Abstract
Fibrotic obliteration of the small airways leading to progressive airflow obstruction, termed bronchiolitis obliterans syndrome (BOS), is the major cause of poor outcomes after lung transplantation. We recently demonstrated that a donor-derived population of multipotent mesenchymal stem cells (MSCs) can be isolated from the bronchoalveolar lavage (BAL) fluid of human lung transplant recipients. Herein, we study the organ specificity of these cells and investigate the role of local mesenchymal progenitors in fibrogenesis after lung transplantation. We demonstrate that human lung allograft–derived MSCs uniquely express embryonic lung mesenchyme–associated transcription factors with a 35,000-fold higher expression of forkhead/winged helix transcription factor forkhead box (FOXF1) noted in lung compared with bone marrow MSCs. Fibrotic differentiation of MSCs isolated from normal lung allografts was noted in the presence of profibrotic mediators associated with BOS, including transforming growth factor-β and IL-13. MSCs isolated from patients with BOS demonstrated increased expression of α-SMA and collagen I when compared with non-BOS controls, consistent with a stable in vivo fibrotic phenotype. FOXF1 mRNA expression in the BAL cell pellet correlated with the number of MSCs in the BAL fluid, and myofibroblasts present in the fibrotic lesions expressed FOXF1 by in situ hybridization. These data suggest a key role for local tissue-specific, organ-resident, mesenchymal precursors in the fibrogenic processes in human adult lungs.
Chronic allograft rejection develops in up to 60% of patients who undergo lung transplantation by 5 years and is the leading cause of poor long-term outcomes after lung transplantation.1 As with other solid organ transplants, chronic allograft rejection in the lung manifests as a fibrotic response to repeated immune and nonimmune insults, leading to narrowing and obliteration of the small airways, a lesion termed bronchiolitis obliterans (BO).2 Subepithelial and/or intraluminal infiltration by myofibroblasts, differentiated mesenchymal cells with augmented collagen secretory and contractile functions,3 is noted in human biopsy specimens that demonstrate BO.4 BO presents clinically as an obstructive ventilatory defect termed BO syndrome (BOS).5 BOS is the major cause of graft failure and long-term mortality, with no effective treatment options.6,7 Understanding the origin of effector myofibroblasts in fibrotic lesions of BO is critical for therapeutic targeting of mechanisms of cell recruitment/differentiation.
One potential source of mesenchymal cells participating in this disorganized repair response is the mesenchymal progenitors residing in the graft. The embryonic lung mesenchyme is derived from splanchnic mesoderm during organogenesis and is marked by expression of several unique mesenchymal transcription factors.8,9 Of these factors, the best studied are the mesenchyme-specific forkhead box (FOXF1) and homeobox (HOX) families.9 The FOXF1 gene (also known as HFH-8 or Freac-1) is expressed in the splanchnic mesoderm during organogenesis,10 and its expression is essential for lung development.11,12 In addition, gene expression of HOXA5 and HOXB5 in the embryonic mesenchyme of the developing lung is necessary for normal branching morphogenesis,13,14 with genetic deletion of HOXA5 leading to respiratory tract defects.13 The critical need for lung-specific mesenchyme to promote lung morphogenesis and the importance of epithelial-mesenchymal cross talk in the embryonic lung suggest that lung-specific mesenchymal precursors may also participate in repair and fibrogenesis during adult life.
Mesenchymal progenitor cells with clonal multilineage differentiation potential, termed mesenchymal stem/stromal cells (MSCs), can be isolated from human lung allografts.15 These cells are of predominantly donor origin in studies of sex-mismatched lung allografts, suggesting they are a lung-resident (LR) population of connective tissue progenitors.15 However, whether these LR-MSCs represent a tissue-specific cell, the potential remnant of embryonic lung mesenchyme remains unknown. Furthermore, whether these cells participate in fibrogenesis in a lung allograft remains to be determined. Herein, we studied the expression of lung-specific mesenchymal transcription factors in LR-MSCs isolated from the bronchoalveolar lavage (BAL) fluid of human lung transplant recipients and investigated their ability to undergo fibrotic differentiation.
Materials and Methods
Isolation of Lung-Derived MSCs and Other Cell Lines
The MSCs were derived from the BAL fluid of lung transplant recipients by plastic adherence and subsequent expansion of colony forming unit-fibroblast, as previously described under a protocol approved by the University of Michigan Institutional Review Board.15,16 Surface marker expression for cells used in the present study was determined using flow cytometry. LR-MSCs were negative for CD45 and positive for CD73, CD105, CD90, and CD44. Furthermore, their multilineage differentiation potential was confirmed by inducing differentiation into osteocytes and adipocytes (see Supplemental Figure S1 at http://ajp.amjpathol.org). Cells were maintained in culture in Dulbecco's modified Eagle's medium with penicillin-streptomycin and 10% fetal calf serum at 37°C in 5% CO2 and used at passages 2 to 6. LR-MSCs obtained from individual BAL samples were treated as separate cell lines. BOS was defined by physiological testing, according to the International Society of Heart and Lung Transplantation guidelines.5 Bone marrow (BM)–derived MSCs were isolated from normal human BM aspirates under an Institutional Review Board–approved protocol at the University of Michigan, Ann Arbor, as previously described.15 Alveolar epithelial cells (A549) and human pulmonary artery endothelial cells were purchased from the American Type Culture Collection (Manassas, VA) and Lonza (Walkersville, MD), respectively. Primary airway epithelial cells were isolated from healthy donors under an Institutional Review Board–approved protocol and cultured in bronchial epithelial cell growth medium (Lonza), as previously described.17,18
Affymetrix and Real-Time qPCR Analysis
Total RNA was prepared using an RNeasy minikit (Qiagen, Inc., Valencia, CA), per manufacturer's instructions. Real-time qPCR analysis was performed on an ABI Prism 7000 SDS (Applied Biosystems, Foster City, CA) using TaqMan PCR Master Mix (Applied Biosystems). The TaqMan real-time PCR primers included Hs00230962_m1 for FOXF1, Hs00430330_m1 for HOXA5, and Hs00357820_m1 for HOXB5 (Applied Biosystems). Affymetrix array hybridization and scanning were performed by the University of Michigan Comprehensive Cancer Center Affymetrix and cDNA Microarray Core Facility, using Human U133 plus 2.0 chips. The expression value for each gene was calculated using a robust multiarray average and stored as log2-transformed data.
