Abstract
Poly(ethylene glycol) (PEG) hydrogels offer numerous advantages in designing controlled 3D environments for cartilage regeneration, but offer little biorecognition for the cells. Incorporating molecules that more closely mimic the native tissue may provide key signals for matrix synthesis and may also help in the retention of neotissue, particularly when mechanical stimulation is employed. Therefore, this research tested the hypothesis that exogenous hyaluronan encapsulated within PEG hydrogels improves tissue deposition by chondrocytes, while the incorporation of Link-N(DHLSDNYTLDHDRAIH), a fragment of link protein which is involved in stabilizing hyaluronan and aggrecan in cartilage, aids in the retention of the entrapped hyaluronan as well as cell-secreted glycosaminoglycans, particularly when dynamic loading is employed. The incorporation of Link-N as covalent tethers resulted in a significant reduction, ~60%, in the loss of entrapped exogenous hyaluronan under dynamic stimulation. When chondrocytes were encapsulated in PEG hydrogels containing exogenous hyaluronan and/or Link-N, the ECM analogs aided in the retention of cell-secreted glycosaminoglycans under loading. The presence of hyaluronan led to enhanced deposition of collagen type II and aggrecan. In conclusion, our results highlight the importance of ECM analogs, specifically hyaluronan and Link-N, in matrix retention and matrix development and offer new strategies for designing scaffolds for cartilage regeneration.
Keywords: hyaluronan, link protein, biomimetic, hydrogel, cartilage tissue engineering
INTRODUCTION
Due to the lack of self-repair of articular cartilage within the body, tissue engineering offers a promising strategy, combining cells, scaffolds, and/or stimuli, to support cell and tissue growth.1–3 The scaffold is intended to provide a 3D framework for the localization of cells and for their newly deposited matrix molecules to organize into a macroscopic tissue. While synthetic based scaffolds allow for tight control of the 3D framework, the incorporation of molecules that more closely mimic the native tissue may provide key signals for matrix synthesis and may also help in the retention and assembly of neotissue.
Towards designing biomimetic environments that more closely mimic native cartilage, hyaluronan-based scaffolds have been explored. Hyaluronan, a variable length polysaccharide consisting of disaccharide repeating units, is particularly attractive because it is one of the main building blocks of aggrecan, a major cartilage macromolecule, and chondrocytes are known to express cell surface receptors specific for hyaluronan.4–6 When chondrocytes were encapsulated in crosslinked hyalyuronan hydrogels and placed in vivo subcutaneously, cartilage-like matrix was deposited after 12 weeks and comprised of sulfated glycosaminoglycans and type II collagen with minimal type I collagen indicating that chondrocytes maintained their phenotype within hyaluronan hydrogels.7 More recently, several studies have shown that hyaluronan-based hydrogels enhance chondrogenesis of mesenchymal stem cells in vitro8,9 and in vivo.10–12 For example, the delivery of mesenchymal stem cells within a hyaluronan hydrogel led to robust cartilage regeneration in an osteochondral defect model in rabbit.13 Together, these and other studies demonstrate that hyaluronan is a promising macromolecule to consider when designing biomimetic environments for cartilage tissue engineering.
In an effort to simulate cues which are thought to be important in functional cartilage growth, a number of studies have incorporated physical stimuli into their tissue engineering strategies14–17 (e.g., dynamic compressive loading), showing enhanced matrix synthesis18–22 and in some studies increased scaffold mechanical properties.23–26 However, several studies have reported loss of matrix molecules from their scaffold and that this loss is accelerated by applications of dynamic loading. For example, a large fraction (~40%) of the glycosaminoglycans (GAGs) synthesized by chondrocytes cultured in agarose27,28 or self-assembled peptide hydrogels24 were released into the surrounding medium. Kisiday et al.24 reported that intermittent cyclic compressive loading applied to self-assembled peptide hydrogels resulted in 50–100% higher GAG loss over free-swelling constructs. Recent studies from our group have shown that for adult bovine chondrocytes cultured in poly(ethylene glycol) (PEG) hydrogels up to 50% of the newly synthesized GAG is lost to the medium under free-swelling cultures.29 When the PEG hydrogels were cultured in a rotating wall vessel, intended to enhance nutrient transport, this dynamic environment further facilitated the loss of matrix from the hydrogels.29 The majority of these studies have examined the loss of matrix based on glycosaminoglycans, which are building blocks of larger proteoglycans like aggrecan. This loss may be a result of simple diffusion of smaller extracellular matrix (ECM) molecules or ECM fragments from the scaffold whereby loading enhances their transport. These findings suggest that, at least during the early stages of neotissue development, many scaffolds are not capable of retaining a significant fraction of the newly synthesized ECM. The significant loss of GAGs may create a critical delay in neo-tissue growth within the hydrogel constructs.
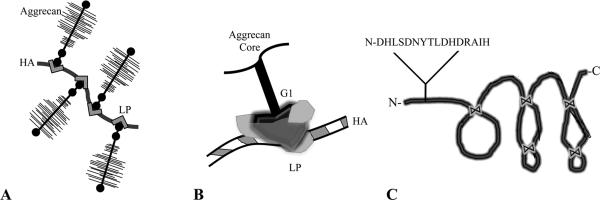
In the extracellular regions of cartilage, there are a number of matrix-matrix interactions that aid in the retention and organization of the ECM components.30 Hyaluronan interacts non-covalently with the G1 domain of aggrecan to form large macromolecules in the extracellular space reaching molecular weights upwards of 100–400 MDa. This complex interaction is stabilized by link protein,31 as shown in Fig. 1. A smaller peptide fragment of link protein, which is produced by MMP-3 cleavage of link protein, has also been shown to facilitate assembly of aggrecan monomers with hyaluronan in articular cartilage explants when delivered exogeneously in solution.32 This small peptide fragment represents the N-terminus of link protein containing the amino acid sequence, DHLSDNYTLDHDRAIH (Link-N). Although the mechanisms are not well understood, it appears Link-N is capable of facilitating matrix-matrix interactions.
Figure 1.
