Abstract
Efficient transfer of progenitor cells without affecting their survival is a key factor in any practical cell therapy. Fibrin microbeads (FMB) were developed as hard biodegradable cell carriers. The FMB could efficiently isolate mesenchymal stem cells (MSCs) from different sources and support the expansion of matrix-dependent cell types in a three-dimensional culture in slow rotation. The cells on FMB could also undergo induced differentiation for their eventual implantation to enhance tissue regeneration. FMB loaded with isolated human MSC (hMSC) were sealed in tubes topped up with medium. Almost full cell survival was recorded when the sealed cells were maintained in room temperature for up to 10 days, followed by a recovery period of 24 hrs at optimal conditions. Assay of cells recovery after such long room temperature storage showed ∼80%–100% survival of the cells on FMB, with only a marginal survival of cells that were kept in suspension without FMB in the same conditions. The hMSC that survived storage at room temperature preserved their profile of mesenchymal cell surface markers, their rate of proliferation, and their differentiation potential. The cell protective effect was not dependent on the presence of serum in the storage medium. It was clearly shown that over-expression of hypoxia induced factor-1α in hMSC with time, which may have protected the sealed cells on FMB at room temperature storage, was not necessarily related to extreme hypoxic stress. Foreskin normal fibroblasts on FMB sealed at room temperature were similarly protected, but with no elevation of their hypoxia-induced factor-1α expression. The results also show that FMB, unlike other commercially available cell carriers, could be used for delivery and shipping of progenitor cells at room temperature for extended time intervals. This could be highly useful for cell transfer for therapeutic application and for simplified cell transfer between different research centers.
Introduction
Cell-based therapies for tissue regeneration may involve a time lag between the preparation of the relevant cells or cellular matrices and their actual implantation. Special conditions should be implemented for the maintenance of cells for this prolonged time interval out of their optimal controlled conditions of the CO2 incubator in 37°C. It is a common understanding that cells in suspension may not survive for extended time intervals while they are being transferred in ambient conditions.1,2 The development of alternative procedures for the transfer and delivery of the progenitor cells is, therefore, crucial.3,4 This may involve complicated and expensive strategies and special cell culturing conditions, but simple methods with minimal manipulation and maximal survival may be superior and more relevant for clinical applications. In spite of the importance of this issue, reports on the prevention of cell death in suboptimal conditions when cells are transported to the clinic are scarce.5,6
Fibrin microbeads (FMB) are human fibrin-based, dense, nonimmunogenic, and slowly biodegradable particles with prolonged shelf life7,8 that were developed as cell carriers. FMB can serve as a simple and highly efficient tool to isolate mesenchymal stem cells (MSCs) from different mixed sources and to support the expansion of many matrix-dependent cells in a three-dimensional (3D) culture with potential application in cell-based regenerative medicine.9–14 Cells loaded on FMB and cultured in slowly rotating 3D conditions in suspension can reach a high density.9–11,13–16 We previously proposed that cell attachment to FMB is probably aided by conserved sequences on the C-termini of fibrin chains beta and gamma,17 which are probably better exposed on the FMB surface than on the surface of fibrin gels. Eventually, MSC on FMB can undergo mesenchymal differentiation into the cell type of interest, such as chondrocytes and osteoblasts, and express the relevant genes. Eventually, these cells can form on the FMB both in-vitro and in-vivo bone and cartilage-like tissue constructs.9,13,14 Adult differentiated cells, such as foreskin fibroblasts (FFs), could also be cultured on FMB for their possible implantation.15 In recent years, a number of other biodegradable microspheres and microcarriers were proposed for cell culturing in 3D,18–21 but unlike FMB, most other carriers are not multifunctional and do not provide all in one: stem cell separation, expansion, and support them while differentiating and functioning as their carrier for cell implantation for tissue regeneration.
The current study presents a simple FMB-based technique for delivery of mesenchymal cells such as fibroblasts and MSC and, possibly, other matrix-dependent cells in sealed vials at room temperature for relatively long time intervals of >10 days, with high survival rate, without any need for further supportive infrastructure. The role of a possible protective mechanism of MSC attached to FMB by hypoxia-induced factor (HIF)-1 is discussed.
Materials and Methods
Fibrin microbeads
FMB were prepared by a procedure previously described in detail.7 For the current work, the FMB were prepared from paste 2 fibrin-enriched fractionated plasma, which was obtained from NABI Biopharmaceuticals. The raw fibrin rich material was further purified by sedimentation in 10% ethanol in 4°C, reconstitution of the sediment in Tris buffer to yield 60–80 mg/mL soluble clotable protein.7 The fibrinogen solution was treated with ∼1 U/mL thrombin (Omrix) with Ca+2 at a final concentration of 3 mM. On initiation of the clot, the mixture was immediately added to fast stirring, MCT pure mineral oil at 80°C was added to form an emulsion with small droplets. Within 7–9 h of fast stirring in heat, very dense dehydrothermally crosslinked beads were formed from the droplets of the fibrin emulsion, as previously described.7 The resulting solid FMB were thoroughly cleaned from oil and soluble salts by a series of rinses in organic solvent and then by water, ethanol, and acetone; dried; and sieved. The main fraction of FMB, which has the size range of 105–180 μm, is used. Before use, the beads are sterilized by incubation for 12 h in 70% ethanol, which is then replaced by the medium in which the FMB are re-hydrated for a few hours.
Foreskin fibroblasts
Foreskin samples were prepared from skin derived from circumcision of normal 7 day-old new-born babies given as a throw-away material. Commercial FF were prepared by Forticell Biosciences (Ortec International). In-house isolated FF were prepared from dermal connective tissue as follows. The source (∼800 mg) was harvested into a full medium of Dulbecco's modified Eagle's medium+10% fetal calf serum (FCS)+10% penicillin-streptomycin for up to 1.5 h in room temperature. The sample was then cleaned from fat, stripped from epidermal cells, and chopped by scalpel and scissors in sterile conditions into pieces of ∼1–3 mm. The pieces were rinsed several times in normal full medium (Dulbecco's modified Eagle's medium+10% FCS+1% penicillin-streptomycin+1% glutamine, 1% nonessential amino-acids+1% vitamins solution; all from Biological Industries). More than 10 pieces were distributed evenly, placed onto the surface of culture plastic flasks, and left standing overnight vertically in the 37°C 7%CO2 incubator. A day later, 2 mL full medium were added to the flask carefully so that the pieces stayed attached to the surface. Medium was replaced twice weekly for 2–4 weeks till downloaded cells formed a monolayer. Cells reaching confluence were split by trypsinization. Cells were used after 2–3 passages, when the culture stabilized into homogenous fibroblast-shaped cells for any experimental purposes. The isolated expanded cells from each source were tested to have normal chromosomal karyotype.
