Abstract
A short-term mouse model was devised to investigate induction of food refusal by the common foodborne trichothecene deoxynivalenol (DON). DON dose-dependently induced anorexia within 2 h of exposure when administered either by intraperitoneal (ip.) injection or by oral gavage. The no observed adverse effect and lowest observed adverse effect levels in this assay were 0.5 mg/kg bw and 1 mg/kg bw for ip. exposure and 1 mg/kg bw and 2.5 mg/kg bw for oral exposure, respectively. DON’s effects on food intake were transient, lasting up to 3 h at 1 mg/kg bw and up to 6 h at 5 mg/kg bw. Interestingly, a dose-dependent orexigenic response was observed in the 14 h following the initial 2 h food intake measurement. Toxin-treated mice exhibited partial resistance to feed refusal when exposed to DON subsequently after 2 d, but not after 7 d suggesting that this modest tolerance was reversible. The short-term mouse bioassay described here was useful in characterizing DON-induced anorexia and should be applicable to elucidating mechanisms underlying this adverse nutritional effect.
Keywords: Anorexia, Deoxynivalenol, Mycotoxin, Trichothecene
1 Introduction
The frequent presence of deoxynivalenol (DON), a trichothecene mycotoxin produced by Fusarium sp., in cereal grains and processed grain products (Lauren and Smith, 2001; Schothorst and van Egmond, 2004; Vesonder et al., 1973) is of public health concern worldwide (Pieters et al., 2002; Schothorst and van Egmond, 2004; Vesonder and Hesseltine, 1980). Current regulatory standards for DON in foods are based on its ability to cause growth suppression- a primary adverse effect observed in experimental animals chronically exposed to the toxin via diet (Amuzie and Pestka, 2010; Forsyth et al., 1977; Pestka et al., 1989) (Amuzie et al., 2011). DON-induced growth suppression results largely in part from its capacity to inhibit food intake (Arnold et al., 1986; Forsell et al., 1986; Forsyth et al., 1977; Trenholm et al., 1984). The observation that pigs refuse food even when the toxin is infused intraperitoneally suggests that other mechanisms unrelated to palatability are contributory to anorexia evoked by this trichothecene (Prelusky, 1997). Notably, several prior research studies have provided evidence supporting a role for neuroendocrine factors such as serotonin (Prelusky, 1993) (Prelusky and Trenholm, 1993) (Fioramonti et al., 1993) (Girish et al., 2008). Furthermore, DON rapidly activates the innate immune response evoking release of proinflammatory cytokines that are widely recognized to cause anorexia (Pestka, 2010).
To better predict DON’s effects on growth in humans and animals, particularly the young, it is critically important to better understand how this toxin causes anorexia. While investigations of DON-induced feed refusal have typically employed sub-chronic and chronic exposure of mice and rats to the toxin via diet (Forsyth et al., 1977; Pestka and Zhou, 2000, 2002; Trenholm et al., 1984), such approaches are largely incompatible with relating transient changes in neuroendocrine and cytokine factor levels in the gut and brain to immediate feed refusal caused by DON because mechanisms of immediate DON-induced feed refusal would occur before before feed refusal takes place. A further complication is that mouse and rat feeding studies often involve the use powdered diet which can lead to overestimation the amount of food consumed because of spillage from a food jar. Even if DON were included in pelleted diet, rodents have the potential to grind brittle food pellets leaving tiny fragments (“orts”) that can cause up to 30 percent overestimation food intake (Cameron and Speakman, 2010). Finally, it has been recently observed that prolonged exposure to DON in a feeding study might cause tolerance through a variety of metabolic and hormonal compensatory mechanisms(Hattori et al., 2011). Thus, initial feeding responses to this trichothecene might be quite different from those occurring hours or days later.
Recent studies in our laboratory have demonstrated that reduced food intake is detectable in mice within the first day that DON is included in an experimental diet (Amuzie et al., 2011; Hattori et al., 2011), suggesting that it might be feasible to develop a short-term feeding assay amenable to mechanistic exploration. The goal of this research was to test the hypothesis that acute administration of DON to the mouse causes a rapid, measurable and reproducible reduction in food intake. The results presented herein indicate that DON’s anorexigenic effects in this novel model were (1) observable within 2 h, (2) transient, (3) detectable following exposure by intraperitoneal (ip.) injection or oral gavage, and (4) succeeded by both a robust orexigenic response and modest short-lived tolerance to further DON challenge.
2 Materials and methods
2.1 Laboratory animals and DON
Mice were maintained according to National Institute of Health guidelines as overseen by the All University Committee on Animal Use and Care at Michigan State University (Approval No. 01/08-007-00). Female, B6C3F1 mice (10 – 11 wk) were purchased from Charles River Breeding (Portage, MI) were housed singly in polycarbonate cages with aspen bedding sifted to uniform size with a flour sifter. Temperature (21°C – 24°C) and relative humidity (40 – 55%) remained constant and room lights were on a 12 h light (6:00-18:00h) /dark (18:00-6:00h) cycle. Mice were fed pelleted High Fat Diet, (45 kcal% fat diet, Research Diets Inc, New Brunswick, NJ) in 2” high glass jars for 1 wk prior to initiating experiments. HFD was chosen because it is (1) softer than 10 kcal% fat pellet thus facilitating rapid feeding (Ford, 1977) and (2) less easily shattered than very high fat diet (60 kcal% fat) making it easier to recover fragments from cage bottom during intake measurements. DON was obtained from Dr. Tony Durst (University of Ottawa, Canada) and its purity verified by H1NMR. For exposure studies, mice received an injection volume of 100 µl of DON dissolved in phosphate buffered saline (PBS) or PBS vehicle.
