Abstract
Proper regulation of nuclear factor-κB (NF-κB) transcriptional activity is required for normal lymphocyte function, and deregulated NF-κB signaling can facilitate lymphomagenesis. We demonstrate that the API2-MALT1 fusion oncoprotein created by the recurrent t(11;18)(q21;q21) in mucosa-associated lymphoid tissue (MALT) lymphoma induces proteolytic cleavage of NF-κB inducing kinase (NIK) at Arg325. NIK cleavage requires the concerted actions of both fusion partners and generates a C-terminal NIK fragment that retains kinase activity and is resistant to proteasomal degradation. The resulting deregulated NIK activity is associated with constitutive noncanonical NF-κB signaling, enhanced B-cell adhesion, and apoptosis resistance. Our study reveals the gain-of-function proteolytic activity of a fusion oncoprotein and highlights the importance of the noncanonical NF-κB pathway in B-lymphoproliferative disease.
MALT lymphoma, the most common extranodal B-cell tumor, accounts for 8% of Non-Hodgkin’s lymphomas (1). The API2-MALT1 fusion oncoprotein present in t(11;18)-positive MALT lymphomas is composed of the N-terminus of API2 (also termed cIAP2) linked to the C-terminus of MALT1 (1). Wild-type MALT1 mediates antigen-induced NF-κB stimulation, which leads to lymphocyte survival and proliferation (2). MALT1 activates canonical NF-κB signaling following auto-oligomerization induced by upstream factors CARMA1 and Bcl10 (3, 4). It is thought that because the API2 moiety mediates auto-oligomerization, API2-MALT1 can stimulate NF-κB independent of upstream signals (5, 6). This may explain why t(11;18)-positive MALT lymphomas are not dependent on antigenic stimulation for progression, whereas t(11;18)-negative tumors require ongoing chronic inflammation for survival. The phenomenon is best exemplified by gastric MALT lymphomas, the majority of which arise in the setting of chronic H. pylori gastritis and are cured by eradication of H. pylori with antibiotics. In contrast, t(11;18)-positive gastric tumors are resistant to this treatment and are associated with advanced-stage disease (1).
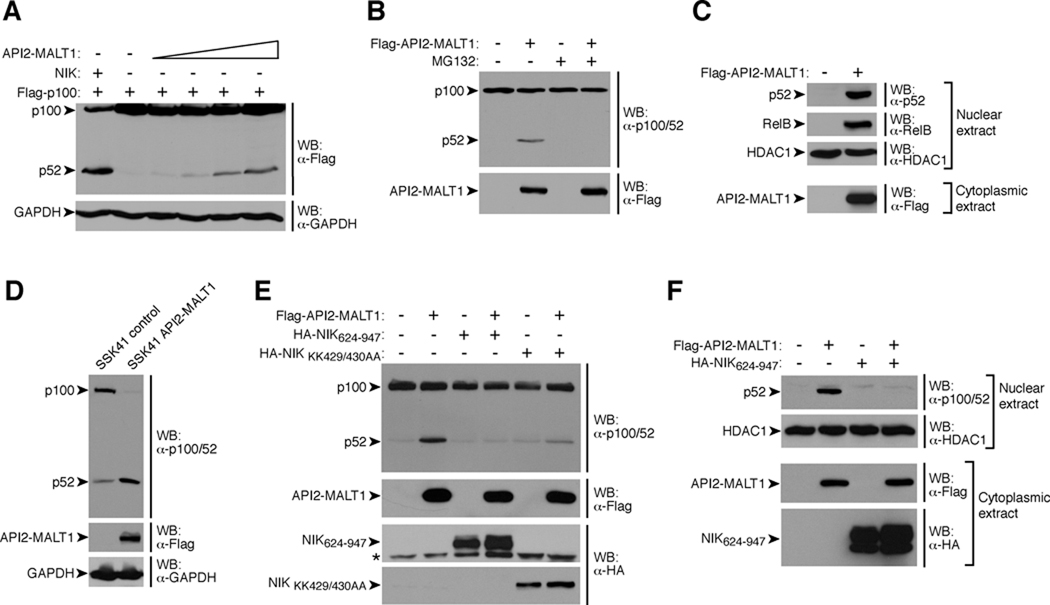
We discovered that besides activating canonical NF-κB, expression of API2-MALT1 in HEK293T or SSK41 B-lymphoma cells induced proteasome-dependent processing of the NF-κB precursor, p100, to its mature form, p52, and stimulated nuclear translocation of p52/RelB (Fig. 1, A–D and fig. S1). These findings indicate that API2-MALT1 activates the “noncanonical” NF-κB pathway, which requires NIK-dependent phosphorylation and activation of IκB kinase-α (IKKα). This in turn triggers proteasome-mediated partial degradation of p100 to p52 and generation of transcriptionally active p52/RelB NF-κB dimers (7). Consistent with this notion, dominant negative NIK mutants (8, 9) blocked API2-MALT1-dependent p100 processing and p52 nuclear translocation (Fig. 1, E and F).