Separation and growth of human MSC from bone marrow
Samples of bone marrow from four different normal young adult volunteers were purchased from Lonza Biologics. Bone-marrow-derived human MSCs (hMSC) were isolated by FMB by a modified protocol that was previously used for mouse and rat MSC. Essentially, the source bone marrow was diluted 1:3 in full hMSC minimum essential medium-α (MEM-α)+1% penicillin-streptomycin, 1% glutamine (all from Biological Industries) with 20% Gibco FCS (Invitrogen). Nutristem™ (supplied by Biological Industries) was used as serum-free medium (SFM) for a relevant experiment with hMSC.
Diluted bone marrow was loaded on FMB in the ratio of 10 mL/150 μL of hydrated FMB in a 50 mL tube with a filter cap for gas exchanging and incubated for 48 h in rotation. After 48 h of incubation, the medium was changed to remove nonattached cells, and FMB with isolated mesenchymal cells were downloaded on plastic to get a monolayer of pure mesenchymal cells (Fig. 1). Cells from isolated hMSC population after two to three passages were tested positive by fluorescence activation cell sorting (FACS) to hMSCs markers CD160, CD105, CD166, CD90, CD73, and CD146 Anti HLA-ABC. They were also examined for CD45, CD3, HLA-DR, and CD117, which are not expected to be expressed in hMSC.
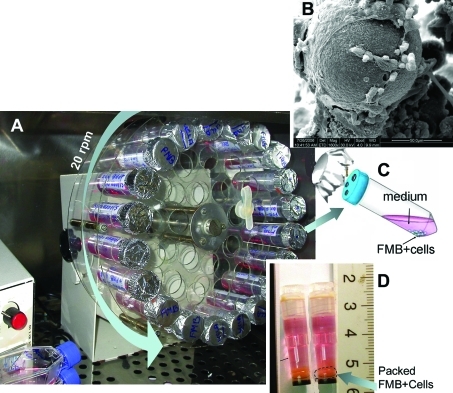
FIG. 1.
The set up of cells cultured on fibrin microbeads (FMB) in regular 50 mL tubes placed in a slow rotator in a CO2 incubator (A). Attached cells on the surface of the FMB are shown in (B) by electron scanning microscopy. The cells grown in special tubes with a covered perforated cover for air exchange with preservation of sterility, as shown in (C). The cells on FMB are loaded for storage in sealed CryoTubes topped up with medium (D). Color images available online at www.liebertonline.com/tec
Extended storage in sealed condition at room temperature
About 50 μL of FMB+cells were loaded into Nunc CryoTubes™ cryovials (Nunc). Subsequently, the tubes were filled with medium with FCS or with Nutristem (volume ∼800 μL) and sealed tightly with special care, avoiding the inclusion of any residual air bubbles. The cells on FMB sealed in CryoTubes were kept in room temperature for different time periods, the cell survival on FMB or in suspension were examined with triplicates at each time point. In addition, the viability of hMSC cells on FMB stored in CryoTubes for 6 days was tested at −4°C, 24°C–27°C (room temperature), and after freezing at −80°C with 10% dimethylsulfoxide (DMSO), bovine serum albumin in different concentration, and/or FCS. At the end of the storage, the frozen cells were thawed by a standard de-freezing procedure. When DMSO was used, the cells on FMB were rinsed after thawing to remove the residual DMSO. The cells on FMB in the different arms were transferred for 24 h recovery in optimal conditions of 7% CO2 incubator at 37°C. The number of cells attached and surviving on the FMB was assayed by the MTS colorimetric cell proliferation assay.
Comparison of cell growth and survival on FMB and diethylaminoethyl-derivatized dextran microspheres (Biosilon)
Biosilon® microspheres (diethyl-aminoethyl-derivatized dextran microspheres, Nalgen; Nunc International) were used for cell culturing in suspension. Five milligram of Biosilon were added to disposable polypropylene tubes and sterilized overnight in ethanol 70%. Then, the beads were washed by culturing medium for hMSC. Cell loading and estimation of cell number on Biosilon were done according to the protocol just described for estimation of cell density relative to a similar amount of FMB by using the modified MTS colorimetric assay.
FACS analysis
For FACS analysis, the cells were trypsinized away from the FMB before storage in room temperature, after 6 days of storage, and 1 week expansion on plastic to reach the adequate cell number needed. For each reading, 20,000 cells were assayed. They were washed in phosphate-buffered saline, suspended in 1 mL of staining buffer (2×106/mL). Each FACS tube contained 60 μL of cell suspension (1.2×105/tube) and 20 μL of antibodies. The cells were incubated with the antibodies for 30 min on ice, rinsed with cold phosphate-buffered saline, and then centrifuged in the cold for 6 min at 1000 rpm. The cell pallet was re-suspended in 400 μL of staining buffer and filtered through 40 μm nylon filter. The antibodies for the different CDs were labeled with either fluorescein isothiocyatate or picoerythrin. The results were compared with adequate isotype controls that served as references. The antibodies were purchased from SA Bioscience.
MTS quantitative assay for cell density, survival, and proliferation
The number of cells on FMB was measured by the MTS assay (CellTiter 96; Promega), which monitors the total number of living cells in the sample. The assay was modified for FMB, as previously described.9 Essentially, tubes of FMB+cells and their matching noncellular negative controls were tested in triplicates. At the end of MTS color development, samples of the supernatant were transferred to 96 wells plate and monitored at OD492 by a computerized OD plate reader (Tecan Sunrise). OD492 results were translated into number of viable cells using a calibration curve of known cell numbers grown on plastic multiwell dishes.
Microscopy
Microscope images were obtained with a DS-R1 color light and fluorescence camera with DS-L2 controller mounted on Eclipse TE200 microscope equipped with Nomarski optics and a fluorescence set-up (all from Nikon). Samples for scanning-electron microscopy were fixed with Karnovsky fixative, pH 7.4, dehydrated in ethanol and Freon, coated with gold, and then subjected to scanning-electron microscopy (FEI Quanta 200).
