Abstract
Satratoxin G (SG) and other macrocyclic trichothecene mycotoxins are potent inhibitors of eukaryotic translation that are potentially immunosuppressive. The purpose of this research was to test the hypothesis that SG-induced apoptosis in the macrophage correlates with binding of this toxin to the ribosome. Exposure of RAW 264.7 murine macrophages to SG at concentrations of 10 to 80 ng/ml induced DNA fragmentation within 4 h that was indicative of apoptosis. To relate these findings to ribosome binding of SG, RAW cells were exposed to different toxin concentrations for various time intervals, ribosomal fractions isolated by sucrose density gradient ultracentrifugation and resultant fractions analyzed for SG by competitive ELISA. SG was found to specifically interact with 40S and 60S ribosomal subunits as early as 5 min. and that, at high concentrations or extended incubation times, the toxin induced polysome disaggregation. While co-incubation with the simple Type B trichothecene DON had no effect on SG uptake into cell cytoplasm, it inhibited SG binding to the ribosome, suggesting that the two toxins bound to identical sites and that SG binding was reversible. Although both SG and DON induced mobilization of p38 and JNK 1/2 to the ribosome, phosphorylation of ribosomal bound MAPKs occurred only after DON treatment. SG association with the 40S and 60S subunits was also observed in the PC-12 neuronal cell model which is similarly susceptible to apoptosis. To summarize, SG rapidly binds small and large ribosomal subunits in a concentration- and time-dependent manner that was consistent with induction of apoptosis.
Keywords: Trichothecene, ribosome, apoptosis, macrocyclic, Stachybotrys
INTRODUCTION
The trichothecene mycotoxins, toxic sesquiterpenoids produced by molds, are encountered in environment and food and are therefore of public health concern (Pestka 2008; Pestka et al. 2008). While trichothecenes share a common structure of a 12, 13 epoxide ring and a 9, 10 double bond, their toxicity varies widely based on differing R groups (Bamburg 1983). Three major types of trichothecene have been characterized. The most toxic of these are the macrocyclic trichothecenes (eg. satratoxins and roridins) (Pestka and Forsell 1988) which possess a cyclic diester or triester ring at C4 and C5 (Grove 1993) as compared to the less toxic Type A and Type B groups which contain simpler acyl substituents (Grove 1988; Grove 2000).
Satratoxin G (SG) is a macrocyclic trichothecene that is produced by the black mold Stachybotrys chartarum, a fungus that often grows on cellulosic building materials in water-damaged buildings. Both S. chartarum and its mycotoxins have been suggested to contribute to a spectrum of adverse immune, respiratory and nervous system effects often referred to as damp-building related illness (Pestka et al. 2008). SG and other trichothecenes cause activation of the mitogen-activated protein kinases (MAPKs) p38, JNK and ERK as well as apoptosis in mononuclear phagocytes (Yang et al. 2000) which could potentially contribute to immunotoxicity. Recently, intranasal instillation of SG has been found to specifically induce apoptosis in olfactory neuronal cells, supporting a possible role in neurotoxicity within the respiratory tracts (Islam et al. 2006; Islam et al. 2007).
Trichothecenes are widely considered to exert their toxicity by binding to the peptidyl transferase center of the 60S ribosomal subunit (Bamburg 1983; Middlebrook and Leatherman 1989a; Middlebrook and Leatherman 1989b). One outcome of this binding is translational arrest which can be differentially classified as initiation inhibition (I-type), elongation inhibition (E-type) and termination inhibition (T-type) (Iordanov et al. 1997). In I-type, there is a rapid conversion of polysomes into monosomes, whereas polysomes are stable in both the E- and T-type. The inhibition class can vary depending on toxin structure, concentration and exposure time.
The ribosome also appears to play a pivotal role in the orchestration of trichothecene-induced toxic responses ranging from elevated gene expression to apoptosis. For example, induction of proinflammatory gene expression and apoptosis by the Type B trichothecene deoxynivalenol (DON) is mediated by mitogen-activated protein kinases (MAPK) (Pestka 2008). Recently, we have demonstrated that DON induces mobilization of p38, JNK and ERK to the ribosome and their subsequent phosphorylation (Bae and Pestka 2008). Interestingly, these effects correlate with cleavage of 18S and 28S rRNA at the peptidyl transferase center of the 60S ribosomal subunit (Li and Pestka 2008).
Many critical questions remain relative to the interactions of trichothecenes with the ribosome and the ensuing ribotoxic stress response. First, the relationship between ribosomal binding and manifestations of cellular toxicity are not fully understood. Second, despite the potential importance of SG and other macrocyclic trichothecenes as etiologic agents in damp building-related illness, their capacities to interact with the ribosome have not been well-characterized. Third, SG has also been reported to covalently bind to proteins in vitro and in vivo (Yike et al. 2006), raising the possibility that this and other macrocyclic trichothecenes might form adducts with the ribosome. Finally, while some trichothecenes have been shown to bind to the 60S ribosomal subunit under cell-free conditions, their potential to bind to the 40S subunit have not been fully addressed.