Immunofluorescence Microscopy and Western Blot Analysis
Immunofluorescence staining for α-smooth muscle actin (α-SMA) and IL-13 receptor α1 was performed on LR-MSCs plated at a density of 50,000 cells per 35-mm cell culture dish using mouse monoclonal anti–α-SMA (clone 1A4; Dako, Carpinteria, CA) and anti–IL-13 receptor α1 antibodies (R&D, Minneapolis, MN). Western blot analysis for α-SMA and collagen I was performed as previously described,4,19 using monoclonal α-SMA (clone 1A4; Dako) at 1:1000 dilution and rabbit polyclonal antibody to collagen I (Cedarlane Laboratories, Burlington, ON, Canada) at 1:500 dilution.
IHC Staining and in Situ Hybridization
Paraffin-embedded sections from biopsy specimens demonstrating organizing pneumonia or BO were obtained under an Institutional Review Board–approved protocol. Immunohistochemical (IHC) staining with α-SMA was performed according to standard clinical laboratory procedures, as previously described.4 Human clone (FOXF1 ORFeome Collaboration Clone; I.D.1000–67187, accession No. EU832158) was purchased from Open Biosystems (Huntsville, AL). Plasmid DNA was purified using a Maxi Prep kit (Qiagen, Inc.) and amplified by PCR using human FOXF1 primers containing EcoR1 and HindIII cutting sites (forward and reverse, respectively) from Integrated DNA Technologies (Coralville, IA) (forward 5′-ATGGAATTCGCGTCGTCCGGCCCGT-3′; reverse 5′-GGGCCAAGCTTTCCACGTTGCCCGG-3′). Purified DNA was linearized and denatured to generate single-stranded DNA. Single-stranded DNA was then labeled with digoxigenin, according to the manufacturer's protocol, using a digoxigenin RNA labeling kit (SP6/T7) from Roche Applied Science (Penzberg, Germany). In situ hybridization was performed according to the manufacturer's protocol using a kit purchased from Biochain Institute Inc. (Hayward, CA). Sections were digested with 20 μg/mL proteinase K (Invitrogen, Carlsbad, CA), and color was developed using Fast Red TR/Napthol AS-MX (Sigma-Aldrich, St Louis, MO). To demonstrate colocalization of α-SMA and FOXF1, antigen retrieval was performed on paraffin-embedded sections, followed by FOXF1 (1:25 dilution; Sigma-Aldrich) and α-SMA (1:1000; Dako) staining using a kit (ABC Elite), according to manufacturer's protocol (Vector Labs, Burlingame, CA). A tyramide signal amplification system from Perkin Elmer (Covina, CA) was used to develop the final stain.
In Vitro Epithelial Mesenchymal Transformation
Human lung epithelial cells (A549) were stimulated with transforming growth factor (TGF)-β (5 ng/mL) and harvested at 0, 0.5, 1, 2, 4, 8, 16, 24, and 72 hours after treatment, as previously described.20 Total RNA was prepared from three biological replicates of each condition, and RNA transcripts were assayed using a chip array (Affymetrix HG-U133 Plus 2.0). Two-way analysis of variance models with effects for three experiments and nine points were fit to the data for each probe set.
Statistics
Student's t-tests were used to determine P values when comparing two groups. When comparing three or more groups, analysis of variance was performed with a post hoc Bonferroni test to determine which groups showed significant differences.
Results
Human Lung Allograft-Derived MSCs Demonstrate Expression of Lung Embryonic Mesenchymal Factors
To investigate if lung allograft-derived MSCs represent a tissue-specific resident mesenchymal cell population, we studied the gene expression profile of mesenchymal transcription factors in LR-MSCs and compared it with that of BM-MSCs (Table 1). Affymetrix analysis demonstrated a significantly higher expression of FOXF1 in LR-MSCs compared with BM-MSCs. FOXF2, another gene from the forkhead family that is seen along with FOXF1 in mesodermal tissues of developing and adult lungs,21,22 was also up-regulated in LR-MSCs compared with BM-MSCs (Table 1). By using a P value threshold of 0.01 and a threefold expression change cutoff, seven HOX genes were differentially expressed between LR-MSCs and BM-MSCs. Three HOX genes (ie, HOXA5, HOXB5, and HOXB6) demonstrated increased expression compared with BM-MSCs. On the other hand, HOXA9, a gene whose expression has been critical in hematopoiesis,23 was significantly down-regulated in LR-MSCs compared with BM-MSCs.
Table 1.
Comparison of Selected Gene Expression between Lung- and BM-Derived MSCs
| Gene title | Gene symbol | P value | Fold⁎ | Expression value† |
|||||
|---|---|---|---|---|---|---|---|---|---|
| LR 1 | LR 2 | LR 3 | BM 1 | BM 2 | BM 3 | ||||
| Forkhead box | |||||||||
| F1 | FOXF1 | 0.001 | 5.4 | 10.48 | 8.69 | 7.62 | 3.29 | 3.52 | 3.95 |
| F2 | FOXF2 | <0.001 | 3.3 | 9.87 | 8.78 | 9.43 | 6.12 | 6.07 | 6.05 |
| Homeobox | |||||||||
| A5 | HOXA5 | <0.001 | 4.0 | 10.19 | 10.22 | 10.97 | 6.42 | 6.37 | 6.62 |
| B5 | HOXB5 | <0.001 | 3.3 | 9.97 | 8.99 | 8.73 | 5.86 | 5.87 | 6.04 |
| B6 | HOXB6 | <0.001 | 3.2 | 9.22 | 9.25 | 9.31 | 6.09 | 5.97 | 6.20 |
| A9 | HOXA9 | <0.001 | -3.3 | 4.79 | 4.79 | 6.02 | 8.52 | 8.73 | 8.35 |
| A10 | HOXA10 | <0.001 | -3.4 | 3.96 | 3.93 | 4.19 | 7.84 | 7.66 | 6.85 |
| C10 | HOXC10 | <0.001 | -3.9 | 4.56 | 4.70 | 4.50 | 8.34 | 8.47 | 8.61 |
| C6 | HOXC6 | 0.001 | -4.8 | 4.25 | 5.86 | 6.76 | 10.31 | 10.55 | 10.45 |
| A9 | HOXA9 | <0.001 | -6.0 | 3.55 | 3.48 | 4.00 | 9.71 | 9.76 | 9.54 |
LR, lung allograft–derived MSCs.
Difference between lung- and BM-derived MSCs.
Expression values are log2-transformed data.