Nuclear staining to evaluate visually cell density on FMB
An aliquot of FMB loaded with cells was fixed and permeabilized, then 2.5 μL of 500 μg/mL propidium iodide (PI) solution (Sigma) was added for an additional 5 min in the dark. The staining solution was subsequently removed, and the sample of FMB+cells was mounted on a slide by mounting solution (Sigma). The red PI-stained nuclei of the cells loaded on FMB were visualized by fluorescent microscopy.
Induction of hMSC differentiation to bone and cartilage
Bone differentiation was induced as previously described.9,11 Essentially, the MEM-α was supplemented with 20% was supplemented with 20% FCS, 1% glutamate, and 1% penicillin-streptomycin-nystatine. For bone differentiation, 10 mM β glycerolphosphate 10−8 M dexamethasone and 50 μg/mL of ascorbic acid were added for 2 weeks before the assay. For chondrogenesis, the medium was supplemented with 3% FCS, 100 ng/mL TGF-β3, 6.35 insulin, and 50 μg/mL ascorbic acid for 3 weeks.
RNA extraction and real-time polymerase chain reaction
RNA was extracted by a modified Trizol-based protocol (Invitrogene). The samples of cells on matrix were placed in small cap-locked Eppendorf tubes with 1 mL Trizol. After vigorous shaking with small metal steel balls for 30 s, a fully homogenized solution was obtained. Then, 200 μL of chloroform was added and mixed well. After 10 min of incubation in 4°C, the samples were centrifuged at 12,000 g for 15 min. The RNA in the colorless upper aqueous phase was transferred to fresh 1.5 mL Eppendorf tube and precipitated from the aqueous phase by mixing with 0.5 mL 100% isopropyl alcohol. After 10 min of incubation of the samples at room temperature and centrifugation by 12,000 g for 15 min at 4°C, the precipitated RNA pellet was collected and washed with >1 mL 70% and 100% ethanol at 4°C. The RNA pellet was air dried for 3 min, then dissolved in purified RNase-free water (20–50 μL), and stored at −80°C.
The RNA integrity was confirmed by electrophoresis on ethidium bromide-stained 1% agarose gel. The RNA concentration was determined by NanoDrop (Thermo Scientific). A sample of RNA (1 μg) was amplified with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) to generate 20 μL of cDNAs. A sample of 1–2 μL of the cDNA was then quantified by real-time polymerase chain reaction (PCR) using the 7900 HT FAST Real-Time PCR System (Applied Biosystems). TaqMan Gene Expression Master Mix and TaqMan were used with Gene Expression Assays (Applied Biosystems). The quantity of PCR product generated from amplification of the gene was standardized using human β actin house keeping gene (Hs 99999903_A1). The probe for HIF-1α was Hs 00936366_A1. For bone differentiation assay, the probe for bone sialoprotein (BSP) was used (Hs-00913374-g1). For cartilage differentiation, probes for both collagen II (Hs0106356) and aggrecan (Hs00153936_A1) were used. For these differentiation studies, the reference housekeeping probe was that of hTATA binding protein-hTBP (Hs99999910_A1).
Evaluation of gases and pH in the medium before and after storage in room temperature
A sample of the medium in which the cells were cultured was taken from the incubator and collected by a thin needle through the wall of the tubes in which the cells were stored at room temperature. The samples were sealed and immediately taken for analysis (Cobas b 221 Blood Gas system; Roche).
Results
FMB isolation of hMSC and their culture in 3D conditions
hMSC were isolated from bone marrow of four donors with the use of the FMB-based adhesion protocol (Fig. 1A). A higher yield of MSC and improved purity is obtained with this protocol compared with plastic adherence.9–11 After 2 days in rotation, the FMB attached and isolated the mesenchymal cells. The hMSC were downloaded from the FMB to plastic plates after 4 days in culture and expanded for two to three passages as previously shown.9,11,12 The purified expended hMSC were stored and when needed they were reloaded on FMB in a slow, rotating suspension culture (Fig. 1A, B).
Survival of stored cells treated at different conditions and protocols
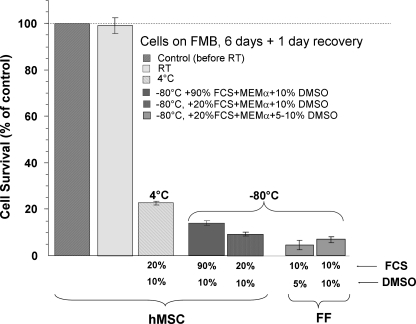
For room temperature storage, hMSC were loaded on FMB by mixing trypsinized cells with sterile FMB and culturing for 1 day, after which the non-FMB attached cells were disposed. The FMB with cells were then sealed in CryoTubes and stored for 6 days at different temperatures; either frozen at −80°C with DMSO and FCS in different concentrations at 4°C or at room temperature (∼24°C). Then, the cells were allowed to recover in a CO2 incubator at 37°C for 24 h; and the number of surviving cells was monitored with the use of the modified MTS assay (Fig. 2). The cells preloaded on FMB and then stored at room temperature survived and were fully recovered. In parallel, cells on FMB were also frozen at −80°C by conventional protocols used for maximal cell recovery with adequate concentrations of DMSO and/or high serum levels, as shown in Figure 2. Only a small fraction of the frozen cells on FMB recovered and survived after the different protocols tested. In parallel, freezing of FF on FMB that was also tested with the same protocol also resulted in poor cell survival. Further, only a small fraction of the cells on FMB that was stored at 4°C for 6 days survived (Fig. 2).
FIG. 2.
Survival of human mesenchymal stem cell (hMSC) attached to FMB and stored tightly sealed in CryoTubes for 6 days at room temperature (∼24°C). The results are compared with storage at 4°C and freezing at −80°C with different levels of fetal calf serum (FCS) and dimethylsulfoxide (DMSO). The results are presented relative to the initial cell number taken from the CO2 incubator, before sealing the cells on FMB in CryoTubes (100%). The advantage of storage of the cells for many days at room temperature is obvious.