2.2 Experimental design
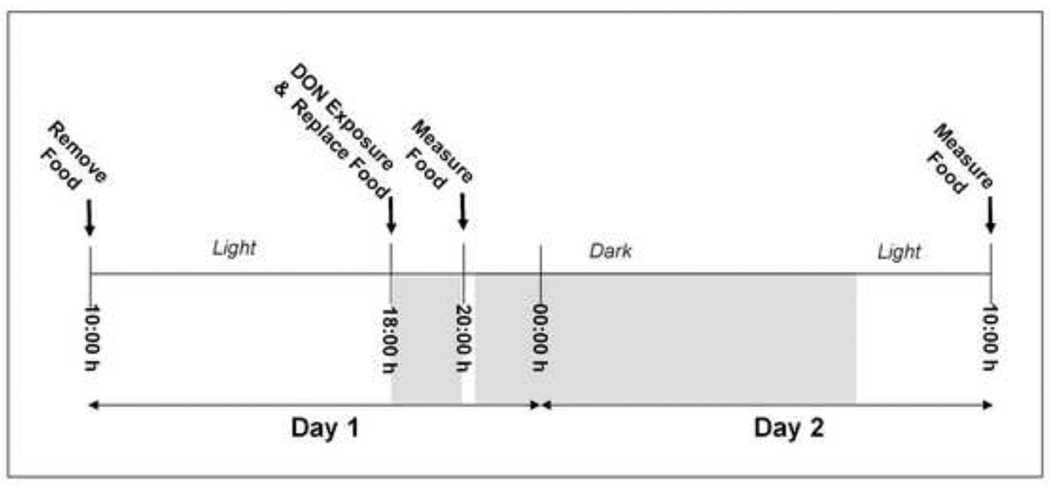
On the day prior to the experiment, mice were randomly distributed into experimental groups based on body weight. On the day of the experiment, mice were fasted from 10:00 h to 18:00 h, but provided free access to water (Fig. 1). For dose response studies, mice were treated at initiation of the dark cycle (18:00 h), by (1) ip. injection of 0, 0.1, 0.5, 1.0, or 2.5 mg/kg bw DON (n = 13/gp) in 100 µl using a sterile 27G, 0.5” needle or (2) oral gavage with 0, 0.5, 1.0, 2.5 or 5.0 mg/kg bw DON (n = 8/gp) in 100 µl using a sterile 22G, 1.5” disposable feeding tube (Instech Solomon; Plymouth Meeting, PA). Following DON exposure, the mice were immediately given two pre-weighed food pellets (≈ 7 g). Lights were turned off and mice were allowed to eat for 2 h. At 20:00 h, lights were turned on for 30 m, food was removed from the food jar and combined with shredded food in the sifted bedding on the cage bottom. The combined food was weighed and replaced back into the feed jar until a second intake measurement (16 h) at 10:00 h on day 2. All procedures were performed quickly and quietly to minimize stress in the animals
Figure 1. Experimental design for mouse feed refusal assay.
Mice are fasted from 10:00 h on Day 1. At 18:00 h, the onset of the dark cycle, mice are treated DON or PBS vehicle and then and then provided with food. At 20:00 h lights were turned on for 30 m and food intake was measured and measured again at 10:00 h on Day 2.
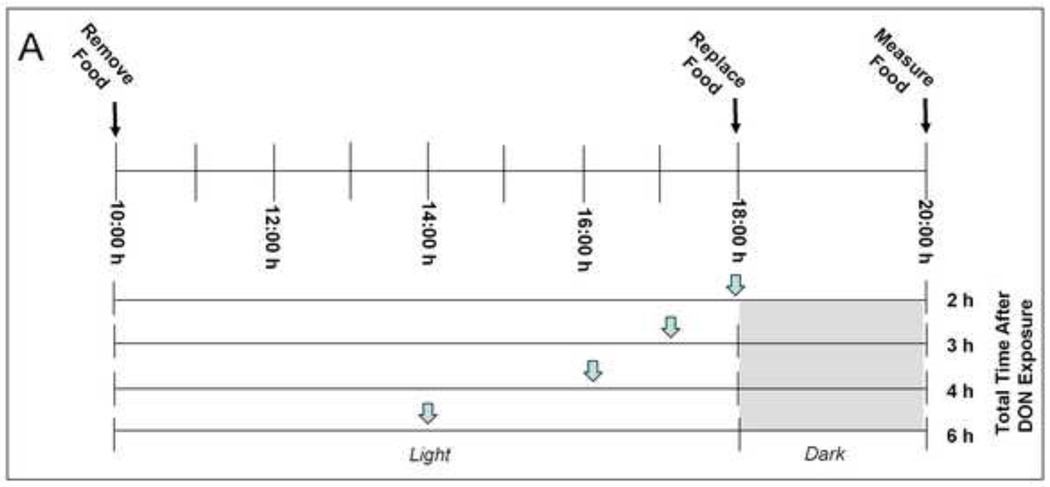
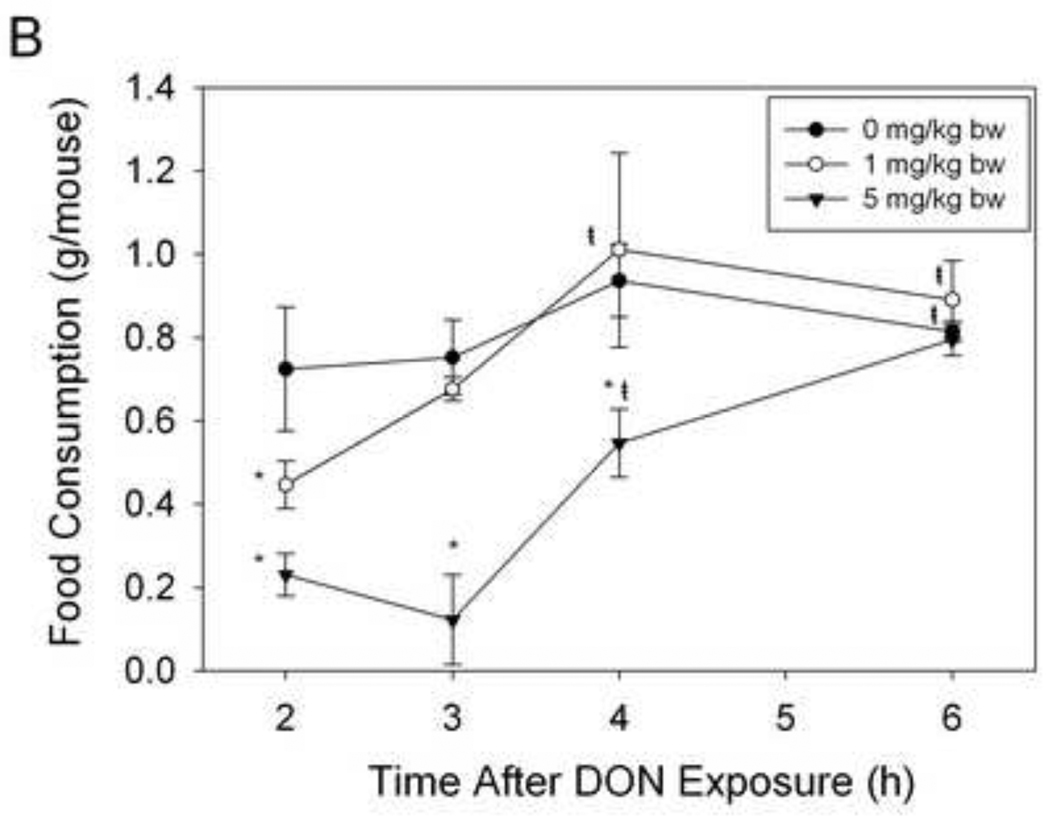
To determine the duration of DON-induced feed refusal, mice were given an ip. injection of 0, 1 or 5 mg/kg bw DON at 0, 1, 2 or 4 h (n = 4 – 5/gp) just prior to the onset of the dark cycle (18:00 h) (Fig. 4A). The remaining food was measured 2 h later. These equated to total DON exposure times of 2, 3, 4 or 6 h upon the completed food intake measurement.
Figure 4. DON-induced feed refusal is transient.