Schematic representing a) the macromolecular organization of hyaluronan (HA), aggrecan and link protein (LP); b) the involvement of link protein in the interaction between the G1 domain of the aggrecan core protein with a hyaluronan binding region; and c) the triple hairpin structure of link protein, held together by disulfide bonds, and the N-terminal cleavage product Link-N peptide.
Synthetic hydrogels based on poly(ethylene glycol) (PEG) were chosen for this study because they have been successfully used to encapsulate chondrocytes, providing a 3D environment that maintains the chondrocyte phenotype and supports the deposition of cartilaginous matrix components33,34 and biological molecules can be incorporated into the hydrogel with relative ease.35 Specifically, this research tested the hypothesis that hyaluronan encapsulated within PEG hydrogels improves tissue deposition by chondrocytes, while the incorporation of small molecules derived from link protein (i.e., Link-N) aid in the retention of the entrapped hyaluronan as well as cell secreted glycosaminoglycans, particularly when dynamic loading is employed. To test this hypothesis, this study is divided into three aims. In the first aim, we examined the release of entrapped hyaluronan in the absence and presence of dynamic compressive loading and assessed whether tethering Link-N to the PEG hydrogels would aid in the retention of hyaluronan. To test for Link-N's specificity, chondroitin sulfate, another one of the main building blocks of aggrecan but which is not known to interact with link protein, was encapsulated and its release evaluated. Because hyaluronan and link protein are involved in the assembly of aggrecan, the second aim assessed the ability of hyaluronan and Link-N to retain cell secreted sulfated glycosaminoglycans. Finally, in the third aim we examined whether the encapsulated hyaluronan under free swelling or dynamic loading conditions would lead to improved cartilage-like matrix deposition and whether the presence of Link-N with its retention capabilities for hyaluronan was an important component.
MATERIALS AND METHODS
Materials
Collagenase type II and papain were from Worthington Biochemical (Lakeshore, NJ). Fetal bovine serum (FBS), Dulbecco's Modified Eagle's Medium (DMEM), 100× penicillin-streptomycin (P/S), fungizone, HEPES-buffer, gentamicin, and MEM-nonessential amino acids (NEAA), goat anti-rabbit IgG Alexa Fluor 488, goat anti-mouse IgG Alexa Fluor 546, and DAPI were from Invitrogen (Carlsbad, CA). L-proline, ascorbic acid, bovine serum albumin (BSA), protease-free chondroitinase ABC, dichloromethane, methacryloyl chloride, diethyl ether, triethylamine (TEA), ammonium persulfate (APS), tetramethylethylenediamine (TEMED), 5-aminofluorescein, 3-(3-dimthylaminopropyl)-1-ethyl-carboiimide (EDC), pyridine, dimethylmethylene blue, and all buffer reagents were from Sigma-Aldrich (St. Louis, MO). Irgacure 2959 was from Ciba Specialty Chemicals (Newport, DE). All amino acids and reagents for peptide synthesis were from Applied Biosystems (Foster City, CA). Acrylate-poly(ethylene glycol)-N-hydroxy succinamide ester (Acrylate-PEG-NHS) was acquired from Nektar Therapeutics Inc. (San Carlos, CA). Medical-grade hyaluronan sodium salt (HA) was from LifeCore Biomedicals (Chaska, MN). Aggrecan antibody (A1059-53F) and collagen II antibody (C5710-20F) were from US Biologicals (Swampscott, MA).
Macromer Synthesis and Hydrogel Formation
Poly(ethylene glycol) dimethacrylate (PEGDM) macromer was synthesized as described elsewhere.36 Briefly, PEG (Fluka, MW~4600) was reacted with 6 molar excess methacryloyl chloride in the presence of TEA in dichloromethane for 24 hrs at 4°C. PEGDM was brought to room temperature and purified by precipitations in chilled diethyl ether. The vacuum dried product was >90% methacrylated as confirmed by 1H NMR (Varian YVR-500S). Hydrogel constructs (5 × 5 mm cylinders) were formed either by redox-initiated polymerization or by photopolymerization. For redox-initiated polymerization, APS (0.05 M) and TEMED (0.05 M) were mixed with 10% PEGDM (w/w) and the solution was allowed to polymerize at room temperature for 15 min. Hydrogels were also formed via photopolymerization by mixing 10% PEGDM (w/w) with 0.05% photoinitiator Irgacure 2959 and exposing to 365 nm light (6 mW/cm2) for 10 min.
Peptide Synthesis and Conjugation
The Link-N peptide (DHLSDNYTLDHDRAIH) was synthesized (Applied Biosystem 433A Peptide Synthesizer) using solid phase Fmoc chemistry on a MHBA Rink Amide Resin (<0.7 mmol/g resin substitution). Peptides were cleaved from their solid support using trifluoroacetic acid/triisopropylsilane/water (95/2.5/2.5%v/v) and allowed to react at room temperature for 2 hrs. The reaction was filtered and the filtrate precipitated and washed (3×) in chilled diethyl ether. Peptides were purified by semipreparative reversed phase HPLC (Waters Delta Prep 4000) using a 70 min linear (5–95%) gradient of acetonitrile in 0.1% trifluoroacetic acid. Peptide purity was confirmed by analytical reversed phase HPLC C18 column and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF, Applied Biosystem DE Voyager). Acrylate-PEG-NHS (3400 Da) was reacted to the N-terminal amine of the Link-N peptide in 0.1 M sodium phosphate buffer pH 8.0 for 4 hrs protected from light. Peptide conjugation was assessed by the fluorescamine free amine assay37 and indicated >70% efficiency. The desired product, Acrylate-PEG-HIARDHDLTYNDSLHD (PEGMA-Link-N), was dialzyed and lyohphilized, resulting in a dry powder (MW~5321 Da).