Fig. 1.
API2-MALT1 induces noncanonical NF-κB signaling through NIK. (A and B) HEK293T cells were transfected as indicated, and p100 processing to p52 was assessed by Western blot (WB) with α-Flag (A) or α-p100/52 (to detect endogenous p100/52) (B). Where indicated, cells were treated with proteasome inhibitor, MG132. (C) After transfection of HEK293T cells, nuclear extracts were prepared and analyzed for p52 and RelB by WB. (D) p100 processing in lysates from control SSK41 cells or SSK41 cells stably expressing API2-MALT1 was analyzed by WB. (E and F) HEK293T cells were transfected with API2-MALT1 in the absence or presence of NIK mutants. Endogenous p100 processing was analyzed by WB (E), or nuclear extracts were analyzed for the presence of p52 (F). * = nonspecific band, HDAC1 = histone deacetylase 1 (loading control for nuclear extract). Data are representative of at least three separate experiments.
cIAP1 and cIAP2 (API2) associate with NIK and promote NIK degradation via RING domain ubiquitin ligase activity (10–12). We hypothesized that API2-MALT1, which lacks the cIAP2 RING domain, stimulates noncanonical signaling through competitive inhibition of cIAP-mediated NIK degradation. In testing this, we discovered that expression of API2-MALT1 instead induced proteolytic cleavage of NIK, generating ~37 kD N-terminal and ~70 kD C-terminal NIK fragments (Fig. 2, A and B and fig. S2).
Fig. 2.
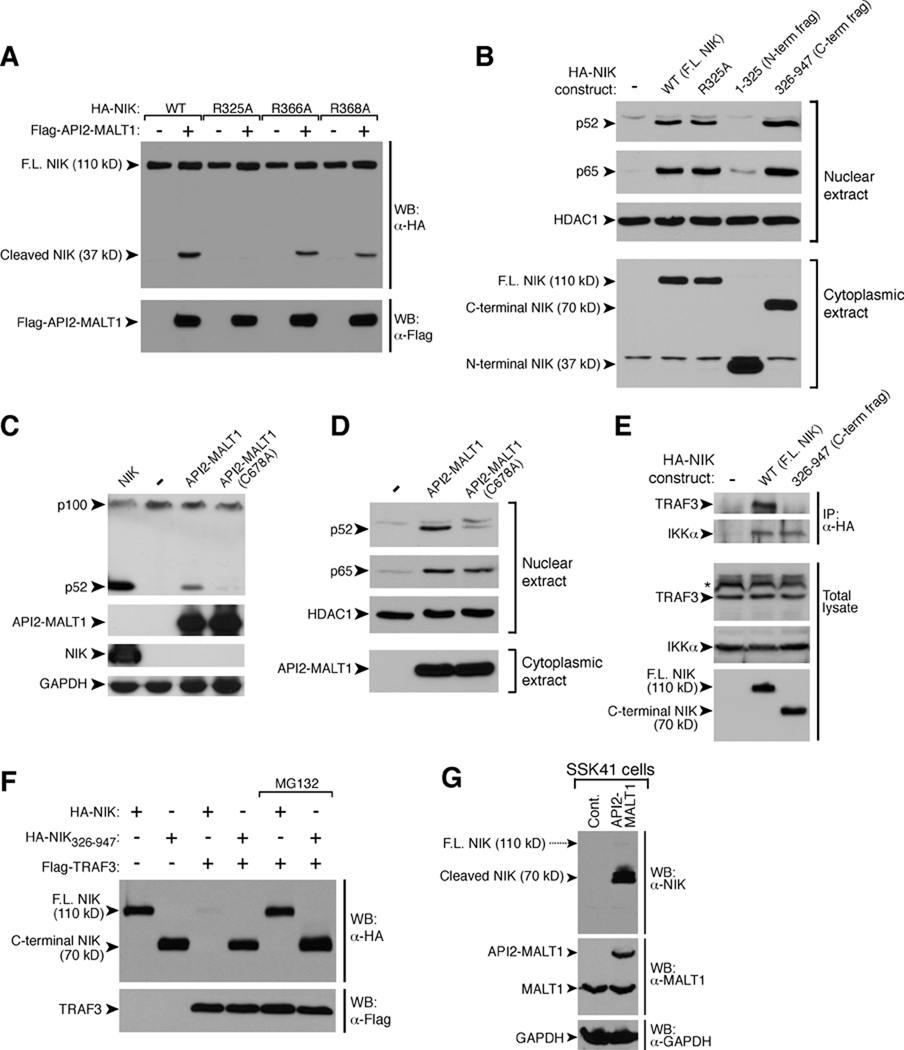
API2-MALT1 induces NIK cleavage, a phenomenon requiring both the API2 moiety and MALT1 protease activity. (A) HEK293T cells were transfected as indicated and NIK cleavage fragments were detected by WB. (B) BJAB cells expressing API2-MALT1 from a tetracycline inducible promoter were treated with doxycycline. WB with an antibody raised against a C-terminal NIK sequence revealed time-dependent generation of an endogenous 70 kD NIK cleavage fragment. To