(A) Experimental design for determining the duration of DON-induced feed refusal. Mice were given an ip. injection of DON either 0, 1, 2 or 4 hours before the dark cycle as indicated by gray inverted arrows. Food intake was measured 2 hr after the injection for a total exposure time of 2, 3, 4 and 6 h. (B) At 1 mg/kg bw DON feed refusal is attenuated within 3 h and at 5 mg/kg bw DON, feed refusal is attenuated within 6 h after exposure. Data are mean ± SEM (n = 4–5/gp). Two-Way ANOVA and Holm-Sidak post hoc tests were employed to determine the effects of dose and time on food intake. Symbols: *Food consumption for a given treatment is significantly different from treatment control at specified time (p < 0.05); ŧFood consumption for a given time is significantly different from 2 hr exposure time at specified dose (p < 0.05).
To assess the effect of short-term repeated DON exposures on feed refusal, naïve mice were given an ip. injection of 0, 0.1, 0.5, 1.0 and 2.5 mg/kg bw DON (n = 5/gp) just prior to the onset of the dark cycle (18:00 h). The remaining food was measured 2 h later. Mice were given the same doses 48 h after the previous DON injection and food intake measured after 2 h (exposure 2). 48 h between exposures was chosen to allow for a 1-day rest period, free of DON exposure. This latter protocol was again repeated a third time (exposure 3).
To ascertain the effect of intermittent repeated DON exposure on food intake, mice were exposed to DON by ip. injection or oral gavage twice, with intervening 7 d rest periods. Briefly, naïve mice were given an ip. injection of 0, 1.0 and 2.5 mg/kg bw DON (n = 8/gp) and food intake measured. For the second exposure, mice were randomized, given an ip. injection of 0, 1.0 and 2.5 mg/kg DON (n = 8/gp) and 2 h food intake measured. Similar experiments were performed using oral gavage, except mice were exposed to 0, 0.5, 1.0, 2.5 or 5.0 mg/kg bw DON (n = 8/gp).
2.3 Statistics
All statistics were performed using SigmaPlot 11 (Jandel Scientific; San Rafael, CA) with α = 0.05 and significance established when p < 0.05. The specific statistical tests performed are described in the relevant figure legends. Data from one mouse was consistently deemed a statistical outlier using Grubb’s Test for Outliers (www.graphpad.com) and not included in the statistical analyses.
3 Results
3.1 Ip. and oral exposure to DON causes rapid and transient feed refusal
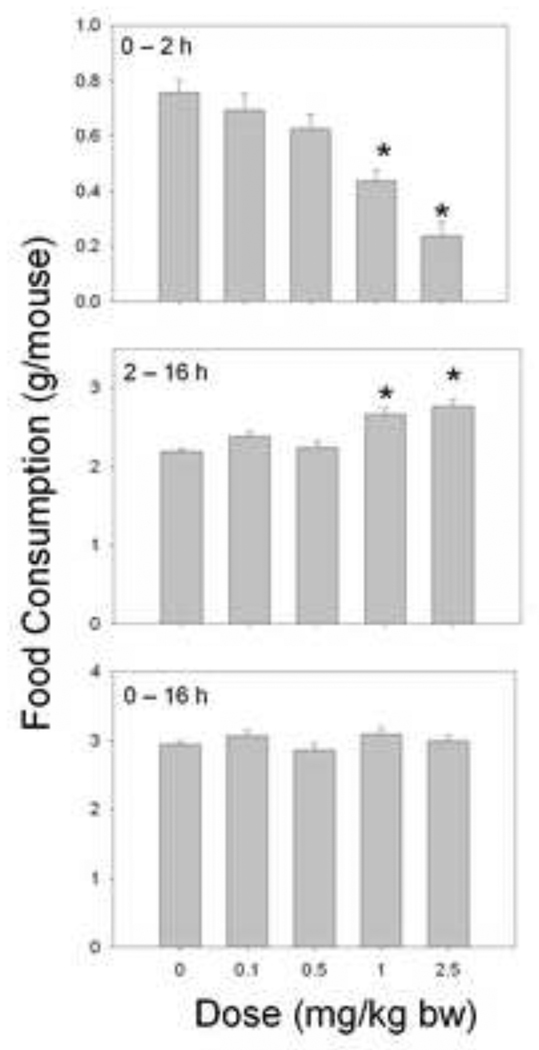
When the effects of ip. injection of DON on food intake were measured using the short-term mouse bioassay (Fig.1), DON was observed to cause significant reduction in food intake within 2 h (Fig. 2). Two hours after exposure to 1 and 2.5 mg/kg bw DON, food intake was reduced by 41% and 69%, respectively, whereas doses of 0.1 and 0.5 mg/kg bw DON were without effect. Conversely, in the 14 h following the initial food intake measurement, 1 and 2.5 mg/kg bw DON caused significant increases in food consumption, eating 21% and 26% more than the control, respectively. When cumulative intakes were compared over the entire 16 h period, no differences were observed in the DON-exposed mice, suggesting that the mice effectively compensated for the initial suppression of food intake.
Figure 2. Intraperitoneal DON exposure in mice induces an early anorexigenic response followed by a delayed orexigenic response.
Mice were given an ip. injection of DON immediately before the dark cycle. Food intake was measured at 2 h and 16 h post injection time and graphically depicted. Data are mean ± SEM (n = 13/gp). A One-way ANOVA using Dunnett’s Test was used to assess significant differences in food intake between doses and the control. Asterisks indicate statistically significant differences in food consumption as compared to the control (p < 0.05).
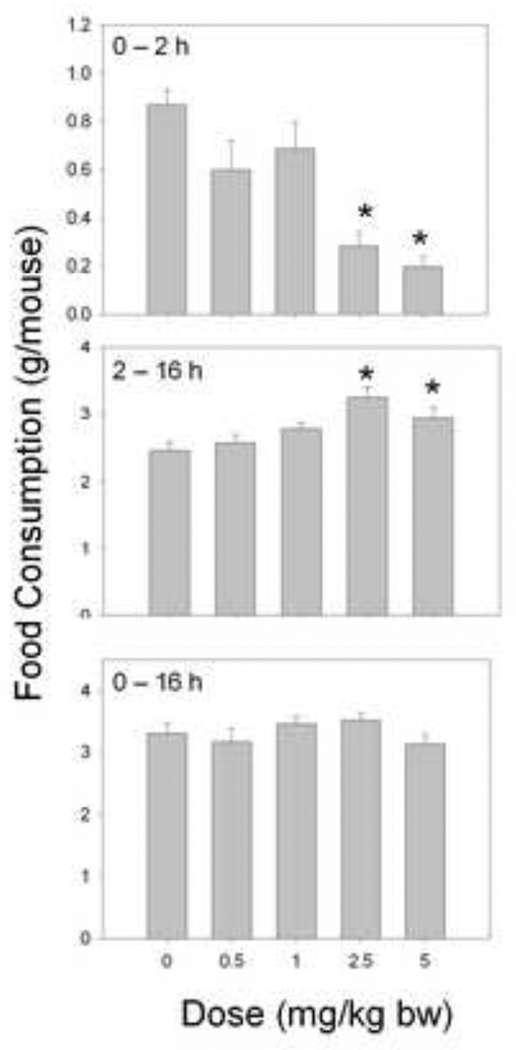
Following administration with 2.5 and 5 mg/kg bw DON by oral gavage, food consumption was reduced by 68% and 77%, respectively, while doses of 0.5 and 1.0 mg/kg bw DON had no effect (Fig.3). In the succeeding 14 h, the 2.5 and 5 mg/kg bw DON groups consumed 32% and 19% more than the control mice, respectively. Again, when cumulative intakes were compared over the entire 16 h period, no differences were observed in the DON-exposed mice.