Model ECM Molecules and Their Release
Fluorescently-labeled hyaluronan (f-HA) was synthesized by methods described by Nagata et al.38,39 Briefly, hyaluronan (Mn~130 kDa, 200 mg sodium salt) was dissolved in 50 mL of a solution containing 75% (v/v) 1 M HCl and 25% (v/v) pyridine and mixed with 10 mL of 5-aminofluorescein (268 mg, 1.6 mole equivalent per disaccharide unit) dissolved in a solution containing 50% (v/v) 1 M HCl and 50% (v/v) pyridine. The pH was adjusted to 4.75 with 37% HCl and EDC (4.52 g, 49 mol equiv. per disaccharide unit) was added to the solution for 2 hrs under agitation at room temperature to facilitate conjugation of 5-aminofluorescein to hyaluronan. The product was purified by dialysis (8 kDa MWCO) overnight and recovered by precipitation in chilled ethanol with 1.25% sodium acetate followed by centrifugation at 1,500× g with additional rinses in chilled ethanol. The pellet was re-dissolved in 20 mL NaOH (0.1 M) for 20 hrs at 37°C, and then neutralized prior to re-precipitating in chilled ethanol with 1.25% sodium acetate followed by centrifugation (2,000× g, 10°C, 10 min). The pellet was dissolved in 100 mL distilled water and dialyzed (8 kDa MWCO) overnight. The final purified product was recovered by lyophilization and stored at −20°C protected from light. The macromer solutions were mixed with PEGMA-Link-N (1 mg/g macromer solution) and either chondroitin sulfate (Mn~36 kDa, 10 mg/g) or f-HA (1 mg/g) prior to redox-initiated polymerization. The redox-initiator system was necessary to permit polymerization and encapsulation of the f-HA due to the presence of fluorescein. Gels were cultured in a shaker bath (40 rpm) at 37°C in 2 mL DI-H2O, which was collected and replenished at each time point. Release of f-HA was measured by spectrophotometric analysis (Ex, 492 nm; Em, 525 nm) against a standard curve (0 μg/mL to 50 μg/mL, R2=0.999). Chondroitin sulfate release was measured by the dimethylmethylene blue (DMMB) assay.40 Cumulative ECM release values are reported as % of total encapsulated and are represented as a mean and standard deviation (n=4).
Chondrocyte Encapsulation
Full depth articular cartilage was harvested from the patellar-femoral groove of 1–3 week old calves (n=2, Research 87, Marlborough, MA) within 24 hrs of slaughter and digested in 500 units/mL collagenase II in DMEM supplemented with 5% FBS for 16 hrs at 37°C on an orbital shaker (40 rpm). The digest was passed through a 100 μm cell-strainer, pelleted and rinsed 3× with PBS containing 1% P/S (Penicillin/Streptomyocin), 0.5 μg/mL fungizone, and 20 μg/mL gentamicin (PBS with antibiotics). Isolated cells were counted using trypan blue exclusion assay. Chondrocytes (50 million cells/mL) were mixed with 10% (w/w) PEGDM and 0.05% (w/w) photoinitiator Irgacure I2959 in PBS and photopolymerized as described above and then placed in chondrocyte medium (DMEM supplemented with 10% FBS (v/v), 0.04 mM L-proline, 50 mg/L L-ascorbic acid, 10 mM HEPES, 0.1 M MEM-NEAA, 1% P/S, 0.5 μg/mL fungizone, and 20 μg/mL gentamicin). Gels were formed under one of four conditions: 1) with PEGMA-Link-N (1 mg/g macromer solution), 2) with hyaluronan (0.5 mg/g), 3) with hyaluronan (0.5 mg/g) and PEGMA-Link-N (1 mg/g or 0.18 mM), or 4) with Acrylate-PEG-NHS (0.6 mg/g or 0.18 mM), which served as a control. It is important to note that the NHS group is readily hydrolyzed in aqueous environments, resulting in a PEG hydrogel with PEG tethers in the control. The concentration of hyaluronan was selected based on proximity to native articular cartilage.22 The Link-N concentration selected was based on the aggregation of aggrecan in cartilage. When hyaluronan is saturated with bound aggrecan, there is approximately one aggrecan (1 aggrecan:1 Link-N) per 32 repeat units on the hyaluronan.30
Mechanical Loading
Following encapsulation, gels were rinsed in PBS with antibiotics, and cultured under free-swelling conditions for 24 hrs, and either removed from culture and analyzed (referred to as time = 0 days), or subjected to dynamic unconfined deformational loading using custom-designed bioreactors described elsewhere.41 All loading studies were performed between permeable platens and bases (Porex 40–70 μm). ECM release studies were performed on acellular gels subjected to a sinusoidal dynamic compression at 1 Hz, 15% peak-to-peak strain (5% tare strain) continuously. Cell-laden gels were subject to intermittent type loading (8 cycles/day with each cycle containing 30 min loading and 90 min rest) using 0.3 Hz and 15% peak-to-peak strain (5% tare strain). Precise displacement control was verified using the on-board linear variable displacement transducer sensor, which showed less than 0.01% deviation. Gels were also cultured under free-swelling conditions (referred to as controls), in which the plates were placed on an orbital shaker rotating at 50 rpm. Cell viability prior to loading and after 2 weeks of loading was qualitatively assessed using a LIVE/DEAD® assay (Invitrogen) per manufacturer. Medium was changed during sampling and stored at −20°C until further analysis. At specified time points, gels were removed from culture and immediately processed.
Characterization of Cell-Laden Hydrogels
At specified time points, constructs were removed from culture and their tangent modulus determined (Bose TestBench 10N) by applying a constant strain rate under unconfined compression (0.5 mm/min). Gels were halved and subsequently processed. To determine the water content in the cell-laden hydrogels, constructs were weighed, lyophilized for 48 hrs and their mass swelling ratio determined by the ratio of the wet mass over the dry mass. Hydrogels were homogenized and enzymatically digested by papain for 16 hrs at 60°C. Gel samples and collected media were assessed for sulfated GAG content by the DMMB dye method40. GAG content within hydrogels were normalized to their corresponding gel wet weight. Data are represented as the mean and standard deviation (n=4).