Real-time PCR analysis confirmed increased expression of FOXF1, HOXA5, and HOXB5 in LR-MSCs compared with BM-MSCs (Figure 1A). A 35,000-fold greater expression of FOXF1 was seen in LR-MSCs than in BM-MSCs (P < 0.0001). Furthermore, a 100-fold increased expression of HOXA5 (P < 0.0001) and a 150-fold increased expression of HOXB5 (P < 0.0001) were noted in LR-MSCs compared with BM-MSCs. The FOXF1 mRNA expression by real-time PCR, cell surface marker expression by flow cytometry, and osteogenic and adipogenic differentiation potential of cells derived from five patients are presented in Supplemental Figure S1 (available at http://ajp.amjpathol.org). This unique expression of fetal lung mesenchyme-associated transcription factors in multipotent MSCs derived from human adult lung suggests that LR-MSCs are derived from embryonic mesenchyme and represent a locally resident tissue-specific progenitor cell.
Figure 1.
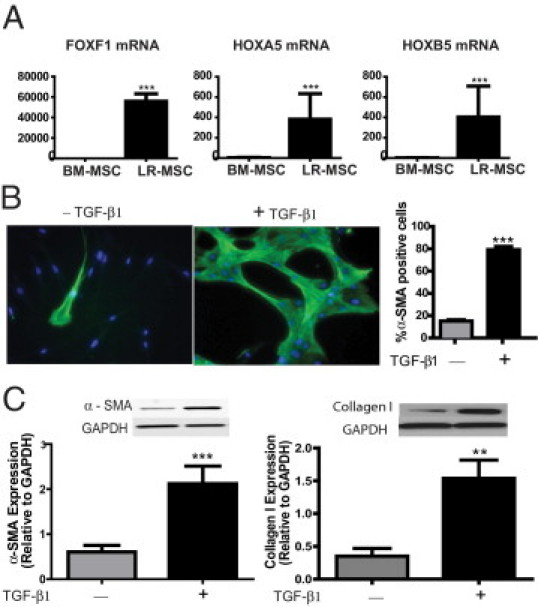
A: Increased expression of embryonic lung mesenchyme-associated transcription factors in lung-derived MSCs. mRNA expression of FOXF1, HOXA5, and HOXB5 in LR-MSCs isolated from BAL fluid of lung allografts (n = 10 LR-MSC lines derived from individual patients) was compared with that in BM-MSCs (n = 3) by real-time PCR. ***P < 0.0001. B and C: LR-MSCs demonstrate myofibroblast differentiation potential in response to the profibrotic mediator TGF-β. LR-MSCs isolated from normal lung allografts, without evidence of acute or chronic rejection, were treated with or without TGF-β1 (2 ng/mL) for 24 hour. In B, immunofluorescence staining of LR-MSCs demonstrated an increase in α-SMA–positive stress fibers in response to TGF-β1. Right: A quantitative analysis of α-SMA–positive cells across 10 high-power fields in three normal cell lines is shown. ***P < 0.0001. C: Effect of TGF-β on α-SMA and collagen I protein expression, analyzed by Western blot analysis. Immunoblots shown are from a representative experiment, with graphical data representing the densitometric ratio of the protein of interest to loading control proteins. Data represent the mean ± SEM of experiments with LR-MSCs derived from 10 lung transplant recipients. ***P = 0.0002 and **P = 0.006. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase.
LR-MSCs Isolated from Normal Human Lung Allografts Demonstrate Myofibroblast Differentiation in Response to Profibrotic Mediators
The myofibroblast, the pivotal effector cell of fibrogenic processes, is a differentiated mesenchymal cell marked by expression of the contractile protein α-SMA and a concomitant increased ability to secrete collagen.3 The availability of MSCs isolated from the BAL fluid of human lung allografts provided an opportunity to investigate whether these resident mesenchymal components of the allograft milieu can undergo profibrotic differentiation by cytokines and mediators thought to be associated with BOS. LR-MSCs isolated from the BAL fluid of normal lung allografts were exposed to TGF-β, a profibrotic mediator implicated in BOS pathogenesis.24,25 Of the LR-MSCs exposed to TGF-β1 (2 ng/mL), 79.31% ± 2.81% demonstrated α-SMA expression by immunofluorescence compared with 15.09% ± 1.18% α-SMA–positive cells noted at baseline (P < 0.0001, Figure 1B). The up-regulation of α-SMA protein expression in LR-MSCs treated with TGF-β1 compared with controls was also noted by Western blot analysis (P = 0.0002, Figure 1C). Similarly, collagen I protein expression demonstrated a significant increase over baseline in LR-MSCs treated with TGF-β1 (P = 0.006, Figure 1C).
The profibrotic type 2 helper T cell cytokine IL-13 is critical in the development of luminal obliteration in animal models of BO,4,26 and increased levels of IL-13 are present in BAL fluid of human lung transplant recipients with BOS.26 Previously, myofibroblasts in human BO lesions expressed IL-13 receptor α1, the receptor chain necessary for signaling by IL-13.4 LR-MSCs demonstrated significant expression of this receptor by both flow cytometry and immunofluorescence microscopy (Figure 2, A and B). Myofibroblast differentiation marked by α-SMA–positive stress fiber organization was noted in 60.84% ± 2.73% of cells in response to IL-13 by immunofluorescence (P < 0.0001, Figure 2C). Immunoblot analysis demonstrates increased expression of both α-SMA and collagen I protein in LR-MSCs treated with IL-13 compared with untreated controls (P = 0.016 and P = 0.034, respectively; Figure 2D). Together, these data demonstrate that profibrotic factors implicated in BOS can drive LR-MSCs to differentiate into myofibroblasts with a robust capacity to elaborate extracellular matrix proteins important in scar formation.
Figure 2.