enhance detection of full-length (FL) NIK, cells were incubated with 25 µM MG132. (C) HEK293T cells were transfected as indicated and the presence of the N-terminal 37 kD and the C-terminal 70 kD NIK cleavage fragments was analyzed by WB. (D) Recombinant purified NIK-V5-bioC and StrepII-flag-tagged MALT1 were incubated in kosmotropic salt buffer for 6 h at 37°C, with or without 100µM MALT1 protease inhibitor, Ac-LSSR-CHO, and analyzed by WB. (E) HEK293Tcells were transfected as indicated and the 37 kD NIK cleavage fragment was detected by WB. (F) HEK293T cells were transfected, and immunoprecipitations were carried out using α-Flag-agarose. For a detailed description of API2-MALT1 mutants, see the legend to fig. S5. * = nonspecific band. Data are representative of at least three separate experiments.
API2-MALT1 fusion transcripts invariably contain three intact baculoviral IAP repeat (BIR) domains from API2 and an intact “caspase-like” domain from MALT1, suggesting that these domains are critical for lymphomagenesis (1). The caspase-like domain of wild-type MALT1 possesses proteolytic activity, and Bcl10 and the NF-κB inhibitor, A20, are the only known substrates (13, 14). We therefore investigated whether the caspase-like domain within API2-MALT1 is also able to cleave NIK. Deletion mutants of API2-MALT1 lacking portions of the caspase-like domain and API2-MALT1-C678A, in which the catalytic cysteine within the MALT1 proteolytic domain is replaced with alanine, were unable to induce NIK cleavage (Fig. 2C and fig. S3, A–C). Furthermore, treatment with z-VRPR-fmk, a MALT1 protease inhibitor (14), blocked API2-MALT1-induced NIK cleavage, whereas z-IETD-fmk, a caspase-8 inhibitor, had no effect (fig. S3, D and E). Finally, an in vitro cleavage reaction using purified recombinant proteins showed that NIK is a direct substrate of the MALT1 protease domain (Fig. 2D and fig. S4).