Histological Analysis
The remaining construct halves were fixed for 24 hrs in 4% paraformaldehyde, dehydrated, paraffin-embedded, and sectioned (10 μm) for immunohistochemistry (IHC). Sections were tested against anti-aggrecan (1:10) and anti-collagen II (1:100). All samples were blocked using 1% BSA for 30 minutes. Fluorescent detection of each protein was achieved using either secondary goat anti-rabbit IgG Alexa Fluor 488 or goat anti-mouse IgG Alexa Fluor 546 antibodies (1:100) and counterstained using DAPI (1:1000). The antibodies were validated using positive controls (articular cartilage) and negative controls (articular cartilage without the application of primary antibody). Sections were mounted and preserved using VectaMount, and a laser scanning confocal microscope (Zeiss LSM 5 Pascal) was used to acquire images. Semi-quantitative analysis of IHC images obtained at 400× was performed by determining the percentage of cells stained positive for aggrecan or collagen II across the entire image. In brief, the cells stained positive for aggrecan or collagen type II were manually selected. ImageJ software with the cell counting add-on was used to determine the number of positive cells. The total number of cells was determined by counting nuclei in the corresponding field of view. Approximately 40–70 cells were counted from three separate images taken in random locations within the hydrogel of three independent samples.
Statistical Analysis
Data are presented as a mean ± standard deviation. One-way or two-way analysis of variance (ANOVA) was performed where stated and analyzed post-hoc using Tukey's HSD. Student t-tests were also performed to determine significance among individual samples. An α=0.05 was considered significant.
RESULTS
Link-N Peptide Synthesis and Characterization
A 16-mer oligopeptide that represents the N-terminal region of the cleaved link protein, shown in Fig. 1, was successfully synthesized. The resulting product was purified by HPLC and MALDI-TOF confirming that the desired peptide (MW 1921 g/mol) (see supplemental figure) and had a purity >99%.
Release of Model Extracelluar Matrix Molecules from Biomimetic Hydrogels
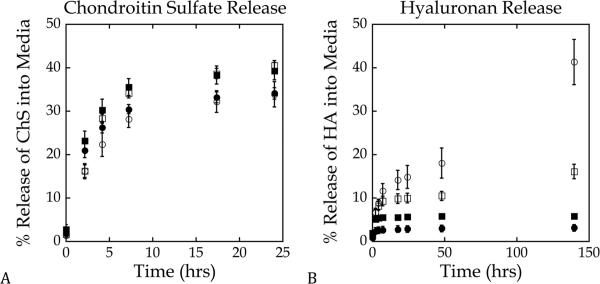
The release of hyaluronan was impacted by dynamic loading (Fig. 2a). Approximately 40% of hyaluronan was released from the hydrogels subjected to dynamic loading and was significantly higher (p < 0.0001) than the hyaluronan released under free swelling conditions (~3%) after 6 days. Tethering Link-N peptide to the hydrogel network affected the release profile of hyaluronan, leading to increased release of hyaluronan from the hydrogels within the first 24 hrs under free swelling cultures (Fig. 2a). However, greater than 95% of the encapsulated hyaluronan was retained within the hydrogel after 6 days of culture in the free swelling cultures. Hydrogels containing Link-N peptide and subjected to dynamic loading resulted in a ~60% reduction in the release of hyaluronan after 6 days of loading (p <0.0001). Chondroitin sulfate was used as a control for specificity (Fig. 2b). The addition of Link-N peptide increased the release rate of chondroitin sulfate similar to that observed with hyaluronan, but dynamic loading had no effect on the release profile of chondroitin sulfate (p > 0.21) regardless of the presence of Link-N peptide.
Figure 2.
Influence of dynamic loading and the covalent incorporation of Link-N on the release of representative ECM molecules. Constructs with (∎,◻) and without (●,◯) Link-N cultured under either free-swelling (∎,●) or dynamic loading at 1 Hz and 15% strain (◻,◯) conditions. a) Release (%) of encapsulated hyaluronan (HA) into the surrounding medium based on the original amount of entrapped hyaluronan; b) Release (%) of encapsulated chondroitin sulfate (ChS) into the surrounding medium based on the original amount of entrapped chondroitin sulfate. Data are reported as mean ± standard deviation.
Properties of Chondrocyte-Laden Hydrogels
Initial properties of the chondrocyte-laden hydrogels were examined by their modulus in compression mode (Table 1) and mass swelling ratio (Table 2) 24 hours post encapsulation (referred to as day 0). Although the average modulus of the hydrogels containing Link-N was 10–20% lower than that of remaining formulations, this apparent difference was not statistically significant (p=0.12). The mass swelling ratio was statistically similar for all hydrogel formulations.
Table 1.
| Compressive Modulus (kPa) in PEG hydrogels containing: | |||||
|---|---|---|---|---|---|
| Day | Culture conditions | No additives | Hyaluronan | Link-N | Hyaluronan and Link-N |
| 0 | Free Swelling | 50 ± 2 | 57 ± 9 | 46 ± 2 | 55 ± 5 |
| 14 | Free Swelling | 49 ± 2 | 47 ± 3 | 38 ± 6 | 53 ± 8 |
| Loaded | 44 ± 8 | 48 ± 8 | 50 ± 3 | 54 ± 4 | |
| 25 | Free Swelling | 39 ± 3 | 46 ± 2 | 41 ± 3 | 43 ± 6 |
| Loaded | 50 ± 5 | 48 ± 3 | 40 ± 1 | 48 ± 2 | |
Table 2.
| Initial mass swelling ratio of PEG hydrogels containing: | |||
|---|---|---|---|
| No additives | Hyaluronan | Link-N | Hyaluronan and Link-N |
| 10 ± 1 | 10 ± 1 | 10 ± 0.2 | 10 ± 0.2 |
The modulus measured in compression mode was evaluated over the course of the culture period (Table 1). Overall, gel formulation (p<0.01) and culture time (p<0.01), but not loading (p=0.07) were factors affecting the compressive modulus. By day 25, the modulus was similar among all gel formulations under free swelling conditions, but under loading conditions PEG hydrogels containing Link-N remained the lowest. Hyaluronan did not affect the mechanical properties of the cell-laden constructs over the course of the study regardless of loading. The water content of the cell-laden constructs was measured throughout the study and no significant differences were observed as a function of gel formulation, culture time, or loading (data not shown).