Follow-up of cells survival on FMB as a function of time: Comparison of hMSC with FF
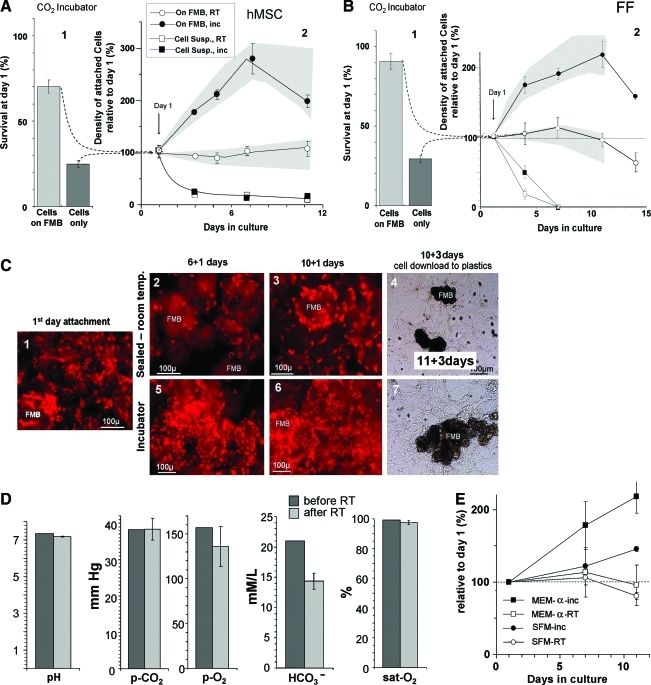
MSCs from bone marrow of four different donors were loaded on FMB, sealed in CryoTubes, and then kept at room temperature for different time intervals. Then, the cells were allowed to recover for 24 h in slow rotation in optimal conditions. In parallel, trypsinized cells were kept in suspension in the same conditions without FMB. Then, the number of cells on FMB or in suspension was evaluated by the MTS assay. A representative full experiment in four replicates with MSC from one donor is given in Figure 3A. Most of the cells that were kept in suspension in slow rotation without FMB died within the first day (∼20% survived). About 80% of the cells incubated with FMB got attached to them and survived the first day (Fig. 3A1). The surviving cells at day 1, both in suspension or on FMB, served as a reference (100%) for further follow-up of cell survival in sealed tubes in room temperature (Fig. 3A2). Tubes with hMSC, both on FMB and in suspension, were cultured in parallel in optimal conditions in a CO2 incubator at 37°C; and their number was assessed at different time points by the MTS assay. At the relevant time points, the sealed vials were opened, and the samples were allowed to recover for 24 h at optimal conditions in rotation in the CO2 incubator. Although the cells on FMB in the incubator continued to proliferate until they reached over-confluence, most hMSCs on FMB in the sealed vials that were kept in room temperature prevailed for up to 10 days with no significant change in their number (Fig. 2B). Most cells that were kept in suspension culture without FMB did not survive more than a few days in both room temperature and optimal conditions at 37°C in the CO2 incubator. The cells that survived were typically organized into aggregates. Similar data were obtained from hMSC isolated from the three donors, and the range of the results was within the shaded areas plotted around the relevant survival curves.
FIG. 3.
Follow-up of the survival of hMSC (A) and foreskin fibroblast (FF) (B) loaded on FMB at different time points after sealing in CryoTubes at room temperature. The results are compared with trypsinized cells in suspension or cells on FMB that were left in optimal conditions in the CO2 incubator 37°C. For each time point, the results are based on triplicate tubes. The range of similar experiments done with four different donors of hMSC (A) or from three FF lines (B) are within the shaded area within each graph. Initially, the cells were mixed with FMB or kept in suspension at 37°C for 24 h in slow rotation; then, the number of residual living cells in each arm was evaluated (A1, B1). The cells on FMB or in suspension were then sealed at room temperature, and MTS assay for the survival at different time points was done. The results were normalized to the initial cell number at day 1 (A2, B2). In parallel, hMSC nuclei stained by propidium iodide (PI) show cell density on minute FMB samples taken and fixed at the different time points (C). A stable density of cells that were kept at room temperature at different time points is evident (C1–C3). The elevated density of the cells that continued to proliferate in the optimal conditions at the incubator to reach confluence are also shown (C1, C5, C6). Eventually, at the end of the follow-up, at day 12, the FMB with cells were placed on a plastic surface, and downloading of functional living cells from both arms was evident (C4, C7). Samples of the medium before and after storage for 6 days in room temperature were taken in syringes and immediately tested for the partial pressure of O2, CO2, and pH (D). The role of the serum in the medium in enhancing survival of hMSC on FMB sealed at room temperature was also evaluated. A comparison of the effect of serum-free medium (Nutristem) relative to the regular FCS containing medium on the survival of cells on FMB sealed at room temperature was evaluated (E). MTS assay showed no significant difference in the survival at room temperature with the different media, though the proliferation rate in optimal conditions was slower with the serum-free Nutristem medium. Color images available online at www.liebertonline.com/tec
As a comparison, similar experiments were done with human normal FFs from three different sources. A similar tendency was seen, suggesting that the phenomenon of cell survival on FMB at room temperature is not limited only to hMSC (Fig. 3B1, 3B2).
To further demonstrate the effect, the cell density on the FMB for both cell types after sealing in room temperature is demonstrated nonquantitatively in fixed samples of FMB with cells taken at different time points and stained with PI to show the density of cells nuclei on the FMB. Although cell density increased in the cells that were allowed to proliferate on FMB in the incubator to reach confluence (Fig. 3C1, 3C5, 3C6), the density of cells on FMB that were kept at room temperature did not change even after 10 days at room temperature (Fig. 3C1–3C3).
Of interest are the findings that long-term storage of cells on FMB sealed in room temperature did not affect both pH and gas level in the medium. When we monitored these parameters in the medium before and after 6 days of room temperature, no major change in the pH and partial pressure of the gases in the medium was recorded in spite of the long-term sealing. The HCO3− level in the medium was the only slightly altered parameter, probably reduced in the process of buffering the pH of the medium within the long-term sealing in room temperature (Fig. 3D).
Effect of SFM on the survival of the sealed hMSC at room temperature
To rule out the possibility of involvement of the FCS in the medium on the survival of hMSC on FMB in room temperature, one of the bone marrow sources was examined for prolonged survival in sealed room temperature conditions using SFM (Nutristem) for stem cells. The survival of the cells on FMB was examined after 5 and 10 days sealing at room temperature, followed by a recovery period of 24 h in the incubator (Fig. 3E). Similar cell survival was recorded in the FCS containing medium and SFM. Only the rate of proliferation of the hMSC on FMB in optimal conditions in the CO2 incubator was somehow slower with SFM.
