Abstract
β3-Adrenoceptors have been demonstrated to mediate urinary bladder smooth muscle relaxation but proof of their expression at the protein level has been missing because of lack of suitable antibodies or radioligands. As among various available radioligands [125I]-iodocyanopindolol ([125I]-ICYP) exhibited the smallest problems in labeling cloned human β3-adrenoceptors in previous studies, we have explored its suitability to label β3-adrenoceptors in rat urinary bladder in saturation and competition radioligand binding experiments. Rat lung was used as an internal control and exhibited all characteristics expected from this tissue with regard to β1/β2-adrenoceptor labeling. Saturation and competition binding studies with [125I]-ICYP in rat bladder yielded saturable binding sites with an affinity compatible with β3-adrenoceptors. In competition experiments various agonists and antagonists largely exhibited a profile compatible with a population consisting largely of β3-adrenoceptors. However, the binding competition properties of ICI 118,551 and SR 59,230A were not easily explained by the idea of labeling a homogeneous β3-adrenoceptor population but interpretation of the data was limited by a high degree of non-specific binding in [125I]-ICYP concentrations required to label the receptors. We conclude that [125I]-ICYP can be used to label tissue β3-adrenoceptors but results obtained with this ligand have to be interpreted with caution.
Keywords: β3-adrenoceptor, [125I]-iodocyanopindolol, rat, urinary bladder, lung
Introduction
β-Adrenoceptors play an important role in the regulation of urinary bladder function. They do so by promoting smooth muscle relaxation (Michel and Vrydag, 2006) but may also act on urothelial mediator release (Otsuka et al., 2008; Masunaga et al., 2010) and afferent nerve function (Leon et al., 2008; Aizawa et al., 2010). The receptor mediating bladder smooth muscle relaxation in humans, and perhaps also those involved in urothelium and afferent nerves, belongs predominantly if not exclusively to the β3-subtype (Michel and Vrydag, 2006). However, demonstrating the presence of this subtype in the bladder or other tissues at the protein level has been difficult in the past, specifically because commercially available antibodies against this receptor may lack the required selectivity (Pradidarcheep et al., 2009) and available radioligands have only low affinity for the β3-adrenoceptor (Vrydag and Michel, 2007).
In a recent study, we have compared radioligands for their ability to label cloned human β-adrenoceptor subtypes, i.e., [3H]-dihydroalprenolol, [3H]-CGP 12,177, and [125I]-iodocyanopindolol ([125I]-ICYP) (Niclauß et al., 2006). While none of them had the desired properties for labeling β3-adrenoceptors, [125I]-ICYP was the most promising among the candidates. [125I]-ICYP is a standard radioligand for the labeling of β1- and β2-adrenoceptors (Engel et al., 1981) but has also been used in other studies for the labeling of cloned human (Hoffmann et al., 2004) or rodent β3-adrenoceptors (Muzzin et al., 1991). Against this background the present study was performed to explore whether [125I]-ICYP may be suitable to label β3-adrenoceptors in rat urinary bladder despite its known short-comings as a radioligand for this receptor subtype. Rat lung was used as an internal control in our experiments because it does not express β3-adrenoceptors (Granneman et al., 1991).
Materials and Methods
Radioligand binding studies
All experiments were performed under the German legislation for the care of experimental animals. Adult Wistar rats of either gender were obtained from the breeding facility at the University of Essen Medical School. They were killed by decapitation under light ether anesthesia. Lung and bladder were rapidly removed, freed from surrounding connective tissues and rapidly frozen in liquid nitrogen. Thereafter, they were stored until use for up to 3 months at −70°C. On the day of the experiment, the tissues were thawed in ice-cold preparation buffer (50 mM Tris, 10 mM MgCl2, 0.5 mM EDTA, pH 7.5), minced with scissors and homogenized with an Ultra-Turrax (Janke & Kunkel, Staufen, Germany) for 10 s at full speed and then twice for 20 s each at 2/3 speed. The homogenates were filtered through medical gauze and then centrifuged for 20 min at 50,000 g at 4°C. The pellets were re-suspended in buffer, re-homogenized shortly (10 s at full speed) and washed by an additional centrifugation step. The final pellets were re-suspended and re-homogenized in binding buffer (see below).