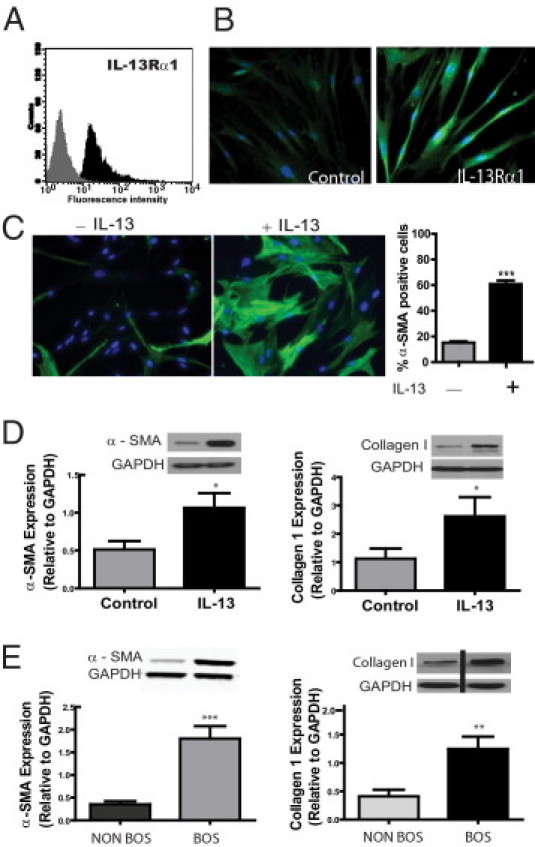
A and B: LR-MSCs express IL-13 receptor α1 (IL-13Rα1). A: Immunophenotyping by flow cytometric analysis demonstrates positive IL-13Rα1 expression on LR-MSCs isolated from human lung allografts. The histogram shows IL-13Rα1 staining in black and isotype control staining in gray (n = 5). B: Immunofluorescent staining of LR-MSCs demonstrates IL-13Rα1–positive staining (compared with control unstained). C and D: LR-MSCs demonstrate profibrotic differentiation in response to IL-13. LR-MSCs isolated from normal lung allografts, without evidence of acute or chronic rejection, were treated with or without IL-13 (10 ng/mL) for 24 hours. C: Expression of α-SMA in LR-MSCs with or without IL-13 is shown using immunofluorescent staining. A quantitative analysis of α-SMA–positive cells across 10 high-power fields in three normal cell lines is shown (right). ***P < 0.0001. D: Effect of IL-13 on α-SMA and collagen I protein expression, analyzed by Western blot analysis. Data represent the mean ± SEM of experiments with LR-MSCs derived from 10 lung transplant recipients. *P < 0.05. E: LR-MSCs isolated from patients with BOS demonstrate a profibrotic phenotype. α-SMA and collagen I protein expression in LR-MSCs isolated from patients with and without BOS was compared by Western blot analysis. Data represent the mean ± SEM of experiments with LR-MSCs derived from 10 lung transplant recipients in each group. ***P < 0.0001 and **P = 0.003. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase.
LR-MSCs Derived from Patients with BOS Demonstrate a Profibrotic Phenotype Marked by Increased α-SMA Expression and Collagen Secretion
The ability of LR-MSCs to undergo fibrotic differentiation in response to profibrotic stimuli led us to investigate if BOS is associated with an altered LR-MSC phenotype. Constitutive α-SMA and collagen expression levels were compared in untreated LR-MSCs obtained from allografts of patients with and without physiological evidence of BOS. Significantly increased α-SMA expression was seen in patients with clinical evidence of BOS compared with time-matched BOS-free control patients (P < 0.0001, Figure 2E). Examination of α-SMA expression over serial passages demonstrated stable α-SMA expression in control and BOS LR-MSCs (data not shown). LR-MSCs isolated from patients with BOS also demonstrated increased baseline collagen I expression (P = 0.003, Figure 2E). Increased collagen synthetic function and α-SMA expression in LR-MSCs isolated from the BAL fluid of transplant recipients with BOS suggest that LR-MSCs might have a pathogenic role in the fibroproliferative response culminating in BOS. To determine whether MSCs isolated from patients with BOS are also lung rather than BM in origin, FOXF1 mRNA expression was assessed in cells isolated from patients with and without BOS. No difference was noted in the expression of FOXF1 in cells from patients with and without BOS (P = 0.31).
FOXF1 Expression in BAL Fluid Correlates with Number of LR-MSCs
FOXF1 expression in LR-MSCs was compared with that in other lung resident cells, such as endothelial and epithelial cells (Figure 3A). Greater than 20,000-fold higher expression of FOXF1 was noted in LR-MSCs compared with human alveolar epithelial cells (A549) and primary bronchial epithelial cells, consistent with the fact that FOXF1 is specifically expressed only in the mesenchyme. Human pulmonary artery endothelial cells demonstrated 40-fold higher FOXF1 expression than epithelial cells, consistent with their embryonic mesenchyme derivation. However, the expression of FOXF1 in LR-MSCs was 500-fold higher than that in endothelial cells.
Figure 3.
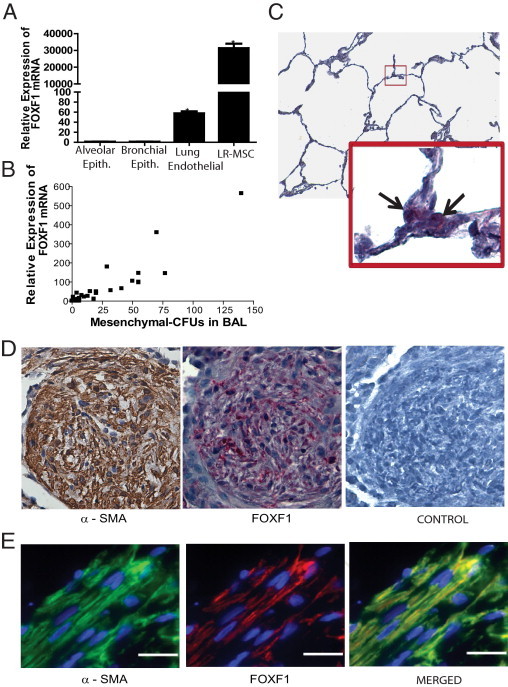
A: FOXF1 expression in other endogenous lung cellular populations. FOXF1 mRNA expression in human lung allograft-derived MSCs (LR-MSCs), human alveolar epithelial (Epith.) cells (A549), human lung primary airway epithelial cells, and human pulmonary artery endothelial cells by real-time qPCR is shown. B: FOXF1 mRNA expression in BAL fluid correlates with the number of LR-MSCs in human lung transplant recipients. FOXF1 expression in 1 × 106 nucleated BAL fluid cells was studied by real-time PCR. The numbers of LR-MSCs in those BAL samples were quantitated by measuring colony forming unit-fibroblast. A significant correlation was noted between number of LR-MSCs and FOXF1 mRNA in the BAL fluid (Pearson r = 0.92; 95% CI, 0. 86 to 0.95; P < 0.001). n = 50 BAL fluid samples. C: FOXF1 expression in normal adult lungs. The expression of FOXF1 in normal human lung was assessed by in situ hybridization using a digoxigenin-labeled RNA probe, followed by hematoxylin counterstaining. The enlarged box indicates the lesion marked by the box within the figure. The black arrows show cells positive for FOXF1. Original magnification: ×100 (top); ×600 (bottom). D: FOXF1 expression in fibrotic lesions in human lung allografts. Representative sections of a transbronchial lung biopsy specimen demonstrated organizing pneumonia in a lung transplant recipient, stained for α-SMA (by IHC staining) and FOXF1 (by in situ hybridization). Left: A discrete area of organizing pneumonia with intense α-SMA staining (brown) signifying infiltration by myofibroblasts. Center: FOXF1 mRNA expression was detected by in situ hybridization in the fibrotic area. Discrete spindle-shaped cells demonstrating red staining, consistent with FOXF1 expression, are noted in the area of organizing pneumonia. Right:In situ hybridization using digoxigenin-labeled control mRNA. Control for α-SMA staining is shown in Supplemental Figure S2 (available at http://ajp.amjpathol.org). Original magnification, ×400. E: Coexpression of FOXF1 and α-SMA in fibrotic lesions. A section from a human lung allograft biopsy specimen demonstrating fibrotic lesions was examined for the expression of FOXF1 and α-SMA using double-immunofluorescence microscopy. Rhodamine and fluorescein tyramide signal amplifications were used to detect the signal for FOXF1 and α-SMA, respectively. Colocalization of FOXF1 and α-SMA in spindle-shaped cells demonstrated FOXF1 expression in myofibroblasts. Original magnification, ×600 (oil). Scale bar = 20 μm.