Cell functions after sealing in room temperature
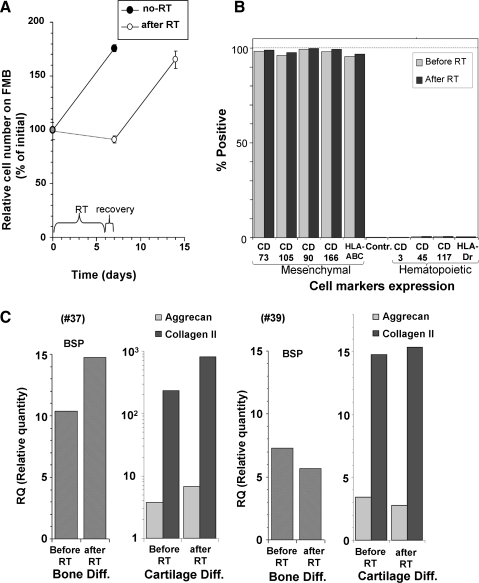
To verify that the cells after room temperature storage and after recovery retained their properties, the rate of proliferation was examined before and after 6 days in room temperature storage. The results indicated that the cells not only fully survived but also retained the same proliferation rate (Fig. 4A). FACS analyses was performed before and after the cell exposure at room temperature. Both mesenchymal and hematopoietic markers were examined. The FACS results showed the purity of the hMSC expressing only the typical mesenchymal markers that were tested (CD105, CD166, CD90, CD73, and HLA-ABC and none of the hematopoietic markers) (Fig. 4B). Moreover, no changes in this profile of markers were recorded after room temperature exposure.
FIG. 4.
The performance and phenotype of the hMSC after 6 days of storage was evaluated. The rate of proliferation was not altered after 6 days at room temperature with 1 day recovery (A). Fluorescence activation cell sorting (FACS) profile of the different typical mesenchymal cell markers and non-mesenchymal markers before and after room temperature storage are presented in (B). In two hMSC samples from different donors, the cells were tested for their gene expression by real-time polymerase chain reaction for osteogeneic and chondrogeneic differentiation before and after storage (C). Both cell lines had a very significant increase in the relevant genes after induction. However, each sample had different levels of the genes expressed. Therefore, the results from these donors are presented separately. Of interest is that storage in room temperature caused no changes in the ability of the cells from both donors to be induced to differentiate to the desired cell types.
The ability of the hMSC to differentiate to both bone and cartilage was fully preserved after room temperature. This issue was examined for cells obtained from two different donors. Though there was a difference between the cells from the two different donors in the degree of the genes expressed for BSP and cartilage (collagen II and aggrecan), after induction for relevant differentiation (Fig. 4C, #37 and #39), in each of the two samples tested, the differentiation potential into these phenotypes was not reduced by the 6 days storing in room temperature and for some genes, it was even somehow elevated.
Correlation of HIF-1α with time of storage in room temperature
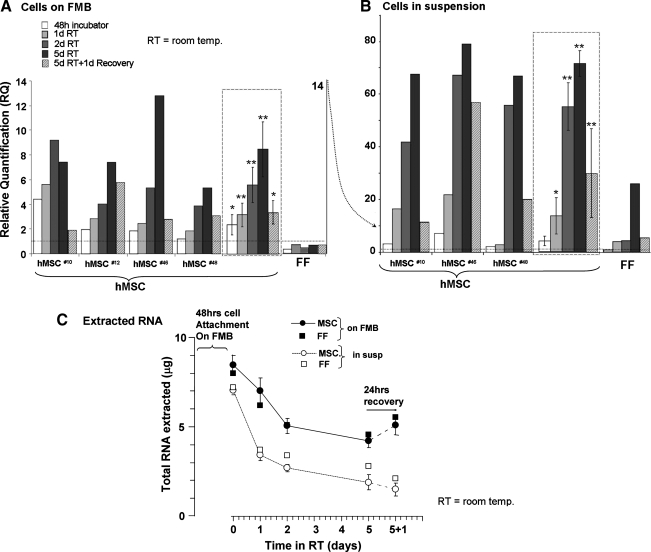
In an attempt to find a major parameter that may be associated with the survival of the cells for extended time intervals on FMB in room temperature, the expression of the HIF1α was examined by real-time quantitative PCR. The reference was the expression of this gene by cells grown in monolayers. Essentially, in all the hMSC loaded on FMB and cultured in 3D at 37°C in the CO2 incubator, a higher expression of HIF1α was observed, even immediately after loading on FMB. After sealing in room temperature, a time-dependent elevation of HIF1α was recorded, reaching a 8.5–12-fold increase in hMSC isolated from the four different donors tested (Fig. 5A). Returning the cells to optimal CO2 incubator conditions for 24 h significantly downregulated the expression of this gene in all the samples as shown for every specific sample and in the average data presented on the right side for each of the cell types tested (Fig. 5A).
FIG. 5.
Follow-up of relative hypoxia-induced factor (HIF)-1α gene expression in hMSC from three different donors sealed at room temperature for up to 5 days, after 1 day recovery, as recorded by real-time polymerase chain reaction. The data derived from the cells from different donors loaded on FMB are presented separately, and the average and errors are given in the frames on the right (A). The data obtained from cell suspension in the same conditions are similarly presented in (B). FF on FMB in the same conditions were tested in parallel (A, B, on the right). The total extractable RNA at different time points and after recovery is also given for both arms of hMSC and FF (C). *p<0.05, **p<0.01.
It is of interest that, unlike hMSC, human FF on FMB which were treated in the same conditions did not show any elevation of HIF1α in 3D culture even after long-term sealing in hypoxic conditions, though they survived in a similar manner (Fig. 5B).
As an additional control, suspended hMSC were sealed in the same conditions at room temperature without FMB. Only a small proportion of hMSC in suspension survived these conditions by spontaneously forming small aggregates. The mRNA was isolated from the cells that survived in such cellular aggregates. The cells in such aggregates were obviously exposed to additional stress of matrix deprivation. When analyzed for HIF1α expression, these survivors showed extremely higher levels of this gene in the range of 40–80-fold, almost an order of magnitude higher than the elevated values recorded for cells on FMB (Fig. 5B). Here again, recovery of the cells for 24 h in optimal conditions significantly reversed the elevation of the expression of HIF1α in these cells. As for FF, unlike the case of cells on FMB, the cells in suspension that survived as aggregate in sealing condition in room temperature, HIF1α expression was elevated, though much less than the elevation recorded for hMSC in the same conditions.