Radioligand binding assays were performed as previously described (Goepel et al., 1997) with minor modifications. Briefly, aliquots of the membrane preparations (approximately 4–10 and 15–30 μg protein per tube for lung and bladder, respectively) were incubated in a total volume of 250 μl binding buffer (10 mM Tris, 150 mM NaCl at pH 7.4) with [125I]-ICYP for 90 min at 37°C. Incubations were terminated by rapid vacuum filtration over Whatman GF/C filters using a Brandell cell harvester. Filters were washed with approximately 20 ml ice-cold incubation buffer. Unless otherwise indicated, non-specific binding was defined using 100 μM isoprenaline. In saturation experiments 6–12 [125I]-ICYP concentrations were used, whereas in competition experiments 21 narrowly spaced competitor concentrations were used; in every case each data point was assessed in duplicate.
Chemicals
[125I]-ICYP (specific activity 2200 Ci/mmol) was obtained from New England Nuclear (Dreieich, Germany). (−)-Adrenaline bitartrate, BRL 37,344 ((±)-R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]-acetic acid sodium), (±)-CGP 12,177 (4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one hydrochloride), CGP 20,712A (1-[2-((3-carbamoyl-4-hydroxy)phenoxy)ethylamino]-3-[4-(1-methyl-4-trifluoromethyl-2-imidazolyl)phenoxy]-2-propanol methanesulfonate), S-(-)-cyanopindolol, ICI 118,551 ((±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol), (−)-isoprenaline hydrochloride, (−)-noradrenaline bitartrate, (±)-propranolol HCl and SR 59,230A (3-(2-ethylphenoxy)-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol oxalate) were obtained from Sigma-Aldrich (Munich, Germany).
Data analysis
Saturation binding data were analyzed by fitting rectangular hyperbolic functions to the experimental data. Competition binding experiments were analyzed by fitting mono- and biphasic sigmoidal curves to the experimental data; a biphasic fit was accepted only if it resulted in a significant improvement of the fit as judged by an F-test. This test was performed for each individual experiment and formed the primary basis of our analysis as shown in Table 1; in contrast, the figures for the competition experiments represent pooled data and hence do not allow conclusions about the superiority of 1- vs. 2-site fits. Resulting IC50 values were converted into Ki values by the Cheng–Prusoff equation (Cheng and Prusoff, 1973). Data are shown as mean ± SEM of n experiments. All curve fitting procedures were performed with the Prism program (Graphpad Software, San Diego, CA, USA).
Table 1.
Estimated drug affinities in rat lung and urinary bladder.
| Lung | Bladder | |||||||
|---|---|---|---|---|---|---|---|---|
| pKi high | pKi low | % High affinity sites | n | pKi high | pKi low | % High affinity sites | n | |
| Adrenaline | – | 5.48 ± 0.39 | – | 3 | – | 5.15 ± 0.07 | – | 4 |
| Noradrenaline | – | 5.58 ± 0.39 | – | 3 | – | 5.50 ± 0.15 | – | 4 |
| Isoprenaline | 8.31 ± 0.76 | 5.87 ± 0.07 | 29 ± 9 | 3 | 8.18 ± 0.13 | 5.09 ± 0.14 | 22 ± 8 | 4 |
| BRL 37,344 | – | 5.87 ± 0.06 | – | 4 | – | 5.10 ± 0.72 | – | 4 |