The purpose of this research was to test the hypothesis that SG-induced apoptosis in the macrophage correlates with the binding of this toxin to the ribosome. A major limitation to studying interaction of SG with the ribosome is the lack of either commercially available radiolabeled macrocyclic trichothecenes or suitable methods to carry out such labeling. Our laboratory has developed a specific antibody to SG and applied it to a highly sensitive ELISA (Chung et al. 2003a). This assay was used here to characterize the interaction of SG with the ribosome in the macrophage relative to kinetics, concentration-dependence, specificity, interacting subunits, and reversibility; these findings were further related to MAPK activation and apoptosis induction.
MATERIALS AND METHODS
Chemicals
SG (kindly provided by B. Jarvis, University of Maryland, College Park, MD) was purified from S. chartarum cultures as previously described (Hinkley and Jarvis 2001) and identity confirmed by electrospray ionization/collision-induced dissociation tandem mass spectroscopy (Tuomi et al. 1998). Purified SG was diluted in Tris-HCl buffer (pH 7.0) and the relative absorbance scanned over 220 to 340 nM using a NanoDrop spectrophotometer ND-1000 (Thermo Scientific, DE). DON and other chemicals were purchased from Sigma Chemical Co. (St Louis, MO) unless otherwise noted.
Cell cultures
RAW 264.7 cells American Type Culture Collection [ATCC] Manassas, VA) were cultured at 37°C in a humidified 6% CO2 incubator in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 10% (vol/vol) heat-inactivated FBS, 100 U/ml penicillin (Sigma), and 100 ug/ml streptomycin (Sigma) (Zhou et al. 2005a). In a typical experiment, RAW 264.7 cells (5 × 105/ml) were cultured overnight to achieve 80% confluence in 100-mm cell culture dishes (Corning, NY), and then treated with SG at various concentrations and time intervals prior to analysis.
PC-12 neuronal cells (ATCC) were grown to approximately 80% confluency on collagen-coated 6-well plates (BD Biosciences Pharmingen, San Diego, CA) containing 2 ml of F-12K medium (ATCC) supplemented with 2.5% (v/v) fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 15% (v/v) horse serum (Atlanta Biologicals), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco-BRL, Rockville, MD)(Islam et al. 2008).
DNA fragmentation ELISA
DNA fragmentation was measured with a Cell Death Detection ELISA (Boehringer Mannheim GmbH, Germany). Briefly, RAW 264.7 cells (1 × 105/ml) in a 12 well cell culture plate were cultured overnight, and then treated with SG at 10, 20, 40 and 80 ng/ml. After 1, 2, 4 and 6 h, cells were lysed and lysates were incubated in anti-histone-coated microtiter plate wells. Each treatment was performed in duplicate. Wells were washed and incubated with anti-DNA-peroxidase conjugates and unbound peroxidase conjugate removed by washing. Bound peroxidase retained in the immunocomplex was determined spectrophotometrically following addition of 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) (ABTS) substrate. Relative increases in mono- and oligo-nucleosomes in cytoplasm indicative of DNA fragmentation were determined by measuring absorbance at 405 nm on a Vmax Microplate Reader (Molecular Devices, Menlo Park, CA).
Confirmation of DNA fragmentation by agarose gel electrophoresis
RAW 264.7 cells (2 × 105/ml) were incubated overnight in a 6 well cell culture plate, and then cultured with SG for 4 and 6 h. Cultures were centrifuged for 10 min (200 × g) at 4°C and the pellet suspended in 0.1 ml cell lysing buffer (10 mM Tris, pH 7.4, 10 mM EDTA, pH 8.0, 0.5% [v/v] Triton X-100)(Islam et al. 2002). Cells were incubated for 10 min at 4°C and the resultant lysate was centrifuged for 30 min (12,000 × g) at 4°C. The supernatant, which contained fragmented DNA, was digested for 1 h at 37°C with 0.4 μg/ml of RNase A (Roche, Indianapolis, IN) and then incubated 1 h at 37°C with 0.4 μg/ml of proteinase K (Roche, Indianapolis, IN). DNA was precipitated with 50% (v/v) isopropanol in 0.5 M NaCl at 4°C overnight. The precipitate was centrifuged at 12,000 × g for 30 min at 4°C. The resultant pellet was air dried and resuspended in 20 μl of 10 mM Tris (pH 7.4), 1 mM EDTA (pH 8.0). DNA samples (10 μl/well) were analyzed by electrophoresis in 2% (w/v) agarose gel in 90 mM Tris–borate buffer containing 2 mM EDTA (pH 8.0) at 50 V for about 3 h. After electrophoresis, the gel was stained with ethidium bromide (0.5 μg/ml), and the nucleic acids were visualized with a UV transilluminator. A 100-bp DNA ladder (GIBCO-BRL, Rockville, MD) was used for molecular sizing.