This uniquely high expression level of FOXF1 mRNA in LR-MSCs led us to investigate the quantitative expression of FOXF1 transcript in the BAL fluid cell pellet as a marker of LR-MSC numbers. BAL fluid from 50 lung transplant recipients was studied for both expression of FOXF1 mRNA and number of LR-MSCs. MSCs were quantitated by the number of colony-forming units isolated from 2 × 106 plated BAL cells. A significant correlation was noted between the number of LR-MSCs in the BAL fluid and FOXF1 mRNA [Pearson r = 0.92; 95% confidence interval (CI), 0.86 to 0.95; P < 0.001; Figure 3B].
FOXF1 Is Expressed in Myofibroblasts in Human Lung Transplant Biopsy Specimens
The markedly greater expression of FOXF1 in LR-MSCs than BM-MSCs also provided us with a tool to investigate if myofibroblasts seen in the fibrotic lesions of lung allografts were derived from locally resident lung-specific mesenchymal progenitor cells. An examination of the normal lung alveolar spaces demonstrated sparse FOXF1 expression, with cells demonstrating FOXF1 mRNA noted predominantly in the triangular corners of the alveoli or the alveolar septa (Figure 3C). The expression of FOXF1 mRNA and α-SMA protein was examined in biopsy specimens demonstrating evidence of either BO or organizing pneumonia (n = 5). Myofibroblasts, identified by their spindle shape and intensely positive α-SMA expression, were present in the fibrotic lesions. Robust expression of FOXF1 mRNA was noted in these lesions by in situ hybridization (Figure 3D). To investigate the coexpression of FOXF1 and α-SMA, dual-immunofluorescence staining was used. Colocalization of α-SMA and FOXF1 was noted in the spindle-shaped cells present in the fibrotic lesions, demonstrating that myofibroblasts in human lung allografts express FOXF1 (Figure 3E).
Because mesenchymal cells can also be potentially derived from local epithelium by epithelial-mesenchymal transformation (EMT), we sought to determine whether this phenomenon, when recapitulated in vitro, is accompanied by expression of FOXF1. The mRNA from A549 epithelial cells treated with TGF-β to induce EMT20,27,28 was analyzed by Affymetrix analysis. Although EMT was associated with a loss of expression of the epithelial gene E-cadherin and an increase in expression of the mesenchymal genes N-cadherin, fibronectin-1, and vimentin, no induction of FOXF1 mRNA was noted (Table 2). The effect of TGF-β1 on FOXF1 expression in BM-MSCs, primary human bronchial cells, and human pulmonary artery endothelial cells was examined; no significant change was noted in FOXF1 mRNA expression by real-time PCR in the presence of this fibrogenic stimuli (P = 0.45, P = 0.26, and P = 0.85, respectively).
Table 2.
Gene Expression in Lung Alveolar Epithelial (A549) Cells Undergoing EMT in Response to TGF-β
| Gene symbol | Name | Fold change from 0 hours⁎ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 16 | 24 | 72 | ||
| FOXF1 | Forkhead box F1 | 0.92 | 0.74 | 0.78 | 1.03 | 1.05 | 1.28 | 0.81 | 1.02 |
| CDH1 | E-cadherin | 1.00 | 1.03 | 0.99 | 0.96 | 0.66 | 0.23 | 0.14 | 0.10† |
| CDH2 | N-cadherin | 1.16 | 1.13 | 1.19 | 1.41 | 2.26 | 3.98 | 4.56 | 6.76† |
| FN1 | Fibronectin 1 | 1.03 | 1.02 | 0.92 | 1.15 | 1.31 | 1.91 | 2.91 | 5.14† |
| VIM | Vimentin | 1.89 | 2.18 | 2.62 | 2.57 | 2.90 | 2.89 | 3.19 | 4.24† |
The amount of TGF-β was 5 ng/mL.
Data are given in hours.
P < 0.01 versus 0 hours.
Discussion
Mesenchymal cell infiltration of the small airways, leading to fibrotic obliteration, is the primary feature of chronic lung allograft rejection or BO. Key profibrotic mediators, such as TGF-β and IL-13, have been linked to the pathogenesis of BO in both human samples and animal modeling.4,24–26 However, the origin of the myofibroblasts critical for driving fibrogenesis in these injured lung allografts is less well understood. In this study, we demonstrate that an MSC population derived from the lung allograft itself is unique in its high-level expression of lung embryonic mesenchymal-associated transcription factors. Markedly greater expression of transcription factors FOXF1 and specific HOX genes in LR-MSCs than BM-MSCs establishes the tissue specificity of solid organ–derived MSCs in humans. The ability of these tissue-specific mesenchymal progenitor cells to contribute to fibrogenesis was demonstrated by their capacity for in vitro myofibroblast differentiation in response to profibrotic mediators and their altered in vivo phenotype (marked by increased α-SMA expression and collagen secretion) in patients with BOS. Finally, FOXF1 expression in myofibroblasts in lung biopsy specimens provides evidence for the local origin of these effector cells of fibrosis in lung transplants. These data, demonstrating the fibrotic differentiation potential of tissue-specific organ-resident MSCs and the local mesenchymal origin of myofibroblasts in fibrotic lung allograft lesions, suggest a key role for local mesenchymal precursor cells in the fibrotic remodeling of a lung allograft.
