Abstract
Genome wide association studies have identified significant association between polymorphisms of the Group 1B phospholipase A2 (PLA2G1B) gene with central obesity in humans. Previous studies have shown that Pla2g1b inactivation decreases postprandial lysophospholipid absorption and as a consequence increases hepatic fatty acid oxidation and protects against diet-induced obesity and glucose intolerance in mice. The current study showed that transgenic mice with pancreatic acinar cell-specific over-expression of the human PLA2G1B gene gained significantly more weight and displayed elevated insulin resistance characteristics, including impaired glucose tolerance, compared to wild type mice when challenged with a high fat/carbohydrate diet. Pre- and post-prandial plasma β-hydroxybutyrate levels were also lower, indicative of decreased hepatic fatty acid oxidation, in the hypercaloric diet-fed PLA2G1B transgenic mice. These, along with earlier observations of Pla2g1b-null mice, document that Pla2g1b expression level is an important determinant of susceptibility to diet-induced obesity and diabetes, suggesting that the relationship between PLA2G1B polymorphisms and obesity may be due to differences in PLA2G1B expression levels between these individuals. The ability of pancreas-specific over-expression of PLA2G1B to promote obesity and glucose intolerance suggests that target phospholipase activity in the digestive tract with nonabsorbable inhibitors should be considered as therapeutic option for metabolic disease therapy.
Keywords: Phospholipase A2, diet-induced obesity, diabetes, fatty acid oxidation
Introduction
Genome wide association studies have identified significant association (P<0.01) between PLA2G1B gene polymorphisms and central adiposity in humans.1 This gene encodes the Group 1B phospholipase A2 protein (PLA2G1B) that hydrolyzes the fatty acyl bond at the sn-2 position of phospholipids to generate free fatty acids and lysophospholipids. More recent lipidomic analyses of obesity-discordant twins revealed lysophosphatidylcholine elevation in plasma is a major obesity risk factor in humans.2 Thus, the obesity differences in humans with PLA2G1B polymorphisms may involve PLA2G1B-mediated lysophospholipid production.
Our earlier studies with Pla2g1b−/− mice showed that the absence of Pla2g1b reduces postprandial lysophospholipid levels due to decreased lysophospholipid absorption, but has no effect on absorption of other fat and fat-soluble nutrients.3 Nevertheless, the Pla2g1b−/− mice are protected against obesity and insulin resistance induced by a high fat/carbohydrate diet.4,5 The reduction in lysophospholipid levels in Pla2g1b−/− mice increases postprandial hepatic fatty acid oxidation, and as a consequence reduces fatty acid availability to extrahepatic tissues resulting in obesity protection.3 The reduced fatty acid availability also increases glucose utilization by extrahepatic tissues, thereby providing protection against glucose intolerance and diabetes.5 These results suggest that PLA2G1B inhibition may be a novel therapeutic strategy for treatment of obesity and diabetes.
The PLA2G1B gene is expressed primarily in the acinar cells of the pancreas with less amounts of mRNA present in lung type II alveolar epithelial cells.6,7 The PLA2G1B protein is also present in insulin secretory granules of pancreatic islet beta cells and is secreted with insulin upon glucose stimulation.8 The importance of pancreatic acinar cell-derived PLA2G1B versus enzymes synthesized in lung and islet β-cells toward diet-induced obesity and hyperglycemia has not been defined. This information is necessary to target PLA2G1B inhibitors to specific anatomic sites where they can be effective in suppressing diet-induced obesity and diabetes with minimal adverse effects. This study examined the impact of transgenic over-expression of the human PLA2G1B gene in mouse pancreatic acinar cells on diet-induced obesity and insulin resistance.
Methods
Animals
A 3.3-kB chimeric gene was constructed in PUC13 vector by ligating a 508-bp DNA fragment containing the −500 to +8 region of the rat elastase-1 gene9 to the 5’-untranslated region of the 2.2-kB human growth hormone (hGH) gene, followed by insertion of a 560-bp human PLA2G1B cDNA into the unique BamHI site at the 5’-untranslated region within exon 1 of the hGH gene (Fig. 1A). The resulting chimeric gene containing the rat elastase-1 promoter and transcription initiation site, the PLA2G1B cDNA, and the hGH gene with its accompanying introns, transcription termination, and polyadenylation signal was excised from the plasmid vector and then used to produce transgenic mice as described previously.10 Founder mice were identified by PCR amplification of genomic DNA with hGH-specific primers, and mated with C57BL/6 mice for 10 generations to obtain transgenic mice in congenic C57BL/6 background. The animals were fed either a basal diet (D12328; Research Diets, New Brunswick, NJ, USA) or a hypercaloric diet (D12331; Research Diets) composed of 58.5% fat, 25% sucrose, and 16.5% protein. Body weight gain was monitored biweekly. All animal protocols were approved by Institutional Animal Care and Use Committee at the University of Cincinnati in accordance with National Institutes of Health guidelines.