Cellular expression of the MALT1 moiety alone was unable to induce NIK cleavage, suggesting that the API2 moiety also contributes in some way (Fig. 2E). Indeed, analyses revealed that NIK physically associates with API2-MALT1 via the API2 moiety (Fig. 2F), and that the region within the API2 moiety that mediates auto-oligomerization of API2-MALT1 (AA 49–98) (5) is required for efficient API2-MALT1-dependent NIK cleavage and p100 processing (Fig. 2F and fig. S5). The collaborative relationship of the API2 and MALT1 moieties in achieving NIK cleavage was further underscored in several different contexts. First, unlike API2-MALT1, induced expression of wild-type MALT1 in BJAB B-cells did not result in NIK cleavage (fig. S6A). Second, NIK cleavage was not observed in SSK41 B-cells, which are characterized by MALT1 gene amplification, overexpression of MALT1 and constitutive MALT1 protease activity (13, 15, 16). In contrast to A20, NIK was cleaved only if SSK41 cells were engineered to express API2-MALT1 (fig. S6B). Third, co-expression of wild-type MALT1 with Bcl10, which triggers MALT1 oligomerization and activation (3), did lead to cleavage of A20 but not of NIK (fig. S6, C and D). Fourth, ligand induced B-cell receptor stimulation, which similarly activates the MALT1 protease (13), did not trigger NIK cleavage (fig. S6E). Furthermore, although MALT1 oligomerization and activation requires Bcl10 (3), API2-MALT1-mediated NIK cleavage occurred in the absence of Bcl10 (fig. S6F). Together, these findings suggest that NIK is a substrate for the MALT1 protease domain, but only when this domain is present within the context of API2-MALT1.
Structural analyses predict that the MALT1 protease should show specificity for substrates with a basic or uncharged residue at P1 (N-terminal to the cleavage site) (17). Furthermore, the MALT1 cleavage sites of Bcl10 and A20 both contain a P2-serine preceding a P1-arginine (13, 14, 18). Thus, we identified candidate P2-Ser/P1-Arg MALT1 cleavage sites within NIK that would generate fragments of ~37 and 70 kD (fig. S7A), and we individually changed each candidate P1-Arg(R) to Ala(A). The R366A and R368A NIK mutants were readily cleaved by API2-MALT1; however, the R325A mutant was resistant (Fig. 3A), suggesting that API2-MALT1-dependent cleavage of NIK occurs at R325 (fig. S7B). Expression of the resulting C-terminal NIK cleavage fragment, NIK(326–947), which retains the kinase domain, induced robust p100 processing (fig. S7C), p52 nuclear translocation (Fig. 3B), and noncanonical NF-κB target gene expression (fig. S7D). NIK(326–947) also induced nuclear translocation of the p65 NF-κB subunit, indicating activation of the canonical NF-κB pathway as well (Fig. 3B). Conversely, API2-MALT1-C678A, the catalytically inactive mutant that cannot induce NIK cleavage to produce NIK(326–947), failed to stimulate p100 processing (Fig. 3C) and p52 nuclear translocation (Fig. 3D and fig. S8).
Fig. 3.
API2-MALT1-dependent cleavage of NIK at R325 generates an active C-terminal fragment. (A) HEK293T cells were transfected as indicated, and the 37 kD N-terminal NIK cleavage fragment was detected by WB. (B) HEK293T cells were transfected as indicated and nuclear translocation of p52 and p65 NF-κB subunits was assessed. (C and D) HEK293T cells were transfected as indicated, and endogenous p100 processing (C) and nuclear translocation of NF-κB subunits (D) were assessed. (E) HEK293T cells were transfected as indicated, and the ability of endogenous TRAF3 or IKKα to co-immunoprecitate with each NIK protein was assessed. (F) HEK293T cells were transfected as indicated and then incubated in the absence or presence of 25 µM MG132. The presence of NIK was detected by WB. (G) Cell lysates were prepared in the absence of MG132 and analyzed by WB to detect full length NIK and the 70 kD C-terminal NIK cleavage fragment. Data are representative of at least three separate experiments.