| CGP 12,177 | – | 10.05 ± 0.04 | – | 3 | – | 6.78 ± 0.33 | – | 7 |
| Propranolol | – | 8.29 ± 0.02 | – | 3 | – | 5.86 ± 0.29 | – | 4 |
| CGP 20,712A | 8.43 ± 0.15 | 4.90 ± 0.04 | 27 ± 1 | 3 | – | 5.59 ± 0.30 | – | 4 |
| ICI 118,551 | 8.42 ± 0.21 | 6.74 ± 0.21 | 69 ± 9 | 5 | 8.92 ± 0.67 | 5.76 ± 0.42 | 39 ± 14 | 4 |
| SR 59,230A | – | 7.22 ± 0.04 | – | 4 | – | 5.66 ± 0.07 | – | 4 |
| Cyanopindolol | – | 9.62 ± 0.09 | – | 6 | – | 6.97 ± 0.49 | – | 8 |
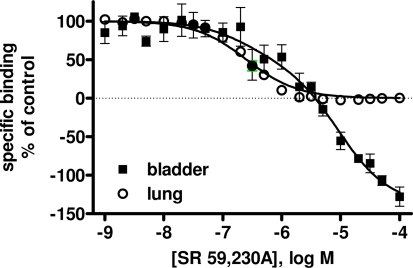
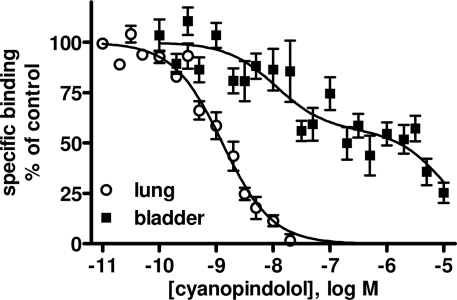
Data are mean ± SEM of n experiments. Results from a biphasic function are shown only if this resulted in a significantly better fit than a monophasic function as judged by an F-test. Note that SR 59,230A competed for more binding sites in bladder membranes than any of the other agents (Figure 1). Note also that competition curves of cyanopindolol were very shallow in rat bladder membranes but not consistently better explained by a two-site model (Figure 2).
Results
Radioligand binding studies rat lung
[125I]-ICYP exhibited saturable high affinity binding in rat lung membranes. The number and affinity of the detectable binding sites was similar regardless whether 1 μM propranolol or 100 μM isoprenaline was used to define non-specific binding in paired experiments (Bmax 251 ± 55 vs. 264 ± 55 fmol/mg protein, Kd 22.1 ± 4.1 vs. 21.3 ± 6.2 pM, n = 4 each).
Competition binding experiments were performed in rat lung membranes using an [125I]-ICYP concentration of 82 ± 2 pM (n = 37), which resulted in 24 ± 1% non-specific binding. The overall competition profile yielded the expected findings (Table 1). Thus, the β1-selective antagonist CGP 20,712A and the β2-selective antagonist ICI 118,551 detected 27 ± 1 and 69 ± 9% high affinity sites, respectively. These experiments confirmed that rat lung contains predominantly if not exclusively propranolol-sensitive [125I]-ICYP binding sites, representing β1- and β2-adrenoceptors, and validate our radioligand binding assay. Of note, isoprenaline was the only agonist for which competition curves were consistently better fit by a two-site model, SR 59,230A had relatively high affinity (pKi 7.22 ± 0.04, Figure 1), and non-iodinated cyanopindolol had monophasic competition curves with an affinity (pKi 9.62 ± 0.09, Figure 2).
Figure 1.
Competition for [125I]-ICYP binding to rat lung (open circles) and urinary bladder (filled squares) by SR 59,320A. Data are mean ± SEM of 4 experiments. Note that SR 59,230A competes for more than 100% of all specific sites as defined by 100 isoprenaline. Quantitative results are shown in Table 1.
Figure 2.
Pseudo-homologous competition binding of non-iodinated cyanopindolol for [125I]-ICYP in rat lung (open circles) and urinary bladder membranes (filled squares). Data are mean ± SEM of 6 to 8 experiments. Of note the competition curves in bladder membranes were very shallow but only in 3 out of 8 experiments were significantly better fitted by a two-site model. Quantitative results are shown in Table 1.