Figure 3. Oral DON exposure in mice induces an early anorexigenic response followed by a delayed orexigenic response.
Mice were orally gavaged with DON immediately before the dark cycle. Food intake was measured at 2 h and 16 h post DON exposure and graphically depicted. Data are mean ± SEM (n = 8/gp). One-way ANOVA and Dunnett’s test were used to assess significant differences in food intake between doses and the control. Asterisks indicate statistically significant differences in food consumption as compared to the control (p > 0.05).
The duration of DON-induced feed refusal was ascertained by dosing mice with the toxin at various intervals prior to onset of the dark phase and measuring food intake (Fig.4A). At 0 mg/kg bw food intake did not significantly change over time (Fig. 4B). At 1 mg/kg bw DON the effects of feed refusal were no longer evident beginning at 3 h post DON exposure, while at 5 mg/kg bw, feed refusal was no longer evident at 6 h. Thus, as time after toxin exposure increased, food intake increased, suggesting again that DON-induced feed refusal was transient.
3.2 Short-term (2 d) repeated DON exposure evokes partial tolerance to feed refusal effects
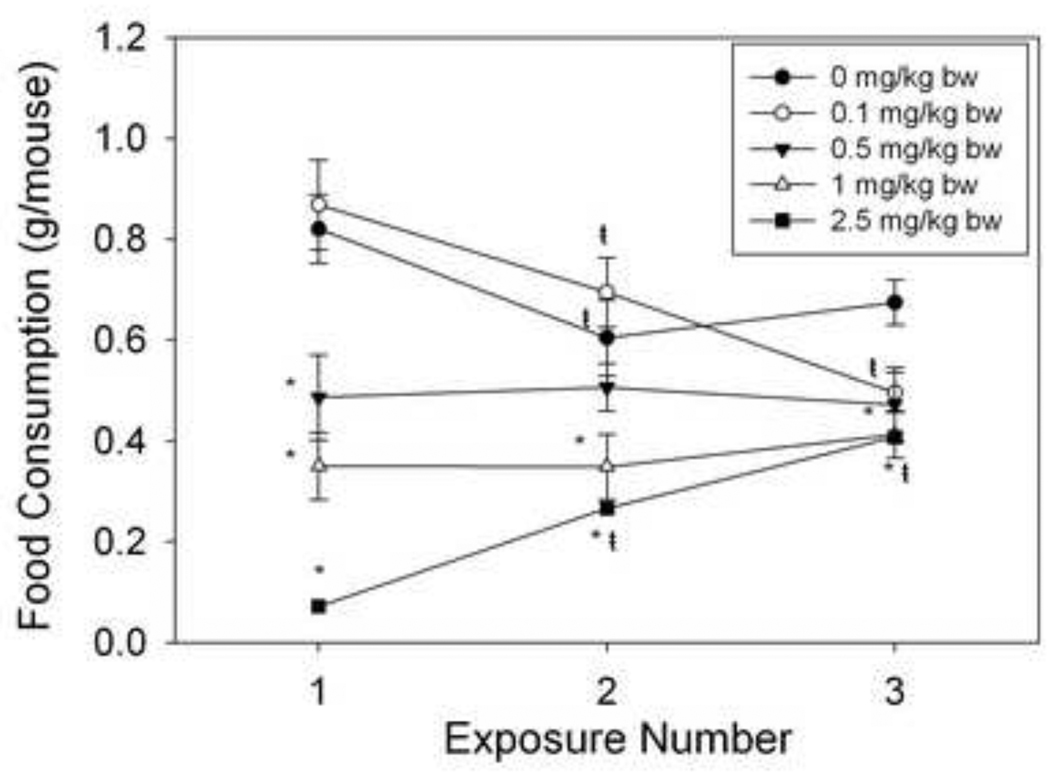
To assess the effect of short-term repeated DON exposure on feed refusal, mice were exposed to DON three separate times, 2 d apart (Fig.5). Mice exposed to 2.5 mg/kg bw DON developed tolerance to DON-induced feed refusal as exposure number increased; In contrast, the opposite was seen for mice exposed to 0.1 mg/kg bw as food intake decreased over multiple exposures.
Figure 5. Short-term repeated DON exposure results in partial tolerance to DON-induced feed refusal.
Mice were given an ip. injection of DON immediately before the dark cycle. Food intake was measured 2 h after injection time. This was repeated twice, 48 h apart. Data are mean ± SEM (n = 5/gp). Two-Way Repeated Measures (one factor) ANOVA and Holm-Sidak post hoc tests were employed to determine the effects of dose and time on food intake. Symbols: *Food consumption for a given treatment is significantly different from treatment control at specified exposure number (p < 0.05). ŧFood consumption for a given exposure number is significantly different from the first exposure at the specified dose (p < 0.05).