Chondrocyte Response to Biomimetic Hydrogels
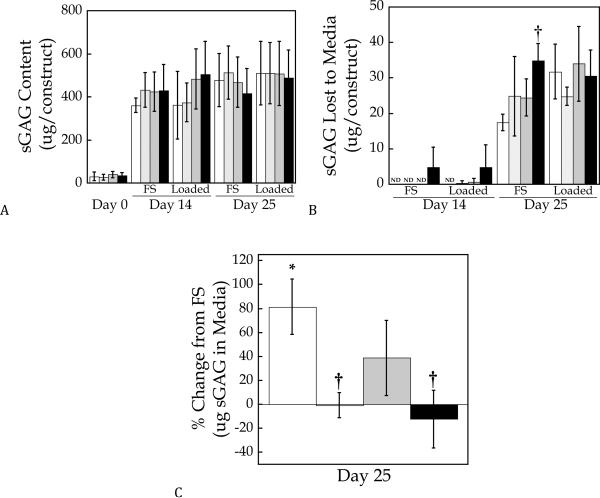
Chondrocyte viability, based on a qualitative membrane integrity assay, was high 24 hrs post-encapsulation and after 14 days of culture within the hydrogels (supplementary figure). Tissue production was assessed in hydrogels prepared from PEG-only and PEG containing entrapped hyaluronan, tethered Link-N peptide, or a combination of Link-N peptide and entrapped hyaluronan. After 24 hours post-encapsulation (i.e. day 0), there was minimal sGAG production within the constructs regardless of their formulation (Fig. 3a). sGAG content reached approximately 500 μg per construct by day 25 and was unaffected by either Link-N peptide, hyaluronan, or by dynamic loading (Fig. 3a). At 25 days of culture there was detectable cumulative sGAG release for all hydrogel formulations approximating 6% of the total sGAG production (Fig. 3b). To assess the impact of gel formulation on sGAG release, cumulative sGAG released for each gel formulation was normalized to their free swelling counterparts (Fig. 3c). The results show that dynamic loading significantly enhanced the amount of sGAG released from the PEG-only hydrogels reaching values that were 60–80% greater than the free swelling hydrogels. Hydrogels containing Link-N, hyaluronan, or both had statistically similar sGAG release in the loaded constructs as their free swelling counterparts. For hydrogels containing hyaluronan, the sGAG released was significantly lower compared to the PEG-only hydrogels (p<0.005).
Figure 3.
Quantification of accumulated sulfated glycosaminoglcyan (sGAG) content a) within cell-laden PEG hydrogels and b) which was released into the culture medium for free-swelling (FS) and dynamically loaded conditions. In c), the % change in sGAG release due to loading is presented (i.e., % change is defined as the difference in sGAG released between the loaded constructs and the free swelling constructs relative to the free swelling constructs). PEG hydrogels contained no additives (◻), hyaluronan (∎), Link-N (∎), or hyaluronan and Link-N (∎). Data are represented as mean ± standard deviation. ND indicates none detected, * indicates difference from free-swelling controls p<0.05, † indicates significant difference from PEG-only gels containing no additives p<0.05.
Spatial Deposition of Cartilage-Specific ECM Molecules in Biomimetic Hydrogels
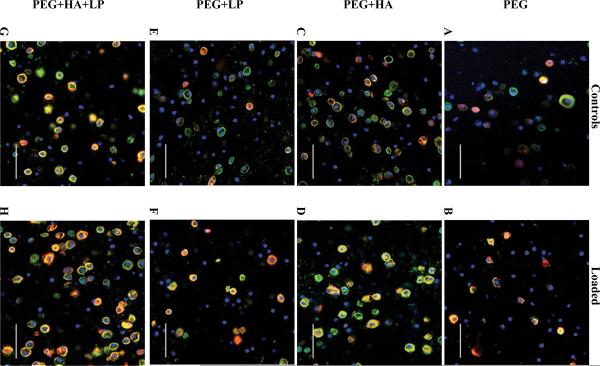
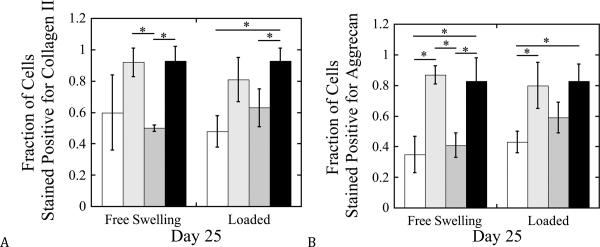
The spatial deposition of the neotissue was analyzed by immunohistochemistry to assess cartilage specific matrix molecules, namely collagen II and aggrecan (Fig. 4). Collagen II and aggrecan were localized to the pericellular matrix (PCM) for all hydrogel formulations, but the degree of staining was dependent on the formulation. Semi-quantitative analysis of the immunohistochemistry (Fig. 5) indicated that approximately 60% and 35% of chondrocytes stained positive for collagen II and aggrecan, respectively, in their pericellular regions in the PEG-only hydrogels when cultured under free swelling conditions. The addition of hyaluronan resulted in 80–90% of the cells staining positive for aggrecan and collagen II in their PCMs, which was maintained with dynamic loading. Incorporation of Link-N peptide showed no significant difference in collagen II or aggrecan from the PEG-only hydrogels (p>0.10). With the application of mechanical loading, the fraction of chondrocytes staining positive for collagen II and aggrecan in Link-N only hydrogels increased by ~25–40%, although not significantly (p=0.07), compared to free swelling constructs.
Figure 4.
Gross examination of cartilage-matrix deposition by immunohistochemical evaluation for chondrocytes encapsulated in PEG hydrogels and cultured for 25 days under free-swelling (a,c,e,g) or dynamic loading conditions (b,d,f,h). PEG hydrogels contained no additives (a,b), hyaluronan (c,d), Link-N (e,f), or hyaluronan and Link-N (g,h). Sections were stained using antibodies against collagen type II (green) and aggrecan (red) and nuclei (blue) were counterstained by DAPI. Images were acquired by laser scanning confocal microscopy. Scale bars represent 50 μm.
Figure 5.