Sucrose density gradient fractionation
To prepare cytoplasmic extract for ribosomal fractionation, cells were washed with ice-cold PBS twice and lysed in ice-cold polysome extraction buffer (PEB) (0.3 M NaCl, 15 mM MgCl2, 15 mM Tris-HCl [pH 7.6], 1% (w/v) Triton X-100, 0.1 mg/ml cycloheximide and 1 mg/ml heparin) (Galban et al. 2003). This was centrifuged at 10,000 × g for 15 min to clear the resultant supernatant of nuclei, mitochondria and debris. Protein was measured using a Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA). Lysate protein (1 mg) was layered over 9 ml linear sucrose gradient solution (10-50%) in a 11.5 ml Sorvall centrifuge tube and centrifuged at 35,000 × g for 3 h at 4°C in Sorvall TH-641 rotor. The gradient was fractioned at a rate of 0.25 to 1 ml per min by upward displacement using an ISCO system consisting of a fraction collector, a needle-piercing device and a syringe pump connected to an EM-1 UV monitor for continuous measurement of the absorbance at 254 nm (Teledyne ISCO, Lincoln, NE).
SG ELISA
SG was quantified by a competitive ELISA (Chung et al. 2003a). SG polyclonal antibodies (100 μl) diluted (0.5 μg/ml) in phosphate buffered saline (PBS) (pH 7.2, 10 mM) were incubated in 96-well ELISA plates (Corning) overnight at 4°C. Plates were aspirated, blocked with 300 μl of 3% (w/v) non-fat dried milk in PBS (NFDM-PBS), covered with parafilm and then incubated 60 min at 37°C. After washing four times with PBS containing 0.05 %(v/v) Tween 20 (PBS-Tween), 50 μl of standard or samples with 50 μl SG-horseradish peroxidase conjugate diluted (0.5 μg/ml) in NFDM-PBS at RT for 60 min. Plate was washed seven times with PBS-Tween, bound peroxidase was determined after incubation for 30 min at 25°C with 100 μl/well of K-Blue Substrate (Neogen, Lansing). The reaction was terminated with 100 μl/well of 6 N sulfuric acid stopping reagent and the plate read at 450 nm by Vmax Kinetic Microplate Reader (Molecular Devices, Menlo Park, CA).
Western analysis
Proteins were separated on 4% (w/v) polyacrylamide gels and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). After incubating with blocking buffer (Li-Cor, Lincoln, NE), membranes containing immobilized proteins were incubated with murine antibodies to phosphorylated p38 and JNK concurrently with corresponding rabbit antibodies to nonphosphorylated p38 or JNK (Cell Signaling Technology, Danvers, MA) overnight at 4°C. After washing, blots were incubated with IRDye 680 goat anti-rabbit IgG conjugates and IRDye 800CW goat anti-mouse IgG conjugates (Li-Cor) for 1 hr at 25°C. Infrared fluorescence of bound secondary antibodies containing the two dyes was simultaneously measured using a Li-Cor Odyssey Infrared Imaging System, and relative phosphorylation determined using Odyssey Analysis Software. Ribosomal fractions were identified with mouse and rabbit antibodies to ribosomal protein S6 (RPS6) (Cell Signaling Technology) and ribosomal protein L7 (RPL7) (Bethyl Laboratories, Inc., Montgomery, TX), respectively, followed by IRDye 680 goat anti-species conjugates (Bae and Pestka 2008).
Statistics
Data were analyzed by Student’s t-test or ANOVA with SigmaStat v 3.1 (Jandel Scientific, San Rafael, CA) with the criterion for significance set at p < 0.05.
RESULTS
SG induces apoptosis in RAW 264.7 cells
The effect of SG concentration on apoptosis induction in RAW 264.7 cells was determined by measuring DNA fragmentation with a specific ELISA. SG-induced apoptosis was evident after 2 h at the two highest concentrations (40 and 80 ng/ml) and after 4 h at all concentrations (10-80 ng/ml) (Fig. 1A). Concentration-dependent fragmentation was confirmed by gel electrophoresis (Figure 1B).
Figure 1. SG induces apoptosis in RAW 264.7 cells.

Cells were treated with different SG concentrations for various time intervals and then assessed for DNA fragmentation by (A) cell-death ELISA in duplicate or (B) agarose gel electrophoresis.
SG binding to ribosomal subunits is concentration-dependent in RAW 264.7 cells
To relate apoptosis to ribosomal binding, RAW 264.7 cells were incubated with SG at 0, 20, 40 and 80 ng/ml for 1 h and binding of the toxin to ribosomal fractions was assessed. Since SG absorbs at 254 nm (Fig. 2), its binding to the ribosomes could be qualitatively followed by monitoring changes in absorbance in eluted sucrose density gradient fractions. This procedure enabled separation of the small subunit (40S), large subunit (60S), intact monosome (80S) composed of the small and large subunits and polysomes composed of multiple monosomes. Both the 40S and 60S absorbance values increased as SG in incubation mixture was elevated whereas the polysome level decreased (Fig. 3A). The relative contributions of SG and protein to absorbance at 254 were assessed by ELISA and protein assay, respectively. Concentration-dependent increases in SG binding pin ooled ribosome subunits and monosomes (RS+M) were confirmed by ELISA (Fig. 3B). Likewise, protein in the 40S and 60S fractions increased proportionally with SG whereas the absorbance in the polysome fraction decreased. Taken together, these findings were suggestive of polysome disaggregation. Since SG binding (1 h) to the ribosome appeared to precede apoptosis induction (2-4 h), this early interaction was characterized further relative to kinetics, specificity, reversibility and relation to MAPK activation.
Figure 2. UV absorption scan for SG.