Radioligand binding studies rat bladder
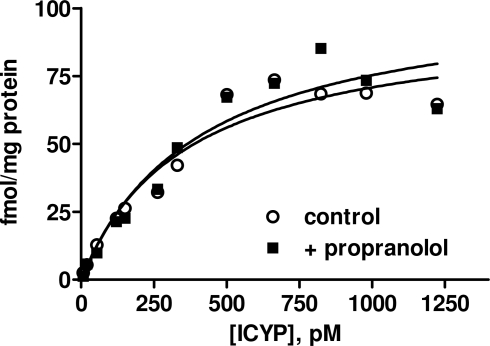
In contrast to the above findings in rat lung, the amount of specific binding in rat bladder defined by 100 μM isoprenaline appeared to be much greater than that defined by 1 μM propranolol in paired experiments, the latter yielding almost no “specific” binding (data not shown). Even when non-specific binding was defined using 100 μM isoprenaline, specific [125I]-ICYP binding to the membrane preparation from rat urinary bladder did not approach saturation in concentrations up to 150 pM (data not shown). Therefore, we performed additional saturation experiments with [125I]-ICYP concentrations up to 1200 pM defining non-specific binding by 100 μM isoprenaline (Figure 3). Under these conditions a Bmax of 149 ± 52 fmol/mg protein and a Kd of 579 ± 147 pM (n = 6) were found. In paired experiments in the additional presence of 1 μM propranolol (definition of non-specific binding also with 100 μM isoprenaline), estimates of Bmax (222 ± 111 fmol/mg protein) and Kd (562 ± 196 pM) were very similar to those determined in the absence of propranolol. These experiments established that [125I]-ICYP binding sites in rat bladder have considerably lower affinity than those in rat lung and are mainly propranolol-resistant, i.e., distinct from classical β1/β2-adrenoceptors. Based on these data, all further experiments were performed with definition of non-specific binding by 100 μM isoprenaline.
Figure 3.
[125I]-ICYP saturation binding in rat urinary bladder. Non-specific binding was defined by 100 μM isoprenaline, and experiments were performed in the absence (control) and presence of 1 μM propranolol. Data are from a representative experiment which was performed 6 times with similar results (see text for mean results).
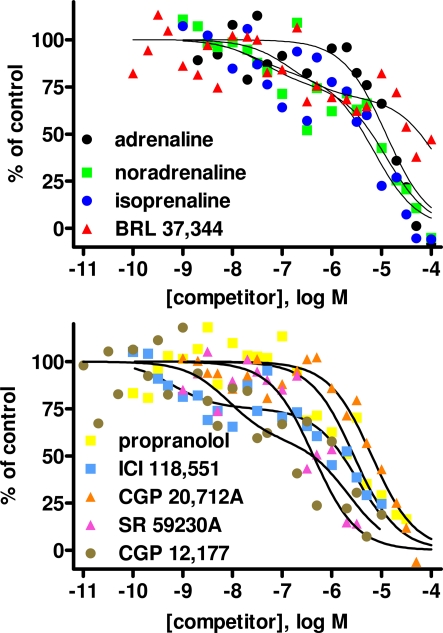
In light of the lower [125I]-ICYP affinity in the saturation binding experiments, competition binding in rat bladder was performed using an [125I]-ICYP concentration of 567 ± 1 pM (n = 62), which resulted in 63 ± 2% of non-specific binding. The competition curves for most compounds in rat bladder membranes were best explained by monophasic fits with all competitors exhibiting relatively low affinity, i.e., less than 1 μM (Figure 4; Table 1). Only CGP 12,177 and cyanopindolol had slightly higher affinity, and only the agonist isoprenaline (but not any of the other agonists) and the β2-selective antagonist ICI 118,551 yielded biphasic competition curves, the latter having 39% high affinity sites. Two other competitors exhibited noteworthy behavior: While SR 59,230A had monophasic competition curves, it competed for far more [125I]-ICYP binding sites than any of the other test compounds and specifically for much more than the isoprenaline concentration used for the definition of non-specific binding (Figure 1). Non-iodinated cyanopindolol exhibited very shallow competition curves (Hill-slope 0.46 ± 0.06, n = 8; Figure 2) but only in 3 out of 8 experiments these were significantly better explained by a two-site model.
Figure 4.
Competition for [125I]-ICYP binding to rat urinary bladder by adrenergic agonists (upper panel) and antagonists (lower panel). Data are mean ± SEM of 4 to 8 experiments. Error bars have been omitted for clarity. Quantitative results are shown in Table 1.