Though the number of living cells on FMB at room temperature was kept constant; we observed that the total amount of extractable cellular RNA was reduced with time. In the case of cells in suspension, there was initially much less extractable RNA, which was further reduced with time (Fig. 5C). At the same time, the gene expression of HIFα was elevated. With the 24 h recovery, the total extractable RNA was restored, whereas the HIFα over-expression in hMSC dropped sharply (Fig. 5A). Although it could be claimed that the general reduction of RNA level in the cells affected the relative calculation of the HIFα levels, it is of notice that the expression level of the reference gene, the human β-actin housekeeping gene, had not changed with time. In FF, a similar decline in the total extractable RNA was recorded in the storage condition at room temperature with no elevation in the HIFα expression.
Comparison of cell storage on FMB relative to Biosilon cell carriers
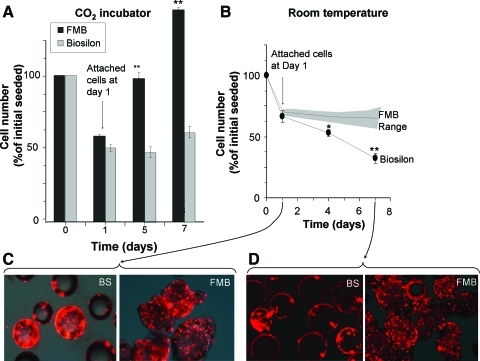
To compare FMB with other carriers, similar quantities of Biosilon microcarriers and FMB were incubated in parallel with the same number of hMSC. The proportion of cells attached to the two types of beads was assayed on the first day, and the rate of proliferation of the cells in the incubator in the CO2 incubator at 37°C was assayed at different time points. In parallel, the cells that attached to the two types of beads were stored at room temperature according to the protocol just mentioned. Although initial cell attachment was similar, the cells proliferated on FMB much faster (Fig. 6A). After sealing of the cells on FMB at room temperature for 6 days, no significant reduction of cell number was recorded on FMB, whereas the survival of cells on Biosilon gradually and significantly decreased with time (Fig. 6B). The difference is also demonstrated in the nuclei-stained cells on both types of carriers, before and after room temperature storage (Fig. 6C).
FIG. 6.
FMB were compared with commercial Biosilon microcarriers for their performance as hMSC carriers and allowing cell survival at room temperature. The proportion of cells attached to the two types of beads was assayed by MTS on the first day, and the rate of proliferation of the cells in the incubator in the CO2 incubator at 37°C was assayed at different time points. The cells attached to the two types of beads were sealed at room temperature according to the protocol just mentioned. Initial cell attachment on Biosilon and FMB and the proliferation rate are shown in (A). Comparison of the survival of hMSCs on FMB and Biosilon after sealing of cells on beads for 6 days at room temperature is shown in (B). The range of data for the hMSC on FMB is taken from the detailed data presented in Figure 3A. PI fluorescence for nuclei staining of cells on FMB or Biosilon with dim light before (day 1) (C) and after sealing in room temperature (day 7) (D), demonstrate the cell density on these beads *p<0.05, **p<0.01. Color images available online at www.liebertonline.com/tec
Discussion
The survival of cells in extreme conditions is highly relevant to cell therapy. Cells in suspension or impregnated within different matrices need to survive the transportation from the laboratory to the clinic or to the operation room where they are expected to be delivered alive and fully functional. The time interval between cell preparation and their implant is not always easily controlled. Strategic planning of the timing and conditions for cell transfer is critical for the success of relevant procedures, and it may affect the efficacy of such treatments. This critical factor may become irrelevant if cells could be transported on FMB sealed at room temperature.
Simple procedures for transport of functional cells for a number of days are needed. This has special relevance for delivery of adult progenitor cells between different centers enabling their maximal survival. So far, the common concept is that to enable the transfer of living functional cells for extended periods, they should be kept in special conditions resembling their normal complex culturing conditions. Another approach is to transfer cells at 4°C for no more than a few hours, similar to the procedures used for organ preservation for implantation. The current study shows that hMSC on FMB, frozen at −80°C by commonly used procedures used for preserving cells in suspension, as well as those cooled in 4°C, did not survive well. Rather, cells on FMB that were just sealed at room temperature fully survived even for many days in such conditions. The phenomenon was dependent on preattachment of the cells to FMB, which provide efficient isolation of MSCs and serve as carriers of choice for matrix-dependent cells grown in suspension culture.8–11,16 The comparison with other gold-standard microcarriers, such as Biosilon, showed the specific advantages of the FMB in enabling faster proliferation of the cells in normal culturing conditions and better survival after being storage sealed at room temperature conditions. Further, the slowly biodegradable FMB could also be used for regenerative medicine, as they enable the isolation of the hMSC from sources such as bone marrow and can eventually support the induced differentiation of the cells and serve as carriers for their implantation.8,13,14
Here, we show that the cells cultured on FMB could also be stored or shipped for >10 days at room temperature with no need for further strategic support. Eventually, after a long period at room temperature, the cells on FMB can fully recover, differentiate, expand, and be transferred for implantation.
Current understanding of the response of mammalian cells to subphysiological temperatures (hypothermia) is less extensive than of the response to heat shock that has been widely studied. Cold shock response in mammalian cells involves the coordination of transcription, translation, cell cycle, metabolism, and cell cytoskeleton organization. However, the exact mechanism by which they are modulated needs to be further elucidated.22 Moreover, mammalian cells respond to mild hypothermia (25°C–35°C) in a different manner than to more severely reduced temperatures (0°C–10°C), and this is reflected in the results obtained in the current study. At moderate temperatures, such as the room temperature used here, cells may show prolonged viability, though cell division and protein synthesis may be slowed down.22 There have been some previous hints on the possible induced protective antiapoptotic effect of hypoxic conditions.23 Cold shock may expose cells to two major types of stress; those related to temperature effect on cellular biochemical processes and those related to the higher oxygen solubility at reduced temperatures.24 The interplay between low temperatures and gases in the medium is complicated and gained specific attention in organ preservation. Although oxygen is necessary for aerobic energy metabolism, it could lead to an increase in reactive oxygen species, resulting in damage to cellular membranes and other cell components. The amount of oxygen consumed by organs in reduced metabolic state at low temperatures is a logarithmic function of temperature.25 Some previous studies showed the advantages of hypoxic preconditioning of MSC to promote their regenerative potential.26 Short-term hypoxic conditions could protect against apoptosis in what was also called metabolic flexibility by a mechanism that has not yet been fully understood.27 Induction of hypoxic mediated increased survival was reported even when the MSC were eventually exposed to low temperature storage.28
It may be suggested that the mechanisms for the drastic protection of the sealed cells on FMB in room temperature may involve the elevation of the expression of the classical hypoxia-related genes such as HIF1α. This indeed happened with hMSC, where a very significant up-regulation of the expression of HIF1α was observed. However, similar prolonged survival was recorded also for FF loaded on FMB in the same conditions, without any significant elevation of the expression of this gene. Moreover, the levels of gases dissolved in the sealed atmosphere-free medium at room temperature have not changed significantly within a week, whereas the pH was found to be stable in the buffered medium. This indicates that sealing cells on FMB at room temperature is not necessary highly stressful in terms of hypoxia. Therefore, the elevation of the expression of HIFα in the sealed hMSC at room temperature may express stress, but probably it is not necessarily the protective mechanism that enables the high cell survival in room temperature.