Purified SG was diluted in phosphate buffer (pH 7.0) and absorbance measured over the UV spectrum.
Figure 3. SG binding to ribosomal subunits is concentration-dependent in RAW 264.7 cells.
Cells were treated with SG (0, 20, 40, 80 ng/ml) for 1 h and then lysed with polysome extraction buffer (PEB). (A) Ribosomal fractions were separated on a sucrose gradient and the A254 monitored and small subunit, large subunit, monosome and ploysomes labeled as 40S, 60S, 80S and P, respectively, (B) Ribosomal subunits and monosome (RS+M) fractions were pooled and analyzed for SG by competitive ELISA and for total protein colorimetrically.
SG binding to ribosomal subunits is rapid and saturable
The kinetics of SG binding to the ribosome were assessed in RAW 264.7 cells treated with low (10 ng/ml) and high (100 ng/ml) SG concentrations. As compared to untreated cells, cells treated with 10 ng/ml SG exhibited increases in A254 peaks corresponding with the 40S and 60S subunits within 30 min and these increased over time (Figure 4A-F). ELISA confirmed that SG binding to specific ribosomal fractions increased from 30 to 240 min (Fig. 4C-F). No increases in colorimetrically-determined protein were observed in cells exposed to 10 ng/ml SG as were seen at higher SG concentrations (Fig. 3B).
Figure 4. Kinetics of SG binding to ribosomal in RAW 264.7 cells.
Cells were untreated or treated (A) with water or SG (10 ng/ml) for 15 (B), 30 (C), 60 (D), 120 (E), and 240 (F) min and then lysed with PEB. Ribosomal fractions were separated on a sucrose gradient A254 and monitored. Individual fractions (0.5 ml) were analyzed for SG (open circle) and total protein (vertical bar). Solid arrows indicate increased absorbances with increasing SG concentration. Dotted arrows indicate increased SG is detectable in ribosomal fractions as toxin concentration ins increased.
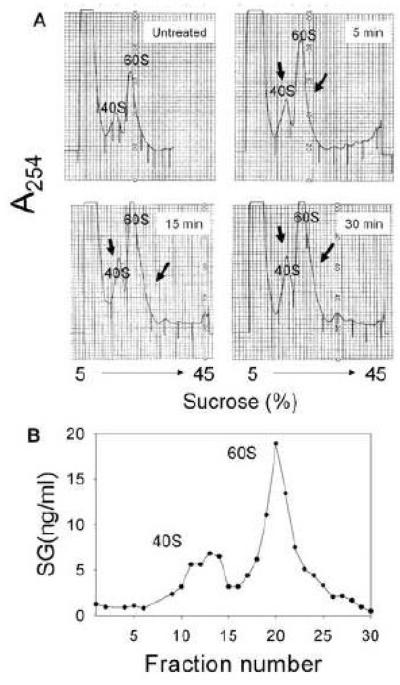
In cells treated with the high SG concentration (100 ng/ml), the toxin was detectable in the 40S and 60S ribosomal fractions as early as 5 min with binding being nearly maximal after 15 min (Fig. 5A). To confirm that SG associated with both subunits, small volume ribosomal fractions (0.25 ml) were collected and analyzed by ELISA to increase resolution of the assay. Increased absorbances in the 40S and 60S peaks closely corresponded with increased SG concentrations in these fractions (Fig. 5B).
Figure 5. High SG concentration induces rapid, saturable binding to 40S and 60S ribosomal subunits in RAW 264.7 cells.
(A)Cells were untreated or treated with SG (100 ng/ml) for 5, 15, 30 min and then lysed with PEB. Ribosomal fractions were separated on a sucrose gradient system and A254 monitored. (B) Cells were treated with SG (100 ng/ml) for 15 min. PEB lysate were separated at high resolution (0.25 ml/fraction) on a sucrose gradient and then fractions analyzed by SG ELISA. Comparable treatment with water vehicle only revealed no detectable SG (data not shown)
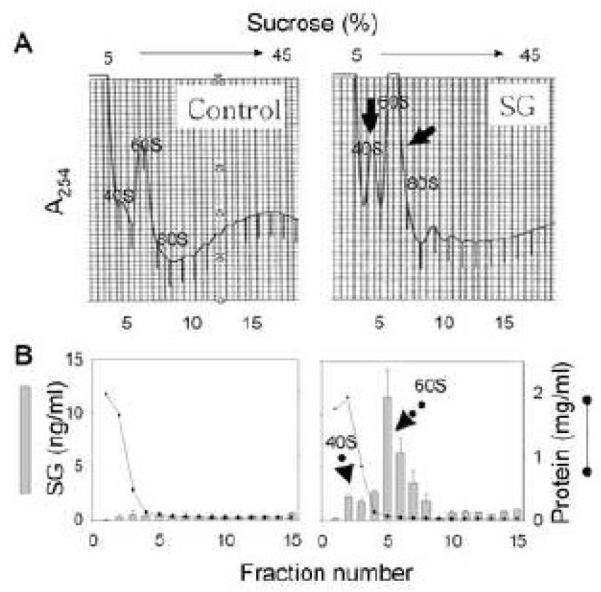
DON blocks SG interaction with the ribosome
Unlike SG, the simpler Type B trichothecene DON does not absorb at 254 nm and does not cross-react in the SG ELISA (Chung et al. 2003a). The capacity of DON to interefere with SG binding to the ribosome fraction was assessed in RAW 264.7 ELISA of the total cell lysate revealed that DON did not alter cytoplasmic SG concentrations (Fig. 6A). As expected, treatment with SG alone increased A254 in 40S and 60S ribosomal subunits (Fig. 6B). However, SG and DON co-treatment generated A254 peaks at 40S and 60S that were comparable to those for the untreated and DON-treated groups. Thus, DON appeared to interfere with SG ribosomal binding, suggesting that the two trichothecenes bind to the same site.