Discussion
β3-Adrenoceptors have been implicated mediating various tissue functions, but detection of corresponding receptor protein expression has been difficult in the past due to lack of suitable antibodies or radioligands (Michel et al., 2010). While none of the available radioligands has optimal properties for the labeling of β3-adrenoceptors, our previous studies (Niclauß et al., 2006) in line with data from other investigators (for review see Vrydag and Michel, 2007) have demonstrated that if anything [125I]-ICYP may be suitable to label β3-adrenoceptors in tissues. Indeed, [125I]-ICYP has been used in one previous study to demonstrate atypical β-adrenoceptors in rat soleus striated muscle (Roberts et al., 1993), but the binding sites identified in that study did not fully match the profile of cloned β3-adrenoceptors. Rat urinary bladder is a tissue which expresses all three β-adrenoceptor subtypes at the mRNA level (Fujimura et al., 1999; Barendrecht et al., 2009) and where both β2- and β3-adrenoceptors contribute to smooth muscle relaxation, but the latter subtype appears to dominate functionally (Michel and Vrydag, 2006). While β3-adrenoceptors may play an even greater relative role in the human detrusor, a tissue with a mixed β2/β3-population was deemed better suited for our purposes as it allows detection of the target subtype against a background of at least one other subtype.
For our radioligand binding studies we have used rat lung as a reference tissue, because this tissue lacks β3-adrenoceptor expression (Granneman et al., 1991). Rather rat lung is well established to express β1- and β2-adrenoceptors in an approximate 30:70% ratio (Minneman et al., 1979; Michel et al., 1987), and our present data, particularly with the β1-selective CGP 20,712A and the β2-selective ICI 118,551, confirm this observation. Moreover, our data show that definition of non-specific binding with 100 μM isoprenaline yields very similar saturation binding as isotherms as that by 1 μM propranolol in rat lung membranes. Hence, our rat lung data validate our approach.
Our experiments in rat urinary bladder yielded a very different picture. Within the concentration range of [125I]-ICYP yielding receptor saturation in rat lung (up to 150 pM), little specific binding was detected in the bladder. However, saturable specific binding was detectable at higher concentrations. The corresponding KD value (579 pM) was in line with previously reported [125I]-ICYP affinities at cloned human and rat β3-adrenoceptors but much higher than those found for β1- and β2-adrenoceptors (Muzzin et al., 1991; Hoffmann et al., 2004). Performing the [125I]-ICYP saturation experiments in the presence of 1 μM propranolol did not affect the estimates of KD and Bmax; while this concentration of propranolol yields little occupancy of β3-adrenoceptors, it should saturate β1- and β2-adrenoceptors (Hoffmann et al., 2004). Therefore, our saturation binding isotherms provided preliminary evidence that [125I]-ICYP predominantly if not exclusively labeled β3-adrenoceptors in rat urinary bladder. Accordingly, this saturable [125I]-ICYP binding was detectable only if non-specific binding was defined by 100 μM isoprenaline and not when it was defined by 1 μM propranolol, although both are equally suitable to define non-specific binding in the β1/β2-tissue rat lung. The density of the specific [125I]-ICYP binding sites in rat bladder (approximately 150–200 fmol/mg protein) was in line with the total β-adrenoceptor expression density in many other tissues. Of note, [125I]-ICYP can also bind to some subtypes of 5-HT receptors (Hoyer et al., 1985), but as we defined non-specific binding by the β-adrenoceptor agonist isoprenaline and because isoprenaline exhibited the expected affinity for binding to β-adrenoceptors potential [125I]-ICYP binding to 5-HT receptors is unlikely to have contributed to our specific binding. However, it may be part of the large non-specific binding reported here for the rather high [125I]-ICYP concentrations required to label the β3-adrenoceptors. The relative contribution of 5-HT receptors to the non-specific binding may differ between tissues and cell types according to their expression of such receptors. However, the observation that the high concentrations of [125I]-ICYP required to label the β3-adrenoceptors is similarly seen in various cell types including those lacking 5-HT receptors such as Chinese hamster ovary (Niclauß et al., 2006) or human embryonic kidney cells (Vrydag et al., 2009) suggests that most of the non-specific binding is indeed non-specific rather than to 5-HT receptors.