3.3 Intermittent (7d) repeated exposure to DON does not evoke tolerance to feed refusal effects
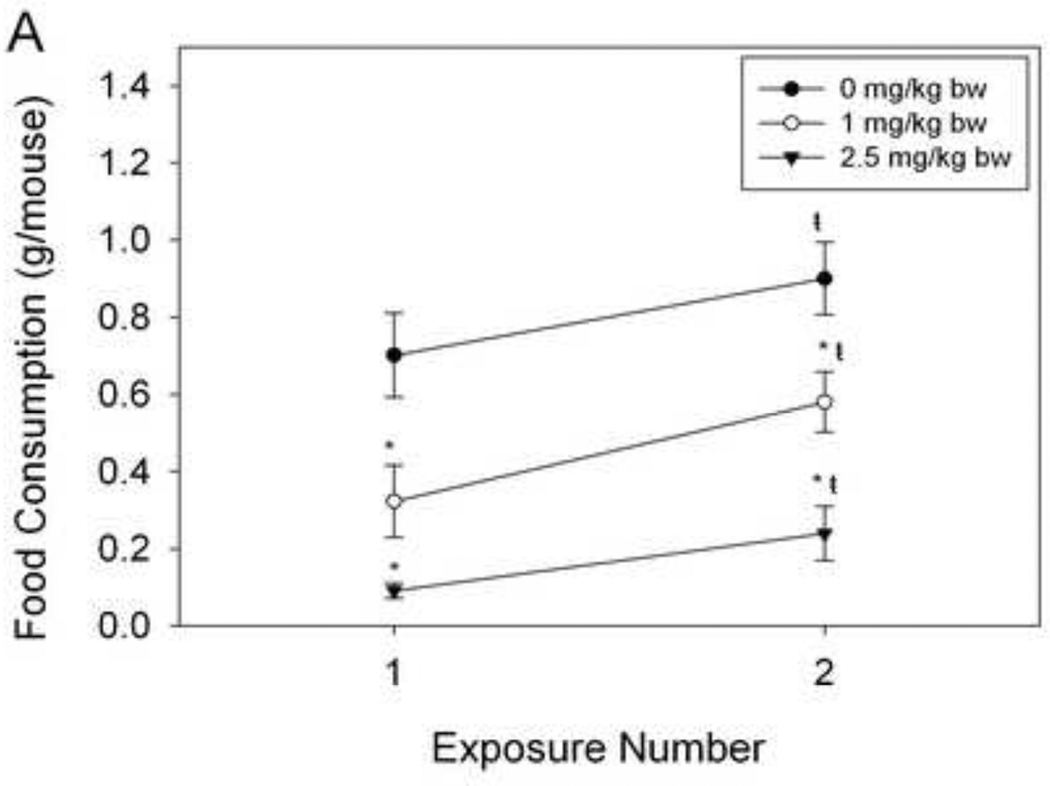
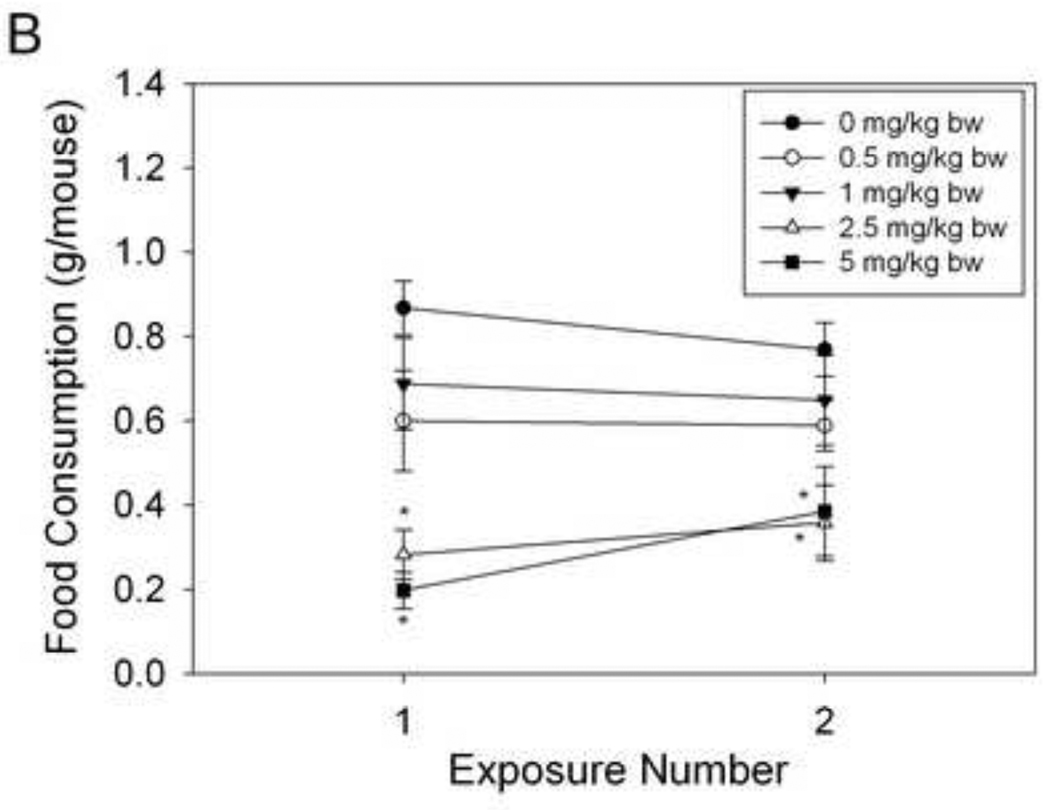
The effects of a longer-term, rest period between ip. injections of DON on food intake were assessed using randomized mice (Fig. 6A). There was a modest increase in food intake after the second DON exposure for 0, 1 and 2.5 mg/kg DON group. However, mice exposed to 1 and 2.5 mg/kg bw exhibited significantly reduced food intake capacity. For oral exposure, as with ip. injection, orally exposed mice significantly reduced food intake as DON dose was increased, independently of exposure number (Fig. 6B). For both exposure methods, DON - treated mice significantly decreased food intake upon the second DON exposure. Thus DON-induced feed refusal persisted after a 7 d rest period.
Figure 6. DON-induced feed refusal tolerance is short-lived.
Mice were treated with DON via (A) ip. injection and (B) oral gavage and food intake measured after 2 h. After randomization, mice were exposed to DON, 7 d later. Data are mean ± SEM (n = 8/gp). Two-Way ANOVA with Holm-Sidak post hoc tests were employed to determine the effects of dose and time on food intake. Symbols: *Food consumption for a given treatment is significantly different from treatment control at specified exposure number (p < 0.05). ŧFood consumption for a given exposure number is significantly different from the first exposure at the specified dose (p < 0.05).
4 Discussion
The results presented herein demonstrate, for the first time, the efficacy of using an acute mouse bioassay to measure DON-induced anorexia and further show that this response is rapid and transient. Specifically, DON-induced feed refusal occurred within 2 h and the duration of the effect was dose-dependent. Mice exposed to DON exhibited increased food intake in the 14 h following the initial food intake measurement thus confirming the transient and reversible nature of DON’s feed refusal effects.
The initial 2 h decrease in food intake may initiate compensatory mechanisms that increase food intake in the 2 – 16 h following DON exposure. Fasted rats compensate for lack of food intake by decreasing the anorexic hormone leptin and by increasing hypothalamic mRNA expression of the orexigenic peptide neuropeptide Y (NPY) (Vuagnat et al., 1998). Recently, we have shown that upon subchronic DON exposure, a decrease in both food intake and weight is correlated to a decrease in serum leptin levels and increased hypothalamic mRNA expression levels of the orexigenic peptide, agouti- related protein (AgRP) (Hattori et al., 2011). Therefore, it is possible that the compensatory increase in food intake exhibited by mice acutely exposed to DON, may be due to changes in leptin and mRNA expression of orexigenic peptides in the hypothalamus.