Figure 6. DON competitively inhibits SG binding to 40S and 60S ribosomal subunits in RAW 264.7 cells.
Cells were treated with vehicle, SG (100 ng/ml), DON (500 ng/ml) or both toxins for 15 min and then lysed with PEB. (A) SG content of cell lysate was analyzed by ELISA. (B) Ribosomal fractions were separated on a sucrose gradient and A254 monitored.
SG interaction with the ribosome is reversible
SG has been previously reported to covalently bind to proteins (Yike et al. 2006) raising the possibility that adducts might form in the ribosome. The capacity of DON to compete with bound SG from free ribosomal subunits was evaluated. RAW 264.7 cells were treated with SG (20 ng/ml) for 1 h and resultant cell lysates subjected to sucrose density gradient fractionation. Pooled ribosome subunits and monosomes (RS+M) containing bound SG, were incubated with additional SG (20 ng/ml), DON (500 ng/ml) or both toxins for 1 h. Following these treatments the mixtures were repeatedly washed and concentrated to remove unbound SG and then subjected to SG ELISA. Supplemental SG treatment did not affect SG interaction with the ribosome, whereas DON treatment dissociated bound SG from the ribosome (Fig. 7). These data suggested that SG association with the ribosome was reversible and therefore non-covalent. In addition, SG (20 ng/ml) and DON (500 ng/ml) co-treatment still resulted in maximum SG binding to the ribosome, suggesting that SG interacts more strongly with the ribosome than DON.
Figure 7. SG binding to the ribosome is reversible in RAW 264.7 cells.

Cells were incubated with SG (20 ng/ml) for 60 min and ribosomal fractions were separated on sucrose gradient. Pooled RS+M fractions , all containing bound SG, were incubated with additional SG (20 ng/ml) and/or (DON 500 ng/ml) for 1 h at 37°C. RS+M were repeatedly concentrated and washed to remove free SG and then analyzed by competitive SG ELISA.
SG induces MAPK association with 40S and 60S subunits in RAW 264.7 cells but not phosphorylation
While incubation with DON at 100 ng/ml for 15 min resulted in marked p38 and JNK phosphorylation being detectable in SDS extracts of whole cells, high SG concentrations (100-500 ng/ml) caused only modest kinase phosphorylation (Fig. 8). The effects of SG and DON on MAPK activation and mobilization to the ribosome were assessed in RAW 264.7 cells. DON-induced p38 and JNK phosphorylation was reduced by co-treatment with SG (Fig. 9A). Both SG (100 ng/ml) and DON induced p38 and JNK association with the pooled RS+M fractions (Fig. 9B). Phosphorylated p38 and JNK were detectable in the pooled RS+M fractions from DON-treated cells, but phosphorylation was not observed in these fractions from SG-treated cells. These data suggest that while both DON and SG could promote MAPK mobilization to the ribosome, only DON could induce MAPK phosphorylation at this site.
Figure 8. SG induces modest p38 and JNK phosphorylation in RAW 264.7 cells.

Cells were treated with SG (100-500 ng/ml) or DON (100 ng/ml) for 30 min and then lysed with SDS buffer. Total protein was analyzed by Western blotting with specific antibodies to non-phosphorylated p38 and JNK and their phosphorylated forms.
Figure 9. SG and DON induce MAPK interaction with the ribosome.
Cells were treated with vehicle, SG (100 ng/ml), DON (500 ng/ml) or both toxins for 15 min, and then lysed with PEB. (A) whole cell lysates were analyzed by Western blotting using specific antibodies to phosphorylated p38 and JNK and their non-phosphorylated forms. (B) Lysates were fractionated on sucrose gradient and pooled ribosomal subunits and monosomes (RS+M) analyzed by Western blotting.
SG binds to 40S and 60S ribosomal protein fractions in PC-12 cells
Since SG has been recently shown to induce apoptosis in the PC-12 neuronal model (Islam et al. 2008), its capacity to bind ribosomes in this cell line was therefore also assessed. SG treatment of PC-12 cells increased the absorbance in both 40S and 60S peaks while the polysome profile decreased (Fig. 10). As observed for the RAW 264.7 cells, ELISA confirmed that SG preferentially bound to the fractions containing ribosomal subunits. Thus, SG treatment evoked similar changes in toxin concentration in 40S and 60S ribosomal fractions of PC-12 cells as described above for RAW 264.7 cells.
Figure 10. SG binds to 40S and 60S ribosomal protein fractions in PC-12 neuronal cells.