By demonstrating the donor origin of multipotent mesenchymal cells derived from human lung allografts, the tissue residence of connective tissue progenitor cells in solid organs was demonstrated.15 The data shown herein extend this observation by demonstrating that MSCs isolated from the lung allografts are resident and tissue-specific progenitor cells, likely remnants of embryonic lung mesenchyme. LR-MSCs demonstrated high-level expression of mesenchymal transcription factors associated with developing lung mesenchyme. When compared with BM-MSCs, LR-MSCs expressed 35,000-fold more FOXF1, a mesenchymal transcription factor whose expression in splanchnic mesoderm is essential for lung development during embryogenesis.8,12 Similarly, the expression of HOX genes in LR-MSCs mirrors that noted during lung development, with markedly greater expression of HOXA5 and HOXB5 in LR-MSCs than BM-MSCs. Recent evidence29 indicates that mesenchymal cells in various adult tissues maintain key features of gene expression patterns established during embryogenesis. Similar expression of FOXF1 in MSCs from patients with and without BOS suggests that this same locally derived population is found in both states of quiescence/organized repair and fibrosis.
We demonstrate that lung allograft–derived MSCs can differentiate into a fibrogenic phenotype by exposure to components of the profibrotic milieu known to be present in BOS. The effect of two important mediators, TGF-β and IL-13, on LR-MSC fibrotic differentiation was examined. Both TGF-β and IL-13 have been strongly linked to the pathogenesis of BO in animal models of tracheal transplantation and have been increased in BAL fluid from patients with BOS.4,24–26 LR-MSCs demonstrated myofibroblast differentiation, marked by both an increased collagen I synthetic ability and α-SMA expression, in response to TGF-β and IL-13. More significantly, mesenchymal cells from the BAL fluid of patients with BOS demonstrated a stable increase in α-SMA and collagen expression, suggesting that the cellular phenotype is skewed toward that of a differentiated myofibroblast during the development of disease. High FOXF1 expression, noted in the cells obtained from patients with BOS, suggests their graft/donor derivation. This is also supported by a previous study15 of cytogenetic analyses in sex-mismatched lung transplant recipients, in which 98% of the cells from patients with BOS were donor in origin. Recent work30 demonstrates that an increase in LR-MSCs in BAL fluid precedes the development of BOS, further reiterating a potential role of these local-mesenchymal cells in BOS pathogenesis. Whether expression of myofibroblast markers in BAL-derived MSCs can be used as a biomarker of disease onset or severity remains to be investigated.
The ability of LR-MSCs to undergo fibrotic differentiation in response to soluble mediators present in an allograft microenvironment during BOS development and the stable fibrotic phenotypic alterations in LR-MSCs in patients with BOS indicate a possible pathogenic role for endogenous MSCs as effector cells in the pathogenesis of BOS. Although this notion of MSCs as a driver of fibrosis appears contradictory to recent literature31–33 ascribing an immunoregulatory/antifibrotic role to this cell, this latter work primarily used models of acute injury. In chronic injury models, a condition more relevant to BOS, exogenous MSCs have contributed to fibrosis.34,35 MSCs, including LR-MSCs, demonstrate an in vitro potential to inhibit T-cell function via secretion of soluble mediators, such as prostaglandin E2.16,36 However, MSCs potentiate fibrotic differentiation of other mesenchymal cells by secreting profibrotic mediators, such as TGF-β1.37 Thus, although MSCs can likely modulate an inflammatory microenvironment, they can also promote fibrogenesis by secreting profibrotic mediators37 or differentiating to myofibroblasts. Regulation of native LR-MSC synthetic function and fibrotic differentiation fate by the local milieu might play a central role in dictating whether these cells serve a predominant immunoregulatory function or promote fibroproliferative events that favor the development of BO.
Myofibroblasts in solid organs can be potentially derived from diverse cellular pools.3 BM, home to mesenchymal precursors such as CD45+Col I+ fibrocytes38 and CD45-Col I+ MSCs,39 is a potential distant reservoir of mesenchymal precursor cells. Organ resident sources include a local pool of mesenchymal progenitor cells or a somatic cell capable of transdifferentiation, such as seen in EMT.40 Biological/signaling mechanisms involved in recruitment/differentiation depend on the compartment from which the participating precursor cells are derived; therefore, it is crucial to distinguish the relative contribution of each compartment in specific diseases. However, the lack of unique markers of mesenchymal progenitor cells from various sources makes the human investigation of this question difficult. Our study identified FOXF1 as a transcription factor uniquely expressed in lung-derived compared with BM-derived MSCs. FOXF1 expression in α-SMA–expressing cells in fibrotic lesions provides a human demonstration of the local origin of myofibroblasts. Thus, these data demonstrating a lung, rather than a BM, origin of myofibroblasts support the studies of BM chimeric animals, in which, despite evidence of recruitment of BM-derived fibroblasts to remodeling lung, myofibroblasts present in the fibrotic lesions were not derived from BM progenitors.41,42 Our human studies also complement the recent elegant animal studies43 of gene-labeled mice demonstrating local tissue resident mesenchymal, rather than epithelial, cells as precursors of collagen-secreting myofibroblasts in the kidney. Interestingly, the fibrotic cells in these kidney injury models arose from resident cells expressing FoxD1, the forkhead transcription factor seen in embryonic mesenchyme fated to become stromal cells of the kidney.43
In summary, this study establishes the tissue specificity and local origin of MSCs isolated from human lungs. The ability of these easily obtainable graft-resident mesenchymal precursor cells to undergo fibrotic differentiation and their altered profibrotic phenotype in BOS suggest a role for these cells in the pathogenesis of this disorder and offer an opportunity to use them as a sentinel cell biomarker of fibroproliferation. Furthermore, the unique expression of FOXF1 in human lung allograft-derived MSCs, the observed failure of up-regulation of FOXF1 in in vitro EMT studies, and FOXF1 expression in myofibroblasts in human tissues in situ provide the first human evidence that local mesenchymal progenitors, likely remnants of the embryonic tissue mesenchyme, are the predominant source of myofibroblasts in lung fibrogenesis after transplantation. These data stress the need to further decipher the niche and biological features of resident mesenchymal progenitor cells in solid organs.