NIK associates with the adaptor protein, TRAF3, via an N-terminal NIK domain (AAs 78–84), and this interaction targets NIK for proteasomal degradation (19–21). Because cleavage of NIK at R325 separates this TRAF3-binding site from the NIK kinase domain, we hypothesized that the active C-terminal NIK cleavage product would be resistant to TRAF3-directed degradation. Indeed, NIK(326–947) retained binding to IKKα but not to TRAF3 (Fig. 3E) and, unlike full-length NIK, was resistant to TRAF3-dependent proteasomal degradation (Fig. 3F). We also demonstrated the unique stability of the API2-MALT1-generated C-terminal NIK cleavage fragment in SSK41 B-lymphoma cells. In the absence of MG132, expression of full-length NIK was very low regardless of whether API2-MALT1 was present, consistent with the fact that NIK is subject to constitutive proteasomal degradation (19, 22) (Fig. 3G). In contrast, high amounts of endogenous C-terminal NIK cleavage fragment were detected in SSK41 cells expressing API2-MALT1 (Fig. 3G).
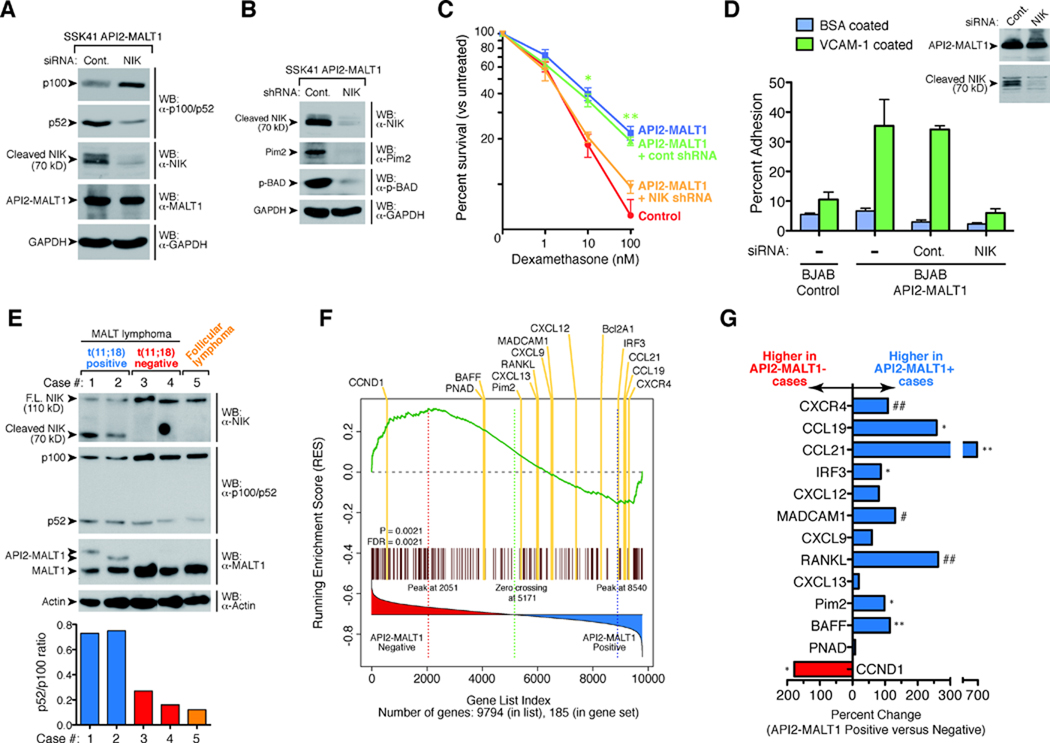
API2-MALT1-dependent generation of the C-terminal NIK cleavage fragment in SSK41 cells was associated with enhanced transcription of noncanonical NF-κB target genes, including Pim-2, an oncogenic kinase that blocks apoptosis by phosphorylating the pro-apoptotic Bcl-2 family member, BAD (figs. S9, A and B) (23–27). RNAi-mediated knock-down of NIK in API2-MALT1 expressing SSK41 cells led to loss of the 70 kD NIK fragment, loss of p100 processing, and loss of API2-MALT1-dependent induction of Pim-2 kinase and BAD phosphorylation (Fig. 4, A and B). In accordance with its impact on anti-apoptotic signal transduction, API2-MALT1 expression protected SSK41 cells from dexamethasone-induced cell death, which was reversed by NIK knock-down (Fig. 4, B and C and fig. S10). Knockdown of IKKα impaired API2-MALT1-dependent protection, supporting a role for the noncanonical NF-κB pathway in mediating this effect of API2-MALT1-induced NIK cleavage (Fig. S11). IKKβ knockdown impaired API2-MALT1-dependent protection as well, implying that the canonical pathway may also contribute (Fig. S11).