Competition experiments were performed with various β-adrenoceptor agonists and antagonists to obtain a more comprehensive characterization of the ligand recognition profile of the [125I]-ICYP binding sites. The endogenous catecholamines noradrenaline and adrenaline and the prototypical β-adrenoceptor agonist isoprenaline had affinities in rat bladder which were in line with those in rat lung (except for the known β1- over β2-selectivity of noradrenaline) and also with the reported values at all human β-adrenoceptor subtypes (Hoffmann et al., 2004). Most importantly, the affinities for the two endogenous catecholamines confirm that the [125I]-ICYP binding sites indeed can be considered to be adrenoceptors. The biphasic competition curves for isoprenaline but not for noradrenaline or adrenaline in bladder (as in lung) most likely reflect agonist high and low affinity states of the receptor for the former, whereas the overall affinities of the latter two were too low to detect those. Although BRL 37,344 is often claimed to be a β3-selective agonist, our data demonstrate a similar affinity for this compound in rat bladder and lung. This is line with reports that BRL 37,344 basically lacks selectivity for cloned human β3-adrenoceptors (Baker, 2010). In rats at the same dose of BRL 37,344 can similarly activate β3- and β2-adrenoceptors (Mori et al., 2010). CGP 12,177 is a high-affinity antagonist at β1- and β2-adrenoceptors (Hoffmann et al., 2004; Baker, 2005), a low affinity agonist at the atypical site on the β1-adrenoceptor (Kaumann and Molenaar, 2008) and a low potency, partial agonist at β3-adrenoceptors (Baker, 2010). Its affinity found in the present study is in line with its reported β3-adrenoceptor affinity (Baker, 2010) but also with its potency at the low affinity site on β1-adrenoceptors (Kaumann and Molenaar, 2008; Galindo-Tovar et al., 2009). However, all other drug affinities detected in our study are compatible with the labeling of a β3- but not a low affinity β1-site. Among the tested antagonists, propranolol, the β1-selective CGP 20,712A and the β2-selective ICI 118,551 all had affinities in line with reported values at β3-adrenoceptors (Hoffmann et al., 2004; Baker, 2005). The affinity estimated for non-iodinated cyanopindolol was also in line with reported values at β3-adrenoceptors (Baker, 2010). All of these data support the view that the specific binding sites labeled by high concentrations of [125I]-ICYP in rat bladder represent a rather homogeneous population of β3-adrenoceptors.
However, there are a number of caveats which need to be considered. Firstly, the competition curves for ICI 118,551 were biphasic and included a minor (39%) fraction of sites with higher affinity. These sites could represent β2-adrenoceptors (Hoffmann et al., 2004; Baker, 2005), but that would raise the question why other compounds with high affinity for β2-adrenoceptors such as propranolol lacked a high affinity component in their competition curves. Secondly, SR 59,230A competed for more [125I]-ICYP sites than any other ligand tested here and hence also for many sites defined as non-specific based on isoprenaline competition. We do not know what these sites represent, but a low affinity [125I]-ICYP binding site insensitive, e.g., to BRL 37,344 has been reported in rat colon (Sugasawa et al., 1997). Thirdly, although non-iodinated cyanopindolol competition curves were not significantly better explained by a two-site model in most experiments, they were very shallow in bladder (but not lung). Of note, Baker has recently reported a biphasic competition curve of non-iodinated cyanopindolol for [3H]-CGP 12,177 binding to β3-adrenoceptor-expressing CHO cells (Baker, 2010). Finally, the high concentration of [125I]-ICYP required to label β-adrenoceptors in rat bladder were associated with a high non-specific binding (63% as compared to 24% in lung). This is a known phenomenon for its binding to cloned human β3-adrenoceptors, probably explained by the low affinity for β3-adrenoceptors as compared to a linear increase in non-specific binding with the required high radioligand concentrations (Niclauß et al., 2006). The presence of such high non-specific binding is a problem as it generates low signal/noise ratios and hence can create problems in data analysis. This may also have contributed to the not fully explained competition curves with ICI 118,551 and cyanopindolol.
In conclusion, under assay conditions validated in rat lung, [125I]-ICYP can label specific and saturable binding sites in rat urinary bladder which in many ways resemble β3-adrenoceptors. However, the match in ligand recognition profile is incomplete and the high degree of non-specific binding associated with the required high concentrations of [125I]-ICYP limits interpretation of the data. These data support the idea that [125I]-ICYP at present is the only radioligand with at least limited suitability to label tissue β3-adrenoceptors, but better radioligands are clearly required to enable a more unequivocal labeling of β3-adrenoceptors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (Mi 294/7-1). Tim Schneider was recipient of a training fellowship from the intramural grant program of the University of Essen Medical School (IFORES). In the β3-adrenoceptor field, MCM has received consultancy honoraria and research support from Astellas and Boehringer Ingelheim, but neither company was involved with this specific study.