Semi-quantitative analysis of cartilage-matrix deposition by immunohistochemical evaluation for chondrocytes encapsulated in PEG hydrogels and cultured for 25 days under free-swelling or dynamic loading conditions. The fraction of cells staining positive for collagen type II (a) and aggrecan (b) found in the pericellular region was normalized to the total number of cells as quantified using DAPI. PEG hydrogels contained no additives (◻), hyaluronan (∎), Link-N (∎), or hyaluronan and Link-N (∎). Data are represented as mean ± standard deviation. * indicates difference p<0.05.
DISCUSSION
While synthetic scaffolds offer numerous advantages in designing a 3D environment with controlled structures, mechanical properties, and degradation behaviors, they offer little biorecognition for the cells. Cells are known to interact with their extracellular matrix receiving numerous insoluble cues, which are likely critical to engineering a functional neotissue. Several studies have reported that incorporating matrix analogs to simulate the native cartilage environment, such as a collagen mimetic protein,42 decorin binding region,43 or chondroitin sulfate,44,45 into PEG hydrogels promoted matrix synthesis. This research tested the hypothesis that exogenous hyaluronan entrapped within PEG hydrogels improves cartilage neotissue deposition, while the incorporation of Link-N aided in the retention of the exogenous hyaluronan and cell secreted glycosaminoglycans, particularly when dynamic loading is employed.
We acknowledge several limitations of our study. First, the release of model ECM molecules were investigated at a loading frequency of 1 Hz, because this frequency is commonly used in studies employing mechanical stimulation for cartilage tissue engineering.16 For the cell-laden experiments, a lower frequency of 0.3 Hz was selected because we have shown more recently that chondrocytes encapsulated in PEG hydrogels are more responsive to loading at 0.3 Hz over 1 Hz.36,46 As a result, the release studies are not able to predict release rates of hyaluronan from cell-laden chondrocytes.47 However, the presence of chondrocytes, which have the ability to bind to hyaluronan through cell surface receptors, may further affect its release making a direct comparison between acellular and cellular experiments not possible. A second limitation is one concentration of Link-N and hyaluronan were investigated. While concentrations were selected to represent physiological levels, optimal concentrations that maximize their function as matrix enhancing and retaining molecules in a tissue engineering model may differ and differ based on chemistry (i.e., hyaluronan versus Link-N). Findings from this study, therefore serve to demonstrate that matrix molecules incorporated into a tissue engineering system are capable of matrix retention and enhancement of cartilage-specific matrix deposition. However, additional studies are necessary to determine optimal concentrations for hyaluronan and Link-N.
One of the primary objectives for this study was to incorporate exogenous hyaluronan within PEG hydrogels to create a biomimetic environment that was more similar to the native environment of chondrocytes. While hyaluronan remained entrapped within PEG hydrogels under free swelling conditions, the application of dynamic loading led to its release and in significant amounts. In an effort to retain hyaluronan and other matrix molecules secreted by the cells, Link-N a derivative of link protein was tethered into the PEG hydrogel. Our findings demonstrate that Link-N is capable of retaining entrapped hyaluronan under dynamic loading suggesting that Link-N maintains some of the functional properties of link protein. This observation is further supported by the fact that Link-N did not aid in the retention of chondroitin sulfate, one of the main glycosaminoglycans in aggrecan monomers, and with which link protein does not natively interact. However, it should be noted that the release kinetics of hyaluronan were significantly slower than that of chondroitin sulfate as a result of the disparate amounts of each matrix molecule encapsulated and their different molecular weights, which may have impacted the findings. Dynamic loading did not affect the release of chondroitin sulfate, although it did affect hyaluronan release. This disparate finding may be due to the smaller size of chondroitin sulfate where diffusion was sufficiently high so that increased fluid transport induced by loading had less of an affect.47,48 It is also important to note that the release kinetics for both hyaluronan and chondroitin sulfate were enhanced in the presence of Link-N, which is attributed to a lower crosslinking density that resulted when Link-N was incorporated into the hydrogel during polymerization (as indicated by the lower modulus of the cell-laden constructs containing Link-N when compared to PEG-only). The reduced crosslinking density may be due to reacting a mono-functional monomer, the presence of a relatively large oligopeptide, Link-N acting as a chain transfer agent thus reducing crosslinking efficiency,49 or slight differences in the manufacturing of the hydrogels.
The incorporation of hyaluronan, and to a lesser extent Link-N, led to a reduction in the loss of cell secreted sulfated glycosaminoglycans under dynamic loading. While the mechanisms are not known, both hyaluronan and link protein natively interact with aggrecan monomers (Fig. 1). In cartilage, sGAGs including chondroitin sulfate are synthesized intracellularly, attach to serine residues on a protein core, and then are glycosylated prior to secretion. Once secreted, the proteoglycan molecules, and specifically aggrecan monomers are assembled in the extracellular space with link protein and hyaluronan to form large aggrecan aggregates. Our findings suggest that hyaluronan is better at retaining sGAGs over Link-N, at least at the concentrations employed in this study. More specifically, exogenous hyaluronan may interact with the hyaluronan binding region within cell-secreted aggrecan monomers.50 In contrast, Link-N represents a small region of the link protein and therefore may have decreased specificity for retention of aggrecan monomers. Nonetheless, these findings demonstrate that incorporating native ECM analogs into the hydrogel aids in the retention of cell secreted matrix under dynamic loading.
The entrapment of exogenous hyaluronan was beneficial to cartilage extracellular matrix formation, evidenced by increased deposition of aggrecan and collagen II. Chondrocytes are known to express cell surface receptors for hyaluronan (e.g, CD44),4–6 which have been shown to be involved in anabolic and catabolic activities of chondrocytes51 and influence the structural organization around chondrocytes.4–6 Therefore, chondrocytes may receive “outside-in” signals from the exogenous hyaluronan, thus stimulating ECM synthesis. In addition to possible receptor-mediated signaling events, the interaction between CD44 and hyaluronan is believed to create cross-bridging that tethers the hyaluronan/proteoglycan-rich pericellular matrix to the cell,4,52 thus contributing to the formation of the PCM. Therefore, it is possible that the exogenous hyaluronan may aid in creating an anchor for secreted proteoglycans to bind and organize. However, the role of Link-N is less clear. Our findings suggest that hyaluronan, when simply entrapped in the hydrogel in the presence of cells, is retained sufficiently under loading to maintain its positive effect on cells. While the incorporation of Link-N clearly led to retention of hyaluronan in the absence of cells, its presence with cells may not be as critical. The incorporation of Link-N with or without hyaluronan did not appear to negatively impact aggrecan nor collagen II deposition.