So far, only a few straightforward differences between hMSC and fibroblasts have been reported, other than the ability of MSC for pluripotent differentiation. In terms of morphology in culture and mesenchymal surface cell markers, they are very similar, though a few reports attempted to demonstrate some specific differences in the balance of surface markers expression on the membranes of the two cell types, which fade in high passages.29 MSC are expected to be able to differentiate into different mesenchymal phenotypes, but there are reports that hint at the possibility that some fibroblasts, especially those from neonates and maternal placenta, could also trans-differentiate to a certain extent to cells of other mesenchymal phenotypes.30–32 In addition, they also showed the ability to induce immunomodulation, which was initially reported as a unique MSC property.30,33–35 We found here clear-cut differences between the FF and hMSC grown on FMB. It could be suggested that since HIF1α expression results from hypoxic or other stress, the MSC may be more sensitive and respond earlier to the hypoxic stress, whereas the conditions were not stressful enough to reach the threshold in fibroblasts. Nevertheless, with matrix deprivation, this gene expression was somehow elevated also in FF.
The use of FMB as a matrix on which cells can be transported at room temperature in sealed conditions with protection of their survival may have enormous implications. These include shipping of living cells in sealed ampoules for long time intervals for clinical regenerative medicine, as well as for cell dispatching between laboratories for prolonged time intervals.
The mechanism of this protection of cells on FMB at room temperature is not straightforward. Further study may find hints as to the possible contribution of specific cell binding epitopes on FMB to this phenomenon. Another major issue that deserves further investigation is the ability to correlate the stemness of mesenchymal cells with their expression of HIF1α in stressful conditions.
Acknowledgments
The study was sponsored by NIH/BARDA grant No. Barda (W.H.M. and R.G.) and partially supported by Hapto Biotech within Bereshith Cell Based Tissue Regeneration Consortium by the Chief Scientist of the MOT, Israel. We would like to thank Irena Pekarski for her technical help in the early stage of this study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Shimony N. Avrahami I. Gorodetsky R. Elkin G. Tzukert K. Zangi L. Levdansky L. Krasny L. Haviv Y.S. A 3D rotary renal and mesenchymal stem cell culture model unveils cell death mechanisms induced by matrix deficiency and low shear stress. Nephrol Dial Transplant. 2008;23:2071. doi: 10.1093/ndt/gfn062. [DOI] [PubMed] [Google Scholar]
- 2.Reddig P.J. Juliano R.L. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 3.Schoenhard J.A. Hatzopoulos A.K. Stem cell therapy: pieces of the puzzle. J Cardiovasc Transl Res. 2010;3:49. doi: 10.1007/s12265-009-9148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenreich M. Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 5.Theus M.H. Wei L. Cui L. Francis K. Hu X. Keogh C. Yu S.P. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Suuronen E.J. Kuraitis D. Ruel M. Improving cell engraftment with tissue engineering. Semin Thorac Cardiovasc Surg. 2008;20:110. doi: 10.1053/j.semtcvs.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Gorodetsky R. Vexler A. Levdansky L. Marx G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. Methods Mol Biol. 2004;238:11. doi: 10.1385/1-59259-428-x:11. [DOI] [PubMed] [Google Scholar]
- 8.Gorodetsky R. Vexler A. Levdansky L. Marx G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. Methods Mol Biol. 2007;238:11. doi: 10.1385/1-59259-428-x:11. [DOI] [PubMed] [Google Scholar]
- 9.Rivkin R. Ben-Ari A. Kassis I. Zangi L. Gaberman E. Levdansky L. Marx G. Gorodetsky R. High-yield isolation, expansion, and differentiation of murine bone marrow-derived mesenchymal stem cells using fibrin microbeads (FMB) Cloning Stem Cells. 2007;9:157. doi: 10.1089/clo.2006.0039. [DOI] [PubMed] [Google Scholar]
- 10.Gurevich O. Vexler A. Marx G. Prigozhina T. Levdansky L. Slavin S. Shimeliovich I. Gorodetsky R. Fibrin microbeads for isolating and growing bone marrow-derived progenitor cells capable of forming bone tissue. Tissue Eng. 2002;8:661. doi: 10.1089/107632702760240571. [DOI] [PubMed] [Google Scholar]
- 11.Zangi L. Rivkin R. Kassis I. Levdansky L. Marx G. Gorodetsky R. High-yield isolation, expansion, and differentiation of rat bone marrow-derived mesenchymal stem cells with fibrin microbeads. Tissue Eng. 2006;12:2343. doi: 10.1089/ten.2006.12.2343. [DOI] [PubMed] [Google Scholar]
- 12.Kassis I. Zangi L. Rivkin R. Levdansky L. Samuel S. Marx G. Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Ari A. Rivkin R. Frishman M. Gaberman E. Levdansky L. Gorodetsky R. Isolation and implantation of bone marrow-derived mesenchymal stem cells with fibrin micro beads to repair a critical-size bone defect in mice. Tissue Eng Part A. 2009;15:2537. doi: 10.1089/ten.tea.2008.0567. [DOI] [PubMed] [Google Scholar]
- 14.Shainer R. Gaberman E. Levdansky L. Gorodetsky R. Efficient isolation and chondrogenic differentiation of adult mesenchymal stem cells with fibrin microbeads and micronized collagen sponges. Regen Med. 2010;5:255. doi: 10.2217/rme.09.90. [DOI] [PubMed] [Google Scholar]