References
- Aizawa N., Igawa Y., Nishizawa O., Wyndaele J.-J. (2010). Effects of CL316,243, a β3-adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol. Urodyn. 29, 771–776 10.1002/nau.20826 [DOI] [PubMed] [Google Scholar]
- Baker J. G. (2005). The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 144, 317–322 10.1038/sj.bjp.0706048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. G. (2010). The selectivity of β-adrenoceptor agonists at human β1-, β2- and β3-adrenoceptors. Br. J. Pharmacol. 160, 1048–1061 10.1111/j.1476-5381.2010.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendrecht M. M., Frazier E. P., Vrydag W., Alewijnse A. E., Peters S. L. M., Michel M. C. (2009). The effect of bladder outlet obstruction on α1- and β-adrenoceptor expression and function. Neurourol. Urodyn. 28, 349–355 10.1002/nau.20642 [DOI] [PubMed] [Google Scholar]
- Cheng Y.-C., Prusoff W. H. (1973). Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 10.1016/0006-2952(73)90196-2 [DOI] [PubMed] [Google Scholar]
- Engel G., Hoyer D., Berthold R., Wagner H. (1981). (±)[125Iodo]cyanopindolol, a new ligand for β-adrenoceptors: identification and quantitation of subclasses of β-adrenoceptors in guinea pig. Naunyn Schmiedebergs Arch. Pharmacol. 317, 277–285 10.1007/BF00501307 [DOI] [PubMed] [Google Scholar]
- Fujimura T., Tamura K., Tsutsumi T., Yamamoto T., Nakamura K., Koibuchi Y., Kobayashi M., Yamaguchi O. (1999). Expression and possible functional role of the β3-adrenoceptor in human and rat detrusor muscle. J. Urol. 161, 680–685 10.1016/S0022-5347(01)61994-3 [DOI] [PubMed] [Google Scholar]
- Galindo-Tovar A., Vargas M. L., Kaumann A. J. (2009). Phosphodiesterases PDE3 and PDE4 jointly control the inotropic effects but not chronotropic effects of (-)-CGP12177 despite PDE4-evoked sinoatrial bradycardia in rat atrium. Naunyn Schmiedebergs Arch. Pharmacol. 379, 379–384 10.1007/s00210-008-0367-7 [DOI] [PubMed] [Google Scholar]
- Goepel M., Wittmann A., Rübben H., Michel M. C. (1997). Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol. Res. 25, 199–206 10.1007/BF00941983 [DOI] [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N., Chaudhry A. (1991). Molecular cloning and expression of the rat β3-adrenergic receptor. Mol. Pharmacol. 40, 895–899 [PubMed] [Google Scholar]
- Hoffmann C., Leitz M. R., Oberdorf-Maass S., Lohse M. J., Klotz K.-N. (2004). Comparative pharmacology of human β-adrenergic receptor subtypes – characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 369, 151–159 10.1007/s00210-003-0860-y [DOI] [PubMed] [Google Scholar]
- Hoyer D., Engel G., Kalkman H. O. (1985). Characterization of the 5-HT1B recognition site in rat brain: binding studies with (-)[125I]iodocyanopindolol. Eur. J. Pharmacol. 118, 1–12 10.1016/0014-2999(85)90657-0 [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Molenaar P. (2008). The low affinity site of the β1-adrenoceptor and its relevance to cardiovascular pharmacology. Pharmacol. Ther. 118, 303–336 10.1016/j.pharmthera.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Leon L. A., Hoffman B. E., Gardner S. D., Laping N. J., Evans C., Lashinger E. S. R., Su X. (2008). Effects of the β3-adrenergic receptor agonist disodium 5[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzodioxole-2,2,dicarboxylate (L-316243) on bladder micturition reflex in spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 326, 178–185 10.1124/jpet.108.138651 [DOI] [PubMed] [Google Scholar]