Footnotes
Supported by grants (R01DK082481 to P.H.K.; RO1HL094311 to M.P.-G.; RO1HL094622 to V.N.L.) from the NIH, The American Thoracic Society Research Award, the American Society of Transportation/Wyeth Clinical Science Faculty Development grant, the Scleroderma Research Foundation Award, and the Brian and Mary Campbell and Elizabeth Campbell Carr research gift fund (V.N.L.).
N.W. and L.B. contributed equally to this work.
None of the authors disclosed any relevant financial relationships.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.01.058.
Supplementary data
Characterization of LR-MSCs from BAL fluid of human lung allografts. Immunophenotypic analysis, multilineage differentiation potential, and FOXF1 expression of mesenchymal cells isolated from BAL fluid of five separate lung transplant recipients is shown. A: Mesenchymal cells isolated from BAL fluid of lung transplant recipients were expanded in culture and immunostained for cell surface markers with specific monoclonal antibodies. The percentage of positive cells relative to the total number of cells analyzed by flow cytometry is shown. These cells were predominantly positive for CD73, CD90, CD105, and CD44 and uniformly negative for the hematopoietic lineage marker CD45. B: The same cell lines were investigated for their in vitro multilineage differentiation capacity by culturing them in either control or differentiation-inducing conditions. Real-time PCR was performed to analyze the expression of mRNAs specifically related to adipogenic and osteogenic activity under inductive culture conditions. The expression levels of peroxidase proliferator–activated receptor (PPAR) γ (indicative of adipogenic activity) and osteopontin (indicative of osteogenic activity) were up-regulated in all five cell lines. Middle: The accumulation of lipid droplets, indicating adipocytic differentiation, was demonstrated by staining with oil red O in treated cells. Right: Osteocytic differentiation was indicated by calcium deposition, as demonstrated by alizarin red staining in treated cells. Left: No staining was observed in control untreated cells. Scale bar = 50 μm. C: mRNA expression of FOXF1 by real-time PCR in the same five cell lines is shown compared with three separate BM-derived MSC lines.
α-SMA staining of human lung biopsy specimens. To identify myofibroblasts in the fibrotic lesions of lung allografts, transbronchial lung biopsy samples from human lung transplant recipients were stained for α-SMA by IHC staining. Left: A representative section demonstrating α-SMA–expressing myofibroblasts and smooth muscle cells in brown is shown. Right: The negative control for α-SMA staining is presented. Scale bar = 200 μm.
References
- 1.Estenne M., Hertz M.I. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 2.Boehler A., Estenne M. Obliterative bronchiolitis after lung transplantation. Curr Opin Pulm Med. 2000;6:133–139. doi: 10.1097/00063198-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lama V.N., Harada H., Badri L.N., Flint A., Hogaboam C.M., McKenzie A., Martinez F.J., Toews G.B., Moore B.B., Pinsky D.J. Obligatory role for interleukin-13 in obstructive lesion development in airway allografts. Am J Pathol. 2006;169:47–60. doi: 10.2353/ajpath.2006.050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estenne M., Maurer J.R., Boehler A., Egan J.J., Frost A., Hertz M., Mallory G.B., Snell G.I., Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 6.Lama V.N. Update in lung transplantation 2008. Am J Respir Crit Care Med. 2009;179:759–764. doi: 10.1164/rccm.200902-0177UP. [DOI] [PubMed] [Google Scholar]
- 7.Wilkes D.S., Egan T.M., Reynolds H.Y. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172:944–955. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa R.H., Kalinichenko V.V., Lim L. Transcription factors in mouse lung development and function. Am J Physiol Lung Cell Mol Physiol. 2001;280:L823–L838. doi: 10.1152/ajplung.2001.280.5.L823. [DOI] [PubMed] [Google Scholar]
- 9.Maeda Y., Dave V., Whitsett J.A. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 10.Peterson R.S., Lim L., Ye H., Zhou H., Overdier D.G., Costa R.H. The winged helix transcriptional activator HFH-8 is expressed in the mesoderm of the primitive streak stage of mouse embryos and its cellular derivatives. Mech Dev. 1997;69:53–69. doi: 10.1016/s0925-4773(97)00153-6. [DOI] [PubMed] [Google Scholar]
- 11.Kalinichenko V.V., Lim L., Stolz D.B., Shin B., Rausa F.M., Clark J., Whitsett J.A., Watkins S.C., Costa R.H. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 12.Mahlapuu M., Enerback S., Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- 13.Mandeville I., Aubin J., LeBlanc M., Lalancette-Hebert M., Janelle M.F., Tremblay G.M., Jeannotte L. Impact of the loss of Hoxa5 function on lung alveogenesis. Am J Pathol. 2006;169:1312–1327. doi: 10.2353/ajpath.2006.051333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpe M.V., Pham L., Lessin M., Ralston S.J., Bhan I., Cutz E., Nielsen H.C. Expression of Hoxb-5 during human lung development and in congenital lung malformations. Birth Defects Res A Clin Mol Teratol. 2003;67:550–556. doi: 10.1002/bdra.10086. [DOI] [PubMed] [Google Scholar]
- 15.Lama V.N., Smith L., Badri L., Flint A., Andrei A.C., Murray S., Wang Z., Liao H., Toews G.B., Krebsbach P.H., Peters-Golden M., Pinsky D.J., Martinez F.J., Thannickal V.J. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvinen L., Badri L., Wettlaufer S., Ohtsuka T., Standiford T.J., Toews G.B., Pinsky D.J., Peters-Golden M., Lama V.N. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajjan U., Keshavjee S., Forstner J. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infect Immun. 2004;72:4188–4199. doi: 10.1128/IAI.72.7.4188-4199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider D., Ganesan S., Comstock A.T., Meldrum C.A., Mahidhara R., Goldsmith A.M., Curtis J.L., Martinez F.J., Hershenson M.B., Sajjan U. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:332–340. doi: 10.1164/rccm.200911-1673OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S., Wettlaufer S.H., Hogaboam C., Aronoff D.M., Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L405–L413. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- 20.Keshamouni V.G., Jagtap P., Michailidis G., Strahler J.R., Kuick R., Reka A.K., Papoulias P., Krishnapuram R., Srirangam A., Standiford T.J., Andrews P.C., Omenn G.S. Temporal quantitative proteomics by iTRAQ 2D-LC-MS/MS and corresponding mRNA expression analysis identify post-transcriptional modulation of actin-cytoskeleton regulators during TGF-beta-induced epithelial-mesenchymal transition. J Proteome Res. 2009;8:35–47. doi: 10.1021/pr8006478. [DOI] [PubMed] [Google Scholar]