Fig. 4.
API2-MALT1-dependent NIK cleavage is associated with upregulation of noncanonical NF-κB target genes, results in an altered B-cell phenotype, and occurs in t(11;18)-positive MALT lymphoma. (A) API2-MALT1 expressing SSK41 cells were transiently transfected with control or NIK siRNA, and p100 processing was assessed by WB. (B and C) API2-MALT1 expressing SSK41 cells were stably infected with control or NIK shRNA lentiviral particles. The 70 kD NIK cleavage fragment, Pim-2 and phospho-Ser112-BAD levels were compared by WB (B). Cells were treated for 48 hours with dexamethasone and percent viability was compared (C). Data are expressed as average +/− SEM for four separate experiments. *p<0.005, **p<0.0001. (D) API2-MALT1 expressing BJAB cells were transfected with control or NIK siRNA, and adhesion to VCAM-1-coated plates was assessed. Data are representative of three separate experiments. (E) Expression of API2-MALT1 in two t(11;18)-positive MALT lymphoma samples was confirmed by WB. p52/p100 ratios were quantified using densitometry. Both t(11;18)-positive cases had the same breakpoint in the API2 gene (between exons 7 and 8), but different breakpoints in the MALT1 gene [between exons 4 and 5 (case 1), and exons 6 and 7 (case 2)]. A Follicular lymphoma and two t(11;18)-negative MALT lymphoma tumor specimens were used as controls. (F) GSEA of NF-κB target genes was performed in a set of MALT lymphomas with t(11;18) vs. without translocation from Collection #1 (34). The distribution of the genes are listed according to rank position, and known noncanonical NF-κB gene targets are highlighted in yellow. Absolute enrichment gave p=0.0021 and FDR=0.0021. (G) Comparison of noncanonical target gene expression in t(11;18)-positive vs. negative cases from Collection #2 (34). Statistical testing for genes differentially expressed between the two types of MALT lymphomas was done by t-test. #p<0.05, ##p<0.01, *p < 0.005, **p < 0.001.
We next investigated the impact of API2-MALT1-dependent NIK cleavage on B-cell adhesion because we had observed that API2-MALT1 induced the expression of B-cell integrins (fig. S9A), known noncanonical NF-κB gene targets (25, 26). API2-MALT1 expression was associated with increased B-cell adhesion to plates coated with the endothelial protein, VCAM-1, and this pro-adhesive phenotype was fully dependent on NIK (Fig. 4D). Lymphocyte adhesion is thought to play role in lymphoma dissemination, thus NIK-cleavage-dependent API2-MALT1-induced adhesion may contribute to the higher rate of tumor spread among t(11;18)-positive MALT lymphomas (1). Again, knockdown of IKKα or IKKβ impaired API2-MALT1-dependent adhesion, suggesting that both noncanonical and canonical pathways contribute to the proadhesive phenotype following API2-MALT1-dependent NIK cleavage (fig. S12).