Cells were treated with water or SG (10 ng/ml) for 60 min and lysed with PEB. Ribosomal fractions were separated on a sucrose gradient system and A254 monitored. Individual fractions were analyzed for SG and protein.
DISCUSSION
Iordanov et al. (1997) proposed that the ribosome can mediate activation of protein kinases after ribotoxic agents bind to or damage 28S rRNA in the 60S ribosomal subunit. Although several studies have investigated for the interaction of trichothecenes with the ribosome (Bamburg 1983; Carter and Cannon 1977; Middlebrook and Leatherman 1989a; Middlebrook and Leatherman 1989b), ribosomal interaction within cultured cells has not been as well-studied. Here we provide evidence for the first time that SG rapidly associated with both the small and large ribosomal subunits prior to the induction of DNA fragmentation, a hallmark of apoptosis.
SG binding to the ribosome was qualitatively evident based on increased A254 and this correlated closely with the ELISA quantitative data. SG was not found in early fractions of the sucrose gradient which contained cytoplasmic proteins, but rather, preferentially bound to the free 40S and 60S ribosomal subunits. The concurrent decreases in polysomes observed after treatment with SG at high concentrations or for prolonged time periods were consistent with previous reports that trichothecenes promote disaggregation of the polysome into 80S or free 40S and 60S subunits (Bamburg 1983). Polysome disassembly likely contributes to translational arrest by inhibiting the elongation process.
Although trichothecenes are known to bind to the 60S ribosomal subunit (Ueno 1985), 40S binding has been heretofore unreported. It was therefore particularly notable that the free 40S ribosomal subunit peak increased after SG treatment in both a concentration- and time-dependent manner. This observation might have biological significance relative to trichothecene effects because dsRNA-activated protein kinase (PKR), a kinase which is capable of driving apoptosis, is primarily localized in free 40S ribosomal subunit (Wu et al., 1998). PKR has been associated with trichothecene-induced MAPK activation and apoptosis in RAW 264.7 (Gray et al. 2008; Zhou et al. 2003b) and PC-12 (Islam et al. 2008) cells. The possibility exists that SG binding to 40S might mediate PKR activation. Relatedly, we recently observed that DON induces migration of p38, JNK and ERK to the 40S ribosomal subunit as well as their phosphorylation (Bae and Pestka 2008). It is possible that the 40S ribosomal subunit might act in concert with PKR as both a sensor and signal transducer for trichothecene-induced toxicity.
The observation that DON competed for SG binding provides additional insight into the mechanisms of this Type B trichothecene. Produced by Fusarium graminearum and commonly found in cereal-based food, DON has been intensively studied relative to its toxicity in vitro and in vivo. The toxin has both immunostimulatory and immunosuppressive effects, depending on dose (Pestka 2008). Regarding the former, DON is rapidly taken up in mononuclear phagocytes where it induces immediate and robust MAPK activation (Bae and Pestka 2008). These kinases, notably p38, drive proinflammatory gene expression, mRNA stability and apoptosis (Chung et al. 2003b; Moon and Pestka 2002; Zhou et al. 2003a; Zhou et al. 2003b; Zhou et al. 2005b).
The observation that DON did not competitively inhibit SG uptake into cells suggests that both SG and DON might freely diffuse into the cell without requiring membrane receptors. Our finding that DON inhibited SG interaction with 40S and 60S ribosomal subunits indicates that DON binds to the same sites as SG. These data further correlate with our recent reports that DON-induced MAPK interaction is mediated through both the 40S and 60S ribosomal subunits (Bae and Pestka 2008).
Concentrations of 10 ng/ml SG induce apoptosis and inhibit over 90% of translation, whereas similar effects require 50 to 100 times more DON (Yang et al. 2000) confirming that this macrocyclic trichothecene is much more cytotoxic than the simpler Type B trichothecene. A critical question that arises relates to what is responsible for the different toxicities of SG and DON. Ueno et al. (1968) first proposed that different binding affinities might affect toxicity among trichothecenes. It was notable that while treatment of the SG-saturated ribosomes with DON alone under cell-free conditions resulted in a reduction of SG binding, co-treatment with both toxins was insufficient to reduce levels of SG bound to the ribosomes. DON was not detectable in ribosomes by a DON-specific ELISA regardless of the concentration employed (data not shown), however, SG association with the ribosome was quite stable during the fractionation. The lower potency of DON for apoptosis induction compared to SG might relate to DON’s lower affinity for the ribosome. A limitation of this interpretation is that ELISAs are dependent on the relative affinity of the SG and DON antibodies employed. It will be desirable in future studies to employ radiolabeled trichothecenes to investigate the relative binding affinities.
Differences in relative toxicity between SG and DON might also result from differential MAPK activation. Importantly, we have observed that DON-induced p38 activation mediates apoptosis in RAW 264.7 cells, whereas SG-induced apoptosis is p38-independent in mononuclear phagocytes (Yang et al. 2000) and PC-12 cultures (Islam et al., 2008). SG did not appear to be as strong of an inducer of MAPK activation as DON. Even though SG induced p38 and JNK migration to the RS+M fraction in this study, subsequent, robust p38 and JNK phosphorylation was not evident suggesting that binding to the ribosome per se is not sufficient to evoke MAPK activation. DON-induced MAPK phosphorylation was inhibited by SG co-treatment. The observation that DON but not SG induced MAPK phosphorylation following ribosomal interaction with these kinases might explain the different toxic effects of these two trichothecenes.
