffolds: a user's guide to the 2D fast Fourier transform approach. J Biomater Sci Polym Ed. 2008;19:603. doi: 10.1163/156856208784089643. [DOI] [PubMed] [Google Scholar]
- 25.Yannas I.V. Hill B.J. Selection of biomaterials for peripheral nerve regeneration using data from the nerve chamber model. Biomaterials. 2004;25:1593. doi: 10.1016/s0142-9612(03)00505-2. [DOI] [PubMed] [Google Scholar]
- 26.Pierucci A. de Duek E.A. de Oliveira A.L. Peripheral nerve regeneration through biodegradable conduits prepared using solvent evaporation. Tissue Eng Part A. 2008;14:595. doi: 10.1089/tea.2007.0271. [DOI] [PubMed] [Google Scholar]
- 27.Katayama Y. Montenegro R. Freier T. Midha R. Belkas J.S. Shoichet M.S. Coil-reinforced hydrogel tubes promote nerve regeneration equivalent to that of nerve autografts. Biomaterials. 2006;27:505. doi: 10.1016/j.biomaterials.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Cao H. McHugh K. Chew S.Y. Anderson J.M. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93:1151. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakar R.G. Ho F. Huang N.F. Liepmann D. Li S. Regulation of vascular smooth muscle cells by micropatterning. Biochem Biophys Res Commun. 2003;307:883. doi: 10.1016/s0006-291x(03)01285-3. [DOI] [PubMed] [Google Scholar]
- 30.Thakar R.G. Cheng Q. Patel S. Chu J. Nasir M. Liepmann D., et al. Cell-shape regulation of smooth muscle cell proliferation. Biophys J. 2009;96:3423. doi: 10.1016/j.bpj.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]