The striking pattern of NIK cleavage and stability observed in API2-MALT1-expressing B-lymphoma cell lines was recapitulated in MALT lymphoma patient specimens. Full-length NIK levels were relatively low in all lymphomas, whereas an endogenous 70 kD C-terminal NIK fragment was detected only in t(11;18)-positive MALT lymphomas expressing API2-MALT1 (Fig. 4E). The presence of the NIK cleavage product was associated with an elevated p52/p100 ratio in the t(11;18)-positive tumors, indicating enhanced noncanonical NF-κB activation (Fig. 4E). We performed absolute gene set enrichment analysis (GSEA) comparing the expression of NF-κB target genes between t(11;18)-positive MALT lymphomas (n=9) vs. those with no translocation (n=8). Analysis revealed a significant difference in the pattern of expression, with most known noncanonical NF-κB target genes over-represented in the t(11;18)-positive cases (Fig. 4F and fig. S13, tables S1 and S2). One exception is cyclin D1, although its categorization as a noncanonical NF-κB target gene is controversial (28). We then compared the expression of these noncanonical target genes in a completely separate group of six t(11;18)-positive and eight t(11;18)-negative MALT lymphomas that were collected at a different institution, and again found that many noncanonical NF-κB target genes were more highly expressed in the t(11;18)-positive tumors (Fig. 4G). CXCR4, which encodes a chemokine receptor whose expression is associated with widespread lymph node involvement in B-lymphomas (29–31), is one intriguing example of a noncanonical gene target that is upregulated in t(11;18)-positive tumors in both tumor collections (Fig. 4F and G).
Deregulated NIK activity has been increasingly implicated in the pathogenesis of B-cell neoplasms. For example, an EFTUD2-NIK fusion oncoprotein that retains the NIK kinase domain but lacks the TRAF3 binding site, and is resistant to proteasomal degradation, was recently identified in a case of multiple myeloma (22). Another recent report described a murine model of B-lymphoproliferative disease in which a NIK mutant lacking the TRAF3 binding domain (NIKΔT3) was expressed in B-cells (32). Compared to control transgenic mice expressing full-length NIK, the NIKΔT3 mice demonstrated increased NIK levels with enhanced p100 processing in B-cells. They also showed expanded MALT and profound splenic marginal zone B-cell hyperplasia, a phenotype that bears similarity to the Eµ-API2-MALT1 transgenic mouse (33). Together with our results, these findings suggest that separating the TRAF3 binding site on NIK from the kinase domain, either through aberrations of the NIK gene or through proteolytic cleavage of NIK protein, may represent a common mechanism for deregulating NIK activity in B-cell neoplasms. Our findings suggest that in API2-MALT1-expressing MALT lymphomas, the API2 moiety mediates auto-oligomerization of API2-MALT1 and recruitment of NIK, and the MALT1 protease domain cleaves NIK, leading to degradation-resistant NIK kinase and deregulated noncanonical NF-κB signaling (see model, fig. S14). Data suggest that NIK cleavage protects API2-MALT1-expressing B-cells from apoptosis and promotes B-cell adhesion, both of which could contribute to the more aggressive phenotype of t(11;18)-positive MALT lymphomas (1). Disrupting the API2-NIK interaction and/or blocking MALT1 protease or NIK kinase activity could represent new treatment approaches for refractory t(11;18)-positive MALT lymphoma.