It should be noted that Nsuetrong et al. (2005) reported that satratoxin H (SH) induces apoptosis in PC-12 cells in a p38-depedent manner. A fundamental difference between this work and our studies was that the PC-12 cells were subjected to 24 h serum deprivation prior to inhibitor and SH treatment, whereas our studies do not employ serum deprivation. The latter is known to directly mediate apoptosis in many cell types and might be a complicating factor when trying to determine mechanisms of trichothecene cytotoxicity.
The capacity of SG and other trichothecenes to induce apoptotic cell death in mononuclear phagocytes can potentially contribute to aberrant immune function. In addition to potential immunotoxic effects, these compounds are also selectively neurotoxic. Islam et al (2006, 2007) demonstrated that intranasal instillation of SG and other macrocyclic trichothecenes specifically induces apoptosis in olfactory sensory neurons in the nose and brain. More recently, PC-12 neuronal cells were used to investigate mechanisms of SG-induced death in neurons (Islam et al., 2008). Exposure to SG at 10 ng/ml or higher for 48 h was found to induce DNA fragmentation characteristic of apoptosis in PC-12 cells. SG-induced apoptosis was confirmed by microscopic morphology, hypodiploid fluorescence and annexin V-FITC uptake. Furthermore, mRNA expression of the proapoptotic genes p53, double stranded RNA-activated protein kinase (PKR), BAX and caspase-activated DNAse (CAD) was significantly elevated from 6 to 48 h after SG treatment. The results presented here suggest, as observed for RAW 264.7, that SG binding to the 40S and 60S subunits in PC-2 cells would be likely to occur prior to the onset of apoptosis.
Taken together, the results presented herein strongly suggest that SG binding to intracellular ribosomes proceeds rapidly, is both concentration- and time-dependent, and precedes apoptosis. In future studies it will be important to determine the specific ribosomal binding sites for SG and other trichothecenes as well as identify the exact linkages between the ribosome and intracellular kinase signaling. It will be further critical to understand how cells respond to different trichothecenes with anti-apoptotic or pro-apoptotic mechanisms. Finally, these in vitro mechanisms must be applied to understanding the pathophysiologic effects of SG and other trichothecenes in vivo.
ACKNOWLEDGMENTS
This study was supported by Public Health Service Grants ES03553 and DK058833 from the National Institutes for Health. We thank Dara Phillips, Grace Tung and Mary Rosner for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Bae HK, Pestka JJ. Deoxynivalenol induces p38 interaction with the ribosome in Monocytes and Macrophages. Toxicol. Sci. 2008;105:59–66. doi: 10.1093/toxsci/kfn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR. Biological and biochemical actions of trichothecene mycotoxins. Prog. Mol. Subcell. Biol. 1983;8:41–110. [Google Scholar]
- Carter CJ, Cannon M. Structural requirements for the inhibitory action of 12,13-epoxytrichothecenes on protein synthesis in eukaryotes. Biochem. J. 1977;166:399–409. doi: 10.1042/bj1660399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YJ, Jarvis BB, Tak H, Pestka JJ. Immunochemical assay for satratoxin G and other macrocyclic trichothecenes associated with indoor air contamination by Stachybotrys chartarum. Toxicol. Mech. Meth. 2003a;13:247–252. doi: 10.1080/713857196. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Zhou HR, Pestka JJ. Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-alpha expression by deoxynivalenol (vomitoxin) Toxicol. Appl. Pharmacol. 2003b;193:188–201. doi: 10.1016/s0041-008x(03)00299-0. [DOI] [PubMed] [Google Scholar]
- Galban S, Martindale JL, Mazan-Mamczarz K, Lopez DS, I, Fan J, Wang W, Decker J, Gorospe M. Influence of the RNA-binding protein HuR in pVHL-regulated p53 expression in renal carcinoma cells. Mol. Cell Biol. 2003;23:7083–7095. doi: 10.1128/MCB.23.20.7083-7095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, Bae H-K, Li JCB, Lau AS, Pestka JJ. Double-stranded RNA-activated proteiin kinase (PKR) mediates induction of IL-8 expression by deoxynivalenol, Shiga toxin 1 and ricin in monocytes. Toxicol. Sci. 2008;105:322–330. doi: 10.1093/toxsci/kfn128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove JF. Non-macrocyclic trichothecenes. Part 2. Prog. Chem. Org. Nat. Prod. 2000;69:1–70. [Google Scholar]
- Grove JF. Macrocyclic trichothecenes. Nat. Prod. Rep. 1993;10:429–448. doi: 10.1039/np9880500187. [DOI] [PubMed] [Google Scholar]
- Grove JF. Non-macrocyclic trichothecenes. Nat. Prod. Rep. 1988;5:187–209. doi: 10.1039/np9880500187. [DOI] [PubMed] [Google Scholar]