- 15.Gorodetsky R. Clark R.A.F. An J. Gailit J. Levdansky L. Vexler A. Berman E. Marx G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. J Invest Dermatol. 1999;112:866. doi: 10.1046/j.1523-1747.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- 16.Shimony N. Gorodetsky R. Marx G. Gal D. Rivkin R. Ben-Ari A. Landsman A. Haviv Y.S. Fibrin microbeads (FMB) as a 3D platform for kidney gene and cell therapy. Kidney Int. 2006;69:625. doi: 10.1038/sj.ki.5000099. [DOI] [PubMed] [Google Scholar]
- 17.Gorodetsky R. Vexler A. Shamir M. An J. Levdansky L. Shimeliovich I. Marx G. New cell attachment peptide sequences from conserved epitopes in the carboxy termini of fibrinogen. Exp Cell Res. 2003;287:116. doi: 10.1016/s0014-4827(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 18.Kang S.W. Seo S.W. Choi C.Y. Kim B.S. Porous poly(lactic-co-glycolic acid) microsphere as cell culture substrate and cell transplantation vehicle for adipose tissue engineering. Tissue Eng Part C Methods. 2008;14:25. doi: 10.1089/tec.2007.0290. [DOI] [PubMed] [Google Scholar]
- 19.Park J.S. Yang H.N. Woo D.G. Jeon S.Y. Park K.H. The promotion of chondrogenesis, osteogenesis, and adipogenesis of human mesenchymal stem cells by multiple growth factors incorporated into nanosphere-coated microspheres. Biomaterials. 2011;32:28. doi: 10.1016/j.biomaterials.2010.08.088. [DOI] [PubMed] [Google Scholar]
- 20.Chung H.J. Park T.G. Injectable cellular aggregates prepared from biodegradable porous microspheres for adipose tissue engineering. Tissue Eng Part A. 2009;15:1391. doi: 10.1089/ten.tea.2008.0344. [DOI] [PubMed] [Google Scholar]
- 21.Acevedo C.A. Weinstein-Oppenheimer C. Brown D.I. Huebner H. Buchholz R. Young M.E. A mathematical model for the design of fibrin microcapsules with skin cells. Bioprocess Biosyst Eng. 2009;32:341. doi: 10.1007/s00449-008-0253-1. [DOI] [PubMed] [Google Scholar]
- 22.Roobol A. Carden M.J. Newsam R.J. Smales C.M. Biochemical insights into the mechanisms central to the response of mammalian cells to cold stress and subsequent rewarming. FEBS J. 2009;276:286. doi: 10.1111/j.1742-4658.2008.06781.x. [DOI] [PubMed] [Google Scholar]
- 23.Li J.H. Zhang N. Wang J.A. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Invest. 2008;31:103. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- 24.Al-Fageeh M.B. Marchant R.J. Carden M.J. Smales C.M. The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production. Biotechnol Bioeng. 2006;93:829. doi: 10.1002/bit.20789. [DOI] [PubMed] [Google Scholar]
- 25.Fuller B. Guibert E. Rodríguez J. Dormancy and resistance in harsh environments. In: Lubzens E., editor; Cerda J., editor; Clark M., editor. Topics in Genetics. Lessons from Natural Cold Induced Dormancy to Organ Preservation in Medicine and Biotechnology: from the Backwoods to the Bedside. Berlin, Heidelberg: Springer-Verlag; 2010. pp. 253–278. [Google Scholar]
- 26.Volkmer E. Kallukalam B.C. Maertz J. Otto S. Drosse I. Polzer H. Bocker W. Stengele M. Docheva D. Mutschler W. Schieker M. Hypoxic preconditioning of human mesenchymal stem cells overcomes hypoxia-induced inhibition of osteogenic differentiation. Tissue Eng Part A. 2010;16:153. doi: 10.1089/ten.TEA.2009.0021. [DOI] [PubMed] [Google Scholar]
- 27.Mylotte L.A. Duffy A.M. Murphy M. O'Brien T. Samali A. Barry F. Szegezdi E. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008;26:1325. doi: 10.1634/stemcells.2007-1072. [DOI] [PubMed] [Google Scholar]
- 28.Daly P.J. Docherty N.G. Healy D.A. McGuire B.B. Fitzpatrick J.M. Watson R.W. The single insult of hypoxic preconditioning induces an antiapoptotic response in human proximal tubular cells, in vitro, across cold storage. BJU Int. 2009;103:254. doi: 10.1111/j.1464-410X.2008.08010.x. [DOI] [PubMed] [Google Scholar]
- 29.Halfon S. Abramov N. Grinblat B. Ginis I.O. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 30.Haniffa M.A. Wang X.N. Holtick U. Rae M. Isaacs J.D. Dickinson A.M. Hilkens C.M. Collin M.P. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 31.in't Anker P.S. Scherjon S.A. Kleijburg-van der Keur C. de Groot-Swings G.M. Claas F.H. Fibbe W.E. Kanhai H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 32.Ishige I. Nagamura-Inoue T. Honda M.J. Harnprasopwat R. Kido M. Sugimoto M. Nakauchi H. Tojo A. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton's jelly explants of human umbilical cord. Int J Hematol. 2009;90:261. doi: 10.1007/s12185-009-0377-3. [DOI] [PubMed] [Google Scholar]
- 33.Wada N. Bartold P.M. Gronthos S. Human foreskin fibroblasts exert immunomodulatory properties by a different mechanism to bone marrow mesenchymal stem cells. Stem Cells Dev. 2010;20:647. doi: 10.1089/scd.2010.0246. [DOI] [PubMed] [Google Scholar]
- 34.Ackema K.B. Charite J. Mesenchymal stem cells from different organs are characterized by distinct topographic Hox codes. Stem Cells Dev. 2008;17:979. doi: 10.1089/scd.2007.0220. [DOI] [PubMed] [Google Scholar]
- 35.Ishii M. Koike C. Igarashi A. Yamanaka K. Pan H. Higashi Y. Kawaguchi H. Sugiyama M. Kamata N. Iwata T. Matsubara T. Nakamura K. Kurihara H. Tsuji K. Kato Y. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun. 2005;332:297. doi: 10.1016/j.bbrc.2005.04.118. [DOI] [PubMed] [Google Scholar]