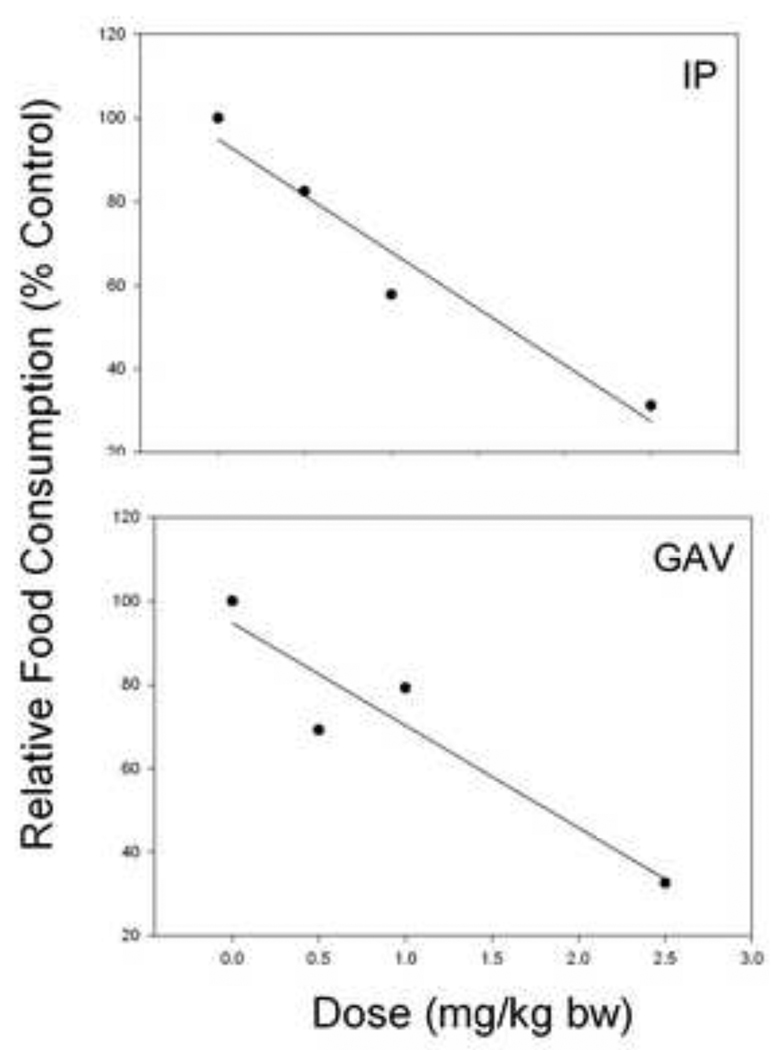
In addition to exposing mice to DON by ip. injection, oral gavage was employed as a complementary method because humans are primarily exposed to DON though consumption of food. It was therefore notable that the NOAEL for DON was 0.5 and 1.0 mg/kg bw following ip. and oral exposure, respectively. Linear regression analysis revealed that ip. injection was slightly more efficacious than gavage at causing 50% feed refusal (Fig.7). A 50% reduction in food intake occurred at 1.66 mg/kg bw DON (r2 = 0.88) for ip. Administration and 1.83 mg/kg bw DON for oral administration (r2 = 0.946). Consistent with our findings here, the minimum emetic dose in swine for DON ip. injection is lower than the minimum emetic dose for the toxin when administered by oral gavage (Forsyth et al., 1977) (Forsell et al., 1987). Not only might these differences relate to the different bioavailabilities of the toxin when administered by the two different methods, but also to the sensitivity of the pig versus the mouse to DON exposure and the respective endpoint measurements (emesis versus feed refusal).
Figure 7. Comparison of feed refusal induction by ip. injection and oral gavage of DON.
To determine the dose at which mice exhibit 50% reduction in food intake relative to the control, a linear regression was calculated for ip. injection (Fig. 2) (y = 94.852 – 26.961x; r2 = 0.94) and for gavage (Fig. 3) (y = 94.741 – 24.496x; r2 = 0.88) with×being dose and y being percent food consumption relative to the control.
Humans are exposed to DON through a variety of grain products (Turner et al., 2008). Since people consume grain products regularly throughout a lifetime, they are likely to be exposed to DON multiple times. Our data suggest that multiple exposures to DON 48 hr apart resulted in partial tolerance to DON-induced feed at the highest dose tested. In contrast, at the lowest DON dose examined, mice exhibited increased sensitivity to DON-induced feed refusal. Thus mice became more or less susceptible to DON’s feed refusal effects depending on dose and exposure number. Since these findings might have implications in assessing the risk posed to humans by DON in food, further research is needed to clarify the effects of dose and exposure number on DON-induced anorexia.
Although the mechanisms for DON’s anorectic effects are not well-understood, they potentially involve altered neuroendocrine and cytokine signaling within the gut-brain axis. The brain, particularly the hindbrain (including the area postrema) and hypothalamus, can signal immediate changes in food intake (Schwartz, 2006). DON exposure has also been reported to cause changes in brain neurochemistry in turkeys, pigs and chickens (Girish et al., 2008; Swamy et al., 2004). Moreover, DON-induced conditioned taste aversion to saccharin in rats can be attenuated by area postrema ablation, suggesting the role of the hindbrain in DON-induced food intake changes (Ossenkopp et al., 1994). Indeed, DON is distributed to brain tissue rapidly (5 min) following oral exposure in the mouse (Pestka et al., 2008) and it might be speculated that this could contribute to altered neuronal signaling that evokes anorexia.
DON is known to rapidly induce in mice the systemic production of interleukin-1β, interleukin-6 and tumor necrosis factor-α (Amuzie et al., 2008; Amuzie et al., 2009; Azcona-Olivera et al., 1995). These proinflammatory cytokines reduce food intake when administered into the brain (Lawrence and Rothwell, 2001; Plata-Salaman et al., 1988; Plata-Salaman et al., 1996; Sonti et al., 1996; Wallenius et al., 2002). Since circulating proinflammatory cytokines can reach the brain (Banks et al., 1994), it could be speculated that the systemic induction of proinflammatory cytokines by DON leads increased cytokine levels in the brain and subsequent feed refusal.
In addition to the ability of proinflammary cytokines to cause anorexia, increased proinflammatory cytokines can lead to altered growth hormone signaling and growth suppression through the activation of suppressors of cytokine signaling 3 (SOCS3) (Boisclair et al., 2000). Acute exposure to DON has been shown to increase hepatic SOCS3 mRNA expression and decrease hepatic mRNA expression of the binding partner of insulin-like growth factor 1 (IGF-1), insulin-like growth factor acid labile subunit (IGFALS) (Amuzie et al., 2009); though decreases in the plasma levels of IGF-1 and IGFALS have not been seen until 7 d DON exposure (Amuzie and Pestka, 2010). DON capacity to decrease food intake may cause decreases in plasma levels of IGF-1 and IGFALS and subsequent growth suppression as it has been well documented that anorexia leads to decreases in plasma IGF-1 and IGFALS (Merimee et al., 1982) (Fukuda et al., 1999).