- Hinkley SF, Jarvis BB. Chromatographic method for Stachybotrys toxins. Methods Mol. Biol. 2001;157:173–194. [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Hegg CC, Pestka JJ. Satratoxin G-induced apoptosis in PC-12 neuronal cells is mediated by PKR and caspase-independent. Toxicol. Sci. 2008;105:142–152. doi: 10.1093/toxsci/kfn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Amuzie CJ, Harkema JR, Pestka JJ. Neurotoxicity and inflammation in the nasal airways of mice exposed to the macrocyclic trichothecene mycotoxin roridin A: Kinetics and potentiation by bacterial lipopolysaccharide co-exposure. Toxicol. Sci. 2007;98:526–541. doi: 10.1093/toxsci/kfm102. [DOI] [PubMed] [Google Scholar]
- Islam Z, Harkema JR, Pestka JJ. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ. Health Perspect. 2006;114:1099–1107. doi: 10.1289/ehp.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Moon YS, Zhou HR, King LE, Fraker PJ, Pestka JJ. Endotoxin potentiation of trichothecene-induced lymphocyte apoptosis is mediated by up-regulation of glucocorticoids. Toxicol. Appl. Pharmacol. 2002;180:43–55. doi: 10.1006/taap.2002.9374. [DOI] [PubMed] [Google Scholar]
- Li M, Pestka JJ. Comparative induction of 28S ribosomal RNA cleavage by ricin and the trichothecenes deoxynivalenol and T-2 toxin in the macrophage. Toxicol. Sci. 2008;105:67–78. doi: 10.1093/toxsci/kfn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook JL, Leatherman DL. Binding of T-2 toxin to eukaryotic cell ribosomes. Biochem. Pharmacol. 1989a;38:3103–3110. doi: 10.1016/0006-2952(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Middlebrook JL, Leatherman DL. Differential association of T-2 and T-2 tetraol with mammalian cells. J. Pharmacol. Exp. Ther. 1989b;250:860–866. [PubMed] [Google Scholar]
- Moon Y, Pestka JJ. Vomitoxin-induced cyclooxygenase-2 gene expression in macrophages mediated by activation of ERK and p38 but not JNK mitogen-activated protein kinases. Toxicol. Sci. 2002;69:373–382. doi: 10.1093/toxsci/69.2.373. [DOI] [PubMed] [Google Scholar]
- Nusuetrong P, Yoshida M, Tanitsu MA, Kikuchi H, Mizugaki M, Shimazu K, Pengsuparp T, Meksuriyen D, Oshima Y, Nakahata N. Involvement of reactive oxygen species and stress-activated MAPKs in satratoxin H-induced apoptosis. Eur. J Pharmacol. 2005;507:239–246. doi: 10.1016/j.ejphar.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. 2008;24:1–13. doi: 10.1080/02652030802056626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Yike I, Dearborn DG, Ward MD, Harkema JR. Stachybotrys chartarum, trichothecene mycotoxins, and damp building-related illness: new insights into a public health enigma. Toxicol. Sci. 2008;104:4–26. doi: 10.1093/toxsci/kfm284. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Forsell JH. Inhibition of human lymphocyte transformation by the macrocyclic trichothecenes roridin A and verrucarin A. Toxicol. Lett. 1988;41:215–222. doi: 10.1016/0378-4274(88)90057-4. [DOI] [PubMed] [Google Scholar]
- Tuomi T, Saarinen L, Reijula K. Detection of polar and macrocyclic trichothecene mycotoxins from indoor environments. Analyst. 1998;123:1835–1841. doi: 10.1039/a803813i. [DOI] [PubMed] [Google Scholar]
- Ueno Y. The toxicology of mycotoxins. Crit. Rev. Toxicol. 1985;14:99–132. doi: 10.3109/10408448509089851. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Hosoya M, Morita Y, Ueno I, Tatsuno T. Inhibition of the protein synthesis in rabbit reticulocyte by Nivalenol, a toxic principle isolated from Fusarium nivale-growing rice. J. Biochem. 1968;64:479–485. doi: 10.1093/oxfordjournals.jbchem.a128919. [DOI] [PubMed] [Google Scholar]
- Wu S, Kumar KU, Kaufman RJ. Identification and requirement of three ribosome binding domains in dsRNA-dependent protein kinase (PKR) Biochemistry. 1998;37:13816–13826. doi: 10.1021/bi981472h. [DOI] [PubMed] [Google Scholar]
- Yang GH, Jarvis BB, Chung YJ, Pestka JJ. Apoptosis induction by the satratoxins and other trichothecene mycotoxins: relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000;164:149–160. doi: 10.1006/taap.1999.8888. [DOI] [PubMed] [Google Scholar]
- Yike I, Distler AM, Ziady AG, Dearborn DG. Mycotoxin adducts on human serum albumin: biomarkers of exposure to Stachybotrys chartarum. Environ. Health Perspect. 2006;114:1221–1226. doi: 10.1289/ehp.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol. Sci. 2005a;87:113–122. doi: 10.1093/toxsci/kfi234. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Jia Q, Pestka JJ. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol. Sci. 2005b;85:916–926. doi: 10.1093/toxsci/kfi146. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. 2003a;72:130–142. doi: 10.1093/toxsci/kfg006. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Lau AS, Pestka JJ. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol. Sci. 2003b;74:335–344. doi: 10.1093/toxsci/kfg148. [DOI] [PubMed] [Google Scholar]