- Masunaga K., Chapple C. R., McKay N. G., Yoshida M., Sellers D. J. (2010). The β3-adrenoceptor mediates the inhibitory effects of β-adrenoceptor agonists via the urothelium in pig bladder dome. Neurourol. Urodyn. 29, 1320–1325 10.1002/nau.20838 [DOI] [PubMed] [Google Scholar]
- Michel M. C., Ochodnicky P., Summers R. J. (2010). Tissue functions mediated by β3-adrenoceptors – findings and challenges. Naunyn Schmiedebergs Arch. Pharmacol. 382, 103–108 10.1007/s00210-010-0529-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. C., Vrydag W. (2006). α1-, α2- and β-adrenoceptors in the urinary bladder, urethra and prostate. Br. J. Pharmacol. 147, S88–S119. 10.1038/sj.bjp.0706619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. C., Wang X. L., Schlicker E., Göthert M., Beckeringh J. J., Brodde O.-E. (1987). Increased β2-adrenoceptor density in heart, lung and kidney of spontaneously hypertensive rats. J. Auton. Pharmacol. 7, 41–51 10.1111/j.1474-8673.1987.tb00132.x [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Hegstrand L. R., Molinoff P. B. (1979). Simultaneous determination of beta-1 and beta-2 adrenergic receptors in tissues containing both receptor subtypes. Mol. Pharmacol. 16, 34–46 [PubMed] [Google Scholar]
- Mori A., Miwa T., Sakamoto K., Nakahara T., Ishii K. (2010). Pharmacological evidence for the presence of functional β3-adrenoceptors in rat retinal blood vessels. Naunyn Schmiedebergs Arch. Pharmacol. 382, 119–126 10.1007/s00210-010-0526-5 [DOI] [PubMed] [Google Scholar]
- Muzzin P., Revelli J.-P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J.-P., Fraser C. M. (1991). An adipose tissue-specific β-adrenergic receptor. Molecular cloning and down-regulation in obesity. J. Biol. Chem. 266, 24053–24058 [PubMed] [Google Scholar]
- Niclauß N., Michel-Reher M. B., Alewijnse A. E., Michel M. C. (2006). Comparison of three radioligands for the labelling of human β-adrenoceptor subtypes. Naunyn Schmiedebergs Arch. Pharmacol. 374, 79–85 [DOI] [PubMed] [Google Scholar]
- Otsuka A., Shinbo H., Matsumoto R., Kurita Y., Ozono S. (2008). Expression and functional role of β-adrenoceptors in the human urinary bladder. Naunyn Schmiedebergs Arch. Pharmacol. 377, 473–481 10.1007/s00210-008-0274-y [DOI] [PubMed] [Google Scholar]
- Pradidarcheep W., Stallen J., Labruyere W. T., Dabhoiwala N. F., Michel M. C., Lamers W. H. (2009). Lack of specificity of commercially available antisera against muscarinic and adrenergic receptors. Naunyn Schmiedebergs Arch. Pharmacol. 379, 397–402 10.1007/s00210-009-0393-0 [DOI] [PubMed] [Google Scholar]
- Roberts S. J., Molenaar P., Summers R. J. (1993). Characterization of propranolol-resistant (-)-[125I]-cyanopindolol binding sites in rat soleus muscle. Br. J. Pharmacol. 109, 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa T., Matsuzaki-Fujita M., Guillaume J.-L., Camoin L., Morooka S., Strosberg A. D. (1997). Characterization of a novel iodocyanopindolol and SM-11044 binding protein, which may mediate relaxation of depolarized rat colon tonus. J. Biol. Chem. 272, 21244–21252 10.1074/jbc.272.34.21244 [DOI] [PubMed] [Google Scholar]
- Vrydag W., Alewijnse A. E., Michel M. C. (2009). Do gene polymorphisms alone or in combination affect the function of human β3-adrenoceptors? Br. J. Pharmacol. 156, 127–134 10.1111/j.1476-5381.2008.00014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrydag W., Michel M. C. (2007). Tools to study β3-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 374, 385–398 10.1007/s00210-006-0127-5 [DOI] [PubMed] [Google Scholar]