Serotonin (5-HT), a neurotransmitter produced primarily in the gastrointestinal tract, has been implicated in DON-induced feed refusal and vomiting. Increased levels of the serotonin metabolite, 5-hydroxy indoleic acid (5-HIAA) are found in the cerebral spinal fluid of DON-exposed pigs (Prelusky, 1993). It was also observed that the 5-HT2 receptor inhibitor, cyropheptadine inhibits DON-induced feed refusal in mice (Prelusky et al., 1997). Interestingly, oral administration of DON causes slowing of gastrointestinal motility in mice and rats, which can be reversed by the 5-HT3 receptor antagonists granisetron and ondansetron (Fioramonti et al., 1993). Although detectable changes in plasma concentrations of serotonin have not been observed in DON-exposed animals (Prelusky, 1994), the potential exists for this neurotransmitter to act locally at the intestinal level by stimulating receptors on vagal afferents, which can signal the hindbrain and ultimately cause changes in appetite.
It is further possible that the presence of DON alters oral-sensory properties of a food. In support of this contention, pigs exposed to DON-amended food exhibited greater feed refusal than those given unamended food and administered DON by infusion (i.v.) (Prelusky, 1997). This feed refusal seen with DON-contaminated food may be due to an inherent objectionable sensory property of DON being found with in food. In support of this contention, it has been reported that many toxic compounds may taste bitter, though the degree of bitterness does not always relate directly to the degree of toxicity (Glendinning, 2007). Even though experiments described herein administered DON though ip. injection and oral gavage, DON might still activate bitter taste receptors (T2Rs) found throughout the gastrointestinal tract (Wu et al., 2002) thus cause feed refusal of even uncontaminated food.
Although mice and other rodents are incapable of vomiting (Ueno et al., 1987), similarities exist between anorexia induction observed here and emetic responses to DON relative to their rapidity and transient nature. For example pigs vomit within 30 min of being injected or gavaged with DON and the response lasts up 1 h (Forsyth et al., 1977) (Pestka et al., 1987; Young et al., 1983). Indeed, even though mice are not capable of vomiting, they have acquired the ability to (1) rapidly develop conditioned taste aversions to toxins, (2) delay gastric emptying and (3) decrease food intake to prevent further delivery of the known emetic stimuli (Andrews and Horn, 2006). Thus, DON-induced feed refusal in the mouse, a non-emetic species, might serve as a surrogate for this toxin’s in species capable of emesis.
To summarize, using a short-term (2 h) mouse bioassay, we have demonstrated that DON causes robust, but transient feed refusal followed a delayed orexigenic response. Additionally, while prior toxin exposures partially attenuated anorexia induced by DON, no such tolerance was evident after 7 d rest from toxin exposure. This short-term model for DON-induced anorexia should be applicable to determining the putative roles of neuroendocrine factors, proinflammatory cytokines and bitter taste receptors in DON-induced feed refusal. Such studies could enable an improved, mechanism-based understanding of the nutritional risks posed to humans and animals from acute and chronic DON exposure.
We model induction of deoxynivalenol-induced anorexia in the mouse. The toxins effects on food intake were transient, lasting up to 6 h. Dose-dependent orexigenic response after 2 h food intake measurement. Bioassay will be used to understand trichothecene-induced anorexia.
Acknowledgements
We would like to acknowledge the technical assistance of Dr. Dale Romsos and the physical assistance of Xiao Pan and Mary Rosner.
This study was supported by United States Department of Agriculture Wheat and Barley SCAB Initiative and by Public Health Service Grants ES03553 and DK058833 from the National Institutes for Health.
Abbreviations
- DON
deoxynivalenol
- ip.
intraperitoneal
- ANOVA
Analysis of Variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared no conflict of interest.
References
- Amuzie CJ, Flannery BM, Ulrich AM, Pestka JJ. Effects of deoxynivalenol consumption on body weight and adiposity in the diet-induced obese mouse. Journal of Toxicology and Environmental Health. 2011;74:658–667. doi: 10.1080/15287394.2011.539119. [DOI] [PubMed] [Google Scholar]
- Amuzie CJ, Harkema JR, Pestka JJ. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: comparison of nasal vs. oral exposure. Toxicology. 2008;248:39–44. doi: 10.1016/j.tox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Amuzie CJ, Pestka JJ. Suppression of insulin-like growth factor acid-labile subunit expression--a novel mechanism for deoxynivalenol-induced growth retardation. Toxicol Sci. 2010;113:412–421. doi: 10.1093/toxsci/kfp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuzie CJ, Shinozuka J, Pestka JJ. Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol Sci. 2009;111:277–287. doi: 10.1093/toxsci/kfp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DL, McGuire PF, Nera EA, Karpinski KF, Bickis MG, Zawidzka ZZ, Fernie S, Vesonder RF. The toxicity of orally administered deoxynivalenol (vomitoxin) in rats and mice. Food Chem Toxicol. 1986;24:935–941. doi: 10.1016/0278-6915(86)90321-2. [DOI] [PubMed] [Google Scholar]
- Azcona-Olivera JI, Ouyang Y, Murtha J, Chu FS, Pestka JJ. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicol Appl Pharmacol. 1995;133:109–120. doi: 10.1006/taap.1995.1132. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Boisclair YR, Wang J, Shi J, Hurst KR, Ooi GT. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1beta on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J Biol Chem. 2000;275:3841–3847. doi: 10.1074/jbc.275.6.3841. [DOI] [PubMed] [Google Scholar]
- Cameron KM, Speakman JR. The extent and function of 'food grinding' in the laboratory mouse (Mus musculus) Lab Anim. 2010;44:298–304. doi: 10.1258/la.2010.010002. [DOI] [PubMed] [Google Scholar]
- Fioramonti J, Dupuy C, Dupuy J, Bueno L. The mycotoxin, deoxynivalenol, delays gastric emptying through serotonin-3 receptors in rodents. J Pharmacol Exp Ther. 1993;266:1255–1260. [PubMed] [Google Scholar]
- Ford DJ. Influence of diet pellet hardness and particle size on food utilization by mice, rats and hamsters. Lab Anim. 1977;11:241–246. doi: 10.1258/002367777780936486. [DOI] [PubMed] [Google Scholar]
- Forsell JH, Jensen R, Tai JH, Witt M, Lin WS, Pestka JJ. Comparison of acute toxicities of deoxynivalenol (vomitoxin) and 15-acetyldeoxynivalenol in the B6C3F1 mouse. Food Chem Toxicol. 1987;25:155–162. doi: 10.1016/0278-6915(87)90149-9. [DOI] [PubMed] [Google Scholar]
- Forsell JH, Witt MF, Tai JH, Jensen R, Pestka JJ. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 1986;24:213–219. doi: 10.1016/0278-6915(86)90231-0. [DOI] [PubMed] [Google Scholar]