- 21.Aitola M., Carlsson P., Mahlapuu M., Enerback S., Pelto-Huikko M. Forkhead transcription factor FoxF2 is expressed in mesodermal tissues involved in epithelio-mesenchymal interactions. Dev Dyn. 2000;218:136–149. doi: 10.1002/(SICI)1097-0177(200005)218:1<136::AID-DVDY12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Pierrou S., Hellqvist M., Samuelsson L., Enerback S., Carlsson P. Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. EMBO J. 1994;13:5002–5012. doi: 10.1002/j.1460-2075.1994.tb06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba S. Homeobox genes in normal hematopoiesis and leukemogenesis. Int J Hematol. 1998;68:343–353. doi: 10.1016/s0925-5710(98)00093-0. [DOI] [PubMed] [Google Scholar]
- 24.Elssner A., Jaumann F., Dobmann S., Behr J., Schwaiblmair M., Reichenspurner H., Fürst H., Briegel J., Vogelmeier C., Munich Lung Transplant Group Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Transplantation. 2000;70:362–367. doi: 10.1097/00007890-200007270-00022. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez A.M., Takagawa S., Sekosan M., Jaffe H.A., Varga J., Roman J. Smad3 deficiency ameliorates experimental obliterative bronchiolitis in a heterotopic tracheal transplantation model. Am J Pathol. 2004;165:1223–1232. doi: 10.1016/S0002-9440(10)63382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane M.P., Gomperts B.N., Weigt S., Xue Y.Y., Burdick M.D., Nakamura H., Zisman D.A., Ardehali A., Saggar R., Lynch J.P., 3rd, Hogaboam C., Kunkel S.L., Lukacs N.W., Ross D.J., Grusby M.J., Strieter R.M., Belperio J.A. IL-13 is pivotal in the fibro-obliterative process of bronchiolitis obliterans syndrome. J Immunol. 2007;178:511–519. doi: 10.4049/jimmunol.178.1.511. [DOI] [PubMed] [Google Scholar]
- 27.Willis B.C., Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 28.Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang H.Y., Chi J.T., Dudoit S., Bondre C., van de Rijn M., Botstein D., Brown P.O. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badri L., Murray S., Liu L.X., Walker N.M., Flint A., Wadhwa A., Chan K., Toews G.B., Pinsky D.J., Martinez F.J., Lama V.N. Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2011;183:1062–1070. doi: 10.1164/rccm.201005-0742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta N., Su X., Popov B., Lee J.W., Serikov V., Matthay M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz L.A., Gambelli F., McBride C., Gaupp D., Baddoo M., Kaminski N., Phinney D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rojas M., Xu J., Woods C.R., Mora A.L., Spears W., Roman J., Brigham K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.di Bonzo L.V., Ferrero I., Cravanzola C., Mareschi K., Rustichell D., Novo E., Sanavio F., Cannito S., Zamara E., Bertero M., Davit A., Francica S., Novelli F., Colombatto S., Fagioli F., Parola M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223–231. doi: 10.1136/gut.2006.111617. [DOI] [PubMed] [Google Scholar]
- 35.Wu G.D., Bowdish M.E., Jin Y.S., Zhu H., Mitsuhashi N., Barsky L.W., Barr M.L. Contribution of mesenchymal progenitor cells to tissue repair in rat cardiac allografts undergoing chronic rejection. J Heart Lung Transplant. 2005;24:2160–2169. doi: 10.1016/j.healun.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 37.Salazar K.D., Lankford S.M., Brody A.R. Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that mediate proliferation and procollagen expression by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1002–L1011. doi: 10.1152/ajplung.90347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucala R., Spiegel L.A., Chesney J., Hogan M., Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 39.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 40.Iwano M., Plieth D., Danoff T.M., Xue C., Okada H., Neilson E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolgachev V.A., Ullenbruch M.R., Lukacs N.W., Phan S.H. Role of stem cell factor and bone marrow-derived fibroblasts in airway remodeling. Am J Pathol. 2009;174:390–400. doi: 10.2353/ajpath.2009.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto N., Jin H., Liu T., Chensue S.W., Phan S.H. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V., Valerius M.T., McMahon A.P., Duffield J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of LR-MSCs from BAL fluid of human lung allografts. Immunophenotypic analysis, multilineage differentiation potential, and FOXF1 expression of mesenchymal cells isolated from BAL fluid of five separate lung transplant recipients is shown. A: Mesenchymal cells isolated from BAL fluid of lung transplant recipients were expanded in culture and immunostained for cell surface markers with specific monoclonal antibodies. The percentage of positive cells relative to the total number of cells analyzed by flow cytometry is shown. These cells were predominantly positive for CD73, CD90, CD105, and CD44 and uniformly negative for the hematopoietic lineage marker CD45. B: The same cell lines were investigated for their in vitro multilineage differentiation capacity by culturing them in either control or differentiation-inducing conditions. Real-time PCR was performed to analyze the expression of mRNAs specifically related to adipogenic and osteogenic activity under inductive culture conditions. The expression levels of peroxidase proliferator–activated receptor (PPAR) γ (indicative of adipogenic activity) and osteopontin (indicative of osteogenic activity) were up-regulated in all five cell lines. Middle: The accumulation of lipid droplets, indicating adipocytic differentiation, was demonstrated by staining with oil red O in treated cells. Right: Osteocytic differentiation was indicated by calcium deposition, as demonstrated by alizarin red staining in treated cells. Left: No staining was observed in control untreated cells. Scale bar = 50 μm. C: mRNA expression of FOXF1 by real-time PCR in the same five cell lines is shown compared with three separate BM-derived MSC lines.
α-SMA staining of human lung biopsy specimens. To identify myofibroblasts in the fibrotic lesions of lung allografts, transbronchial lung biopsy samples from human lung transplant recipients were stained for α-SMA by IHC staining. Left: A representative section demonstrating α-SMA–expressing myofibroblasts and smooth muscle cells in brown is shown. Right: The negative control for α-SMA staining is presented. Scale bar = 200 μm.