While findings from the cell-laden studies are largely attributed to biochemical effects of hyaluronan, it is important to recognize that changes in hydrogel formulation can impact the properties of the hydrogel and subsequently affect cellular response. For example, it has been shown that substrate stiffness can affect cellular differentiation.53 and crosslinking density can alter tissue synthesis and deposition.54 However, the initial modulus and swelling of the cell-laden hydrogels with and without hyaluronan were similar suggesting that the improved matrix deposition was likely due to biochemical effects of hyaluronan. The positive effects of hyaluronan may be due to chondrocytes interacting directly with hyaluronan through cell surface receptors or may be due to indirect interactions, such as extracellular signaling molecules (e.g. cytokines, growth factors) interacting with hyaluronan. Since hyaluronan carries a net negative charge, albeit ~105 times lower than that of native cartilage, the presence of negative charges may sequester other signaling molecules leading to improved tissue deposition.55 While the incorporation of Link-N did lead to a reduced modulus, the presence of Link-N had little effect on the encapsulated chondrocytes. Link-N similarly carries a net negative charge (~106 times lower than that of native cartilage), but this charge was not likely sufficient to have any effects on encapsulated chondrocytes.
For future considerations, there are several important observations worth noting. To further investigate the interaction of hyaluronan and Link-N, a scrambled peptide sequence of Link-N would help to elucidate if the mechanism is indeed sequence specific thus capturing the native function of link protein or is due to non-native interactions. A second observation from this study is that many of the large cartilage-specific matrix molecules deposited by the cells remain localized to the pericellular regions in the non-degrading PEG hydrogels. This observation suggests that the gel crosslinked structure limits diffusion and organization of the large proteoglycan aggregates, which can reach upwards of ~1–4 million Da, and collagen II matrix molecules in the extracellular space. Previous studies by Bryant et al.56–58 demonstrated the importance of hydrogel degradation in matrix elaboration and the development of a macroscopic engineered tissue, particularly with respect to collagen secretion. Therefore, the incorporation of Link-N may have more pronounced effects in a degradable system where its ability to capture and interact with both exogenous hyaluronan as well as larger cell-secreted aggrecan monomers may lead to enhanced matrix content. Future studies will combine matrix retaining ECM analogs with degradable hydrogels which are currently being explored in our laboratory.
CONCLUSIONS
The incorporation of hyaluronan positively impacted matrix retention and matrix synthesis in PEG hydrogels. Hyaluronan and Link-N were capable of reducing load-induced cell-secreted sGAG loss suggesting that the incorporation of these ECM analogs may help to retain matrix molecules and aid in macroscopic tissue evolution particularly when loading is applied. These findings support the idea that cells receive insoluble biochemical cues from their extracellular matrix and that these cues may be important for not only tissue homeostasis, but also in supporting neo-tissue secretion and deposition.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institute of Health (grant number K22DE016608), Department of Education's Graduate Assistantships in Areas of National Need (GAANN) fellowships to GDN and JJR, a NIH Biotechnology Training Fellowship to JJR, and the NSF REU program in the Department of Chemical and Biological Engineering at the University of Colorado for support of SG. We would like to especially thank Dr. Alex Aimetti and Dr. Brian Polizotti for their technical assistance in synthesizing and purifying the link protein peptide.
REFERENCES
- 1.Chung C, Burdick JA. Engineering cartilage tissue. Advanced Drug Delivery Reviews. 2008;60(2):243–262. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Engineering Part B-Reviews. 2008;14(2):149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoddart MJ, Grad S, Eglin D, Alini M. Cells and biomaterials in cartilage tissue engineering. Regenerative Medicine. 2009;4(1):81–98. doi: 10.2217/17460751.4.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Knudson W, Aguiar DJ, Hua Q, Knudson CB. CD44-anchored hyaluronan-rich pericellular matrices: an ultrastructural and biochemical analysis. Exp Cell Res. 1996;228(2):216–28. doi: 10.1006/excr.1996.0320. [DOI] [PubMed] [Google Scholar]
- 5.Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002;21(1):15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 6.Knudson CB, Knudson W. Hyaluronan and CD44: modulators of chondrocyte metabolism. Clin Orthop Relat Res. 2004;427(Suppl):S152–62. [PubMed] [Google Scholar]
- 7.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. Journal Of Biomedical Materials Research Part A. 2006;77A(3):518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30(26):4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Abatangelo G. Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res. 2000;50(2):101–9. doi: 10.1002/(sici)1097-4636(200005)50:2<101::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Sharma B, Williams CG, Khan M, Manson P, Elisseeff JH. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plastic and Reconstructive Surgery. 2007;119(1):112–20. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 11.Angele P, Kujat R, Nerlich M, Yoo J, Goldberg V, Johnstone B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 1999;5(6):545–54. doi: 10.1089/ten.1999.5.545. [DOI] [PubMed] [Google Scholar]
- 12.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YC, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Engineering. 2006;12(12):3405–3416. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 14.Darling EM, Athanasiou KA. Articular cartilage bioreactors and bioprocesses. Tissue Engineering. 2003;9(1):9–26. doi: 10.1089/107632703762687492. [DOI] [PubMed] [Google Scholar]