- Forsyth DM, Yoshizawa T, Morooka N, Tuite J. Emetic and refusal activity of deoxynivalenol to swine. Appl Environ Microbiol. 1977;34:547–552. doi: 10.1128/aem.34.5.547-552.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda I, Hotta M, Hizuka N, Takano K, Ishikawa Y, Asakawa-Yasumoto K, Tagami E, Demura H. Decreased serum levels of acid-labile subunit in patients with anorexia nervosa. J Clin Endocrinol Metab. 1999;84:2034–2036. doi: 10.1210/jcem.84.6.5737. [DOI] [PubMed] [Google Scholar]
- Girish CK, MacDonald EJ, Scheinin M, Smith TK. Effects of feedborne fusarium mycotoxins on brain regional neurochemistry of turkeys. Poult Sci. 2008;87:1295–1302. doi: 10.3382/ps.2008-00025. [DOI] [PubMed] [Google Scholar]
- Glendinning JI. How do predators cope with chemically defended foods? Biol Bull. 2007;213:252–266. doi: 10.2307/25066643. [DOI] [PubMed] [Google Scholar]
- Hattori KK, Amuzie CJ, Flannery BM, Pestka JJ. Body Composition and Hormonal Effects Following Exposure to Mycotoxin Deoxynivalenol in the High Fat Diet-Induced Obese Mouse. Molecular Food and Nutrition Research. 2011 doi: 10.1002/mnfr.201000640. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren DR, Smith WA. Stability of the fusarium mycotoxins nivalenol, deoxynivalenol and zearalenone in ground maize under typical cooking environments. Food Addit Contam. 2001;18:1011–1016. doi: 10.1080/02652030110052283. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Rothwell NJ. Anorexic but not pyrogenic actions of interleukin-1 are modulated by central melanocortin-3/4 receptors in the rat. J Neuroendocrinol. 2001;13:490–495. doi: 10.1046/j.1365-2826.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors in the fed and fasted states. J Clin Endocrinol Metab. 1982;55:999–1002. doi: 10.1210/jcem-55-5-999. [DOI] [PubMed] [Google Scholar]
- Ossenkopp KP, Hirst M, Rapley WA. Deoxynivalenol (vomitoxin)-induced conditioned taste aversions in rats are mediated by the chemosensitive area postrema. Pharmacol Biochem Behav. 1994;47:363–367. doi: 10.1016/0091-3057(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol. 2010;84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Islam Z, Amuzie CJ. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol Lett. 2008;178:83–87. doi: 10.1016/j.toxlet.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Lin WS, Miller ER. Emetic activity of the trichothecene 15-acetyldeoxynivalenol in swine. Food Chem Toxicol. 1987;25:855–858. doi: 10.1016/0278-6915(87)90264-x. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Moorman MA, Warner RL. Dysregulation of IgA production and IgA nephropathy induced by the trichothecene vomitoxin. Food Chem Toxicol. 1989;27:361–368. doi: 10.1016/0278-6915(89)90141-5. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR. Interleukin-6-deficient mice refractory to IgA dysregulation but not anorexia induction by vomitoxin (deoxynivalenol) ingestion. Food Chem Toxicol. 2000;38:565–575. doi: 10.1016/s0278-6915(00)00041-7. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR. Effects of tumor necrosis factor type 1 and 2 receptor deficiencies on anorexia, growth and IgA dysregulation in mice exposed to the trichothecene vomitoxin. Food Chem Toxicol. 2002;40:1623–1631. doi: 10.1016/s0278-6915(02)00153-9. [DOI] [PubMed] [Google Scholar]
- Pieters MN, Freijer J, Baars BJ, Fiolet DC, van Klaveren J, Slob W. Risk assessment of deoxynivalenol in food: concentration limits, exposure and effects. Adv Exp Med Biol. 2002;504:235–248. doi: 10.1007/978-1-4615-0629-4_25. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Oomura Y, Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1988;448:106–114. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Sonti G, Borkoski JP, Wilson CD, French-Mullen JMb. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- Prelusky DB. The effect of low-level deoxynivalenol on neurotransmitter levels measured in pig cerebral spinal fluid. J Environ Sci Health B. 1993;28:731–761. doi: 10.1080/03601239309372851. [DOI] [PubMed] [Google Scholar]
- Prelusky DB. The effect of deoxynivalenol on serotoninergic neurotransmitter levels in pig blood. J Environ Sci Health B. 1994;29:1203–1218. doi: 10.1080/03601239409372923. [DOI] [PubMed] [Google Scholar]
- Prelusky DB. Effect of intraperitoneal infusion of deoxynivalenol on feed consumption and weight gain in the pig. Nat Toxins. 1997;5:121–125. doi: 10.1002/1522-7189(1997)5:3<121::AID-NT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Rotter BA, Thompson BK, Trenholm HL. Effect of the appetite stimulant cyproheptadine on deoxynivalenol-induced reductions in feed consumption and weight gain in the mouse. J Environ Sci Health B. 1997;32:429–448. doi: 10.1080/03601239709373096. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Trenholm HL. The efficacy of various classes of anti-emetics in preventing deoxynivalenol-induced vomiting in swine. Nat Toxins. 1993;1:296–302. doi: 10.1002/nt.2620010508. [DOI] [PubMed] [Google Scholar]
- Schothorst RC, van Egmond HP. Report from SCOOP task 3.2.10 "collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states". Subtask: trichothecenes. Toxicol Lett. 2004;153:133–143. doi: 10.1016/j.toxlet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14 Suppl 1:1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- Sonti G, Ilyin SE, Plata-Salaman CR. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol. 1996;270:R1394–R1402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- Swamy HV, Smith TK, MacDonald EJ. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on brain regional neurochemistry of starter pigs and broiler chickens. J Anim Sci. 2004;82:2131–2139. doi: 10.2527/2004.8272131x. [DOI] [PubMed] [Google Scholar]
- Trenholm HL, Hamilton RM, Friend DW, Thompson BK, Hartin KE. Feeding trials with vomitoxin (deoxynivalenol)-contaminated wheat: effects on swine, poultry, and dairy cattle. J Am Vet Med Assoc. 1984;185:527–531. [PubMed] [Google Scholar]
- Turner PC, Rothwell JA, White KL, Gong Y, Cade JE, Wild CP. Urinary deoxynivalenol is correlated with cereal intake in individuals from the United kingdom. Environ Health Perspect. 2008;116:21–25. doi: 10.1289/ehp.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41:513–518. doi: 10.1016/0024-3205(87)90229-3. [DOI] [PubMed] [Google Scholar]
- Vesonder RF, Ciegler A, Jensen AH. Isolation of the emetic principle from Fusarium-infected corn. Appl Microbiol. 1973;26:1008–1010. doi: 10.1128/am.26.6.1008-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesonder RF, Hesseltine CW. Vomitoxin: Natural Occurrence on Cereal Grains and Significance as a Refusal and Emetic Factor to Swine. Process Biochemistry. 1980;16 [Google Scholar]
- Vuagnat BA, Pierroz DD, Lalaoui M, Englaro P, Pralong FP, Blum WF, Aubert ML. Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology. 1998;67:291–300. doi: 10.1159/000054326. [DOI] [PubMed] [Google Scholar]
- Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LG, McGirr L, Valli VE, Lumsden JH, Lun A. Vomitoxin in corn fed to young pigs. J Anim Sci. 1983;57:655–664. doi: 10.2527/jas1983.573655x. [DOI] [PubMed] [Google Scholar]