Supplementary Material
References and Notes
- 1.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 2.Thome M. Multifunctional roles for MALT1 in T-cell activation. Nat Rev Immunol. 2008;8:495. doi: 10.1038/nri2338. [DOI] [PubMed] [Google Scholar]
- 3.Lucas PC, et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappaB signaling pathway. J Biol Chem. 2001;276:19012. doi: 10.1074/jbc.M009984200. [DOI] [PubMed] [Google Scholar]
- 4.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 5.Lucas PC, et al. A dual role for the API2 moiety in API2-MALT1-dependent NF-kappaB activation: heterotypic oligomerization and TRAF2 recruitment. Oncogene. 2007;26:5643. doi: 10.1038/sj.onc.1210342. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H, Du MQ, Dixit VM. Constitutive NF-kappaB activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell. 2005;7:425. doi: 10.1016/j.ccr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 8.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 9.Song HY, Régnier CH, Kirschning CJ, Goeddel DV, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen SL, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Coornaert B, et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol. 2008;9:263. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- 14.Rebeaud F, et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol. 2008;9:272. doi: 10.1038/ni1568. [DOI] [PubMed] [Google Scholar]
- 15.Noels H, et al. A Novel TRAF6 binding site in MALT1 defines distinct mechanisms of NF-kappaB activation by API2-MALT1 fusions. J Biol Chem. 2007;282:10180. doi: 10.1074/jbc.M611038200. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Izquierdo D, et al. MALT1 is deregulated by both chromosomal translocation and amplification in B-cell non-Hodgkin lymphoma. Blood. 2003;101:4539. doi: 10.1182/blood-2002-10-3236. [DOI] [PubMed] [Google Scholar]
- 17.Uren AG, et al. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell. 2000;6:961. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 18.McAllister-Lucas LM, Lucas PC. Finally, MALT1 is a protease! Nat Immunol. 2008;9:231. doi: 10.1038/ni0308-231. [DOI] [PubMed] [Google Scholar]
- 19.Liao G, Zhang M, Harhaj EW, Sun S-C. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 20.Vallabhapurapu S, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarnegar BJ, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annunziata CM, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonizzi G, et al. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. Embo J. 2004;23:4202. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JL, Limnander A, Rothman PB. Pim-1 and Pim-2 kinases are required for efficient pre-B-cell transformation by v-Abl oncogene. Blood. 2008;111:1677. doi: 10.1182/blood-2007-04-083808. [DOI] [PubMed] [Google Scholar]
- 25.Enzler T, et al. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Guo F, Weih D, Meier E, Weih F. Constitutive alternative NF-kappaB signaling promotes marginal zone B-cell development but disrupts the marginal sinus and induces HEV-like structures in the spleen. Blood. 2007;110:2381. doi: 10.1182/blood-2007-02-075143. [DOI] [PubMed] [Google Scholar]
- 27.Woodland RT, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witzel II, Koh LF, Perkins ND. Regulation of cyclin D1 gene expression. Biochem Soc Trans. 2010;38:217. doi: 10.1042/BST0380217. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Giral S, et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76:462. doi: 10.1189/jlb.1203652. [DOI] [PubMed] [Google Scholar]
- 30.Nie Y, et al. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luftig M, et al. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc Natl Acad Sci USA. 2004;101:141. doi: 10.1073/pnas.2237183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki Y, et al. NIK overexpression amplifies, whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R-mediated survival signals in B cells. Proc Natl Acad Sci USA. 2008;105:10883. doi: 10.1073/pnas.0805186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baens M, et al. Selective expansion of marginal zone B cells in Emicro-API2-MALT1 mice is linked to enhanced IkappaB kinase gamma polyubiquitination. Cancer Res. 2006;66:5270. doi: 10.1158/0008-5472.CAN-05-4590. [DOI] [PubMed] [Google Scholar]
- 34.See Materials and Methods in Supporting Online Materials for description of the Tumor Collections # 1 and 2.
- 35.We thank M. Dyer for the SSK41 cells, R. Renne for the BJAB Tet-On cells, and G. Nunez for several expression plasmids. L.M. is a recipient of the Nancy Newton Loeb Pediatric Cancer Research and Helen L. Kay Pediatric Cancer Research Awards, and received support from NICHD T32-HD07513. L.M. and S.R. were both supported by NHLBI T32-HL007622-21A2. M.B. is supported by grants from the Research Foundation – Flanders (FWO) and Belgian Foundation against Cancer. H.N. was an aspirant of the FWO-Vlaanderen. P.V.L. is a postdoctoral researcher of the FWO. This work was supported by the Shirley K. Schlafer Foundation, the Elizabeth Caroline Crosby Fund, and grants from the University of Michigan Comprehensive Cancer Center (G007839), Leukemia & Lymphoma Research UK, and NIH/NCI (RO1CA124540).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.