- 15.Darling EM, Athanasiou KA. Biomechanical strategies for articular cartilage regeneration. Annals of Biomedical Engineering. 2003;31(9):1114–1124. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- 16.Hung CT, Mauck RL, Wang CCB, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Annals of Biomedical Engineering. 2004;32(1):35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 17.Kuo CK, Li WJ, Mauck RL, Tuan RS. Cartilage tissue engineering: its potential and uses. Current Opinion in Rheumatology. 2006;18(1):64–73. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 18.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. Journal of Orthopaedic Research. 1997;15(2):181–188. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 19.Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis and Cartilage. 2006;14(4):323–330. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Engineering. 2003;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury TT, Bader DL, Shelton JC, Lee DA. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Archives of Biochemistry and Biophysics. 2003;417(1):105–111. doi: 10.1016/s0003-9861(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 22.Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. Journal of Orthopaedic Research. 2002;20(4):842–848. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 23.Lima EG, Bian L, Ng KW, Mauck RL, Byers Ba, Tuan RS, Ateshian Ga, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta 3. Osteoarthritis and Cartilage. 2007;15(9):1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kisiday JD, Jin MS, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. Journal of Biomechanics. 2004;37(5):595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Annals of Biomedical Engineering. 2002;30(8):1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 26.Mauck RL, Soltz MA, Wang CCB, Wong DD, Chao PHG, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. Journal of Biomechanical Engineering-Transactions of the ASME. 2000;122(3):252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 27.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte agarose culture. Journal of Cell Science. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 28.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in Agarose Culture Synthesize a Mechanically Functional Extracellular-Matrix. Journal of Orthopaedic Research. 1992;10(6):745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 29.Villanueva I, Klement BJ, von Deutsch D, Bryant SJ. Cross-Linking Density Alters Early Metabolic Activities in Chondrocytes Encapsulated in Poly(Ethylene Glycol) Hydrogels and Cultured in the Rotating Wall Vessel. Biotechnology and Bioengineering. 2009;102(4):1242–1250. doi: 10.1002/bit.22134. [DOI] [PubMed] [Google Scholar]
- 30.Hardingham TE. Proteoglycans and Glycosaminoglycans. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. Elsevier; Burlington: 2006. pp. 85–98. [Google Scholar]
- 31.Hardingham TE. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979;177(1):237–47. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dean MF, Lee YW, Dastjerdi AM, Lees P. The effect of link peptide on proteoglycan synthesis in equine articular cartilage. Biochim Biophys Acta. 2003;1622(3):161–8. doi: 10.1016/s0304-4165(03)00138-7. [DOI] [PubMed] [Google Scholar]
- 33.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. Journal Of Biomedical Materials Research. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 34.Bryant SJ, Anseth KS, Lee DA, Bader DL. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. Journal of Orthopaedic Research. 2004;22(5):1143–1149. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Materials Research. 1998;39(2):266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Nicodemus GD, Bryant SJ. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. Journal of Biomechanics. 2008;41(7):1528–1536. doi: 10.1016/j.jbiomech.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Udenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972;178(63):871–2. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- 38.Nagata H, Kojima R, Sakurai K, Sakai S, Kodera Y, Nishimura H, Inada Y, Matsushima A. Molecular-weight-based hyaluronidase assay using fluorescent hyaluronic acid as a substrate. Analytical Biochemistry. 2004;330(2):356–358. doi: 10.1016/j.ab.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 39.Ogamo A, Matsuzaki K, Uchiyama H, Nagasawa K. Preparation and Properties of Fluorescent Glycosaminoglycuronans Labeled with 5-Aminofluorescein. Carbohydrate Research. 1982;105(1):69–85. doi: 10.1016/s0008-6215(00)81855-8. [DOI] [PubMed] [Google Scholar]
- 40.Farndale RW, Buttle DJ, Barrett aJ. Improved Quantitation and Discrimination of Sulfated Glycosaminoglycans by Use of Dimethylmethylene Blue. Biochimica Et Biophysica Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 41.Villanueva I, Hauschulz DS, Mejic D, Bryant SJ. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis Cartilage. 2008;16(8):909–18. doi: 10.1016/j.joca.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27(30):5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Salinas CN, Anseth KS. Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells. Journal of Biomedical Materials Research Part A. 2009;90A(2):456–464. doi: 10.1002/jbm.a.32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villanueva I, Gladem SK, Kessler J, Bryant SJ. Dynamic loading stimulates chondrocyte biosynthesis when encapsulated in charged hydrogels prepared from poly(ethylene glycol) and chondroitin sulfate. Matrix Biol. 2009 doi: 10.1016/j.matbio.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant SJ, Arthur JA, Anseth KS. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomaterialia. 2005;1(2):243–252. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Nicodemus GD, Bryant SJ. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage. 2010;18(1):126–37. doi: 10.1016/j.joca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Gardiner B, Smith D, Pivonka P, Grodzinsky A, Frank E, Zhang L. Solute transport in cartilage undergoing cyclic deformation. Comput Methods Biomech Biomed Engin. 2007;10(4):265–78. doi: 10.1080/10255840701309163. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Szeri AZ. Transport of neutral solute in articular cartilage: effects of loading and particle size. Proceedings of the Royal Society a-Mathematical Physical and Engineering Sciences. 2005;461(2059):2021–2042. [Google Scholar]
- 49.Gauthier MA, Klok HA. Peptide/protein-polymer conjugates: synthetic strategies and design concepts. Chem Commun (Camb) 2008;(23):2591–611. doi: 10.1039/b719689j. [DOI] [PubMed] [Google Scholar]
- 50.Yang B, Zhang L, Turley EA. Identification of Hyaluronan Binding Motifs in a Novel Hyaluronan Receptor Termed Rhamm. Molecular Biology of the Cell. 1992;3:A73–A73. [Google Scholar]
- 51.Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum. 2000;43(5):1165–74. doi: 10.1002/1529-0131(200005)43:5<1165::AID-ANR27>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120(3):825–34. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 54.Bryant SJ, Nicodemus GD, Villanueva I. Designing 3D photopolymer hydrogels to regulate biomechanical cues and tissue growth for cartilage tissue engineering. Pharmaceutical Research. 2008;25(10):2379–2386. doi: 10.1007/s11095-008-9619-y. [DOI] [PubMed] [Google Scholar]
- 55.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20(1):9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 56.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. Journal Of Biomedical Materials Research Part A. 2003;64A(1):70–79. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 57.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating Chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnology And Bioengineering. 2004;86(7):747–755. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 58.Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. Journal of Biomedical Materials Research Part A. 2003;67A(4):1430–1436. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.