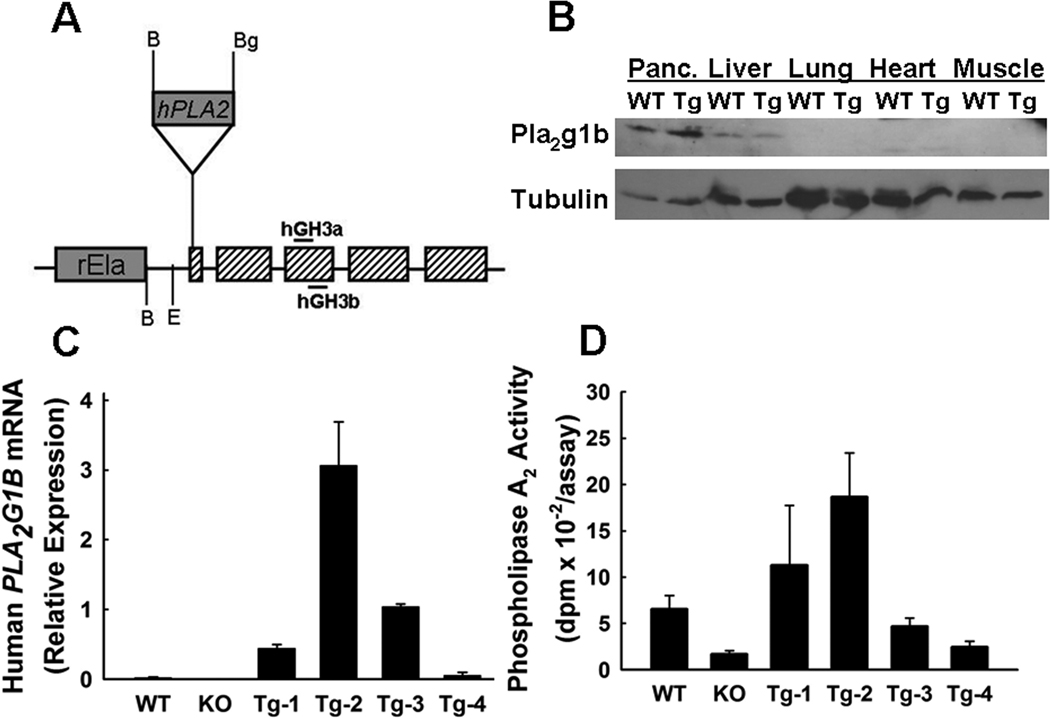
Figure 1.
Transgenic expression of human PLA2G1B in mice. Panel A shows the schematic diagram of transgene. The shaded boxes represent exons of hGH gene connected by their respective introns. The rEla box represents −500 to +8 region of the rat pancreatic elastase-1 gene, and the hPLA2 box represents the 560-bp BamHI/BglII fragment of human PLA2G1B cDNA inserted into exon 1 of the hGH gene. The positions of the primers used for PCR amplification detection of the transgene are indicated as hGH3a and hGH3b, respectively. B denotes BamHI restriction site and E denotes EcoRI restriction site in the chimeric transgene. Panel B shows Western blot of extracts from wild type (WT) and transgenic (Tg) pancreas (Panc.), liver, lung, heart, and skeletal muscle with rabbit antibodies against PLA2G1B. Blots with anti-tubulin antibodies were used as loading control. Panel C shows human PLA2G1B mRNA detected in pancreas of wild type (WT), Pla2g1b-null (KO), and the 4 transgenic lines (Tg1–4) relative to expression levels of cyclophilin detected by RT-PCR. Panel D shows phospholipase A2 eyzmatic activity in 50 µg pancreatic extracts from the respective mouse lines based on the hydrolysis of 1-palmitoyl-2-[14C]oleoyl phosphatidylcholine. Each bar represents mean ± s.e. of 3–4 animals in each group.
Human PLA2G1B expression in transgenic mice
Offsprings of founder mice were screened for expression of the chimeric transgene by reverse polymerase-PCR amplification of mRNA isolated from the pancreas and other tissues and normalized to the expression levels of cyclophilin A. Pancreatic phospholipase A2 activity was determined by monitoring the hydrolysis of 6.5 mM 1-palmitoyl-2-[14C]oleoyl-phosphatidylcholine (0.05 µCi) with 50 µg of pancreatic extracts in 50 µl buffer containing 500 µM Tris-HCl, pH 8.0, 135 mM NaCl, 1 mM CaCl2, and 7.95 mM cholic acid. Protein expression was detected by Western blot analysis with anti-phospholipase A2 antibodies that react with both mouse and human Pla2g1b.11 Pancreas from Pla2g1b+/+ and Pla2g1b−/− C57BL/6 mice were used as positive and negative controls, respectively.
Blood chemistry and glucose tolerance tests
Blood was collected from the tail vein of mice after an overnight fast. Blood glucose level was determined with an Accu-Chek Glucometer (Roche Applied Science, Indianapolis, IN, USA). Insulin and β-hydroxybutyrate levels in plasma were measured by ELISA and colorimetric assay kits as described previously.3 Glucose tolerance tests were conducted by measuring blood glucose levels before and at various time periods after feeding a bolus glucose-lipid mixed meal containing 50% glucose, 2.6 mM egg phosphatidylcholine, 13.33 mM triolein, and 2.6 mM cholesterol.5 Insulin sensitivity and resistance were estimated by homeostasis model assessment index, calculated as (fasting insulin concentration in µU/mL × fasting glucose concentration in mmol/L)/22.5.12
Data analysis
All results are reported as means ± se with the sample size as indicated. Significance (P<0.05) was evaluated using unpaired 2-tailed Student’s t test assuming equal variance. All statistical analyses, including the Pearson correlation, were conducted using SigmaPlot 10.0 (Systat Software, San Jose, CA, USA).
Results
Western blot analysis of various tissues in wild type and PLA2G1B-transgenic mice showed increased PLA2G1B in pancreas but not in the liver, lung, heart, and muscle tissues of the transgenic mice (Fig. 1B). Reverse transcriptase-PCR analysis of pancreatic mRNA isolated from the 4 independent transgenic mouse lines revealed different levels of human PLA2G1B expression, with the Tg-2 line showing the most robust expression level whereas expression of the transgene in the Tg-4 line was minimal (Fig. 1C). Phospholipase A2 enzymatic activities in the pancreas of the transgenic mice were consistent with the mRNA expression data, with pancreas from Tg-2 mice displaying ~3.5-fold higher phospholipase A2 activity compared to the non-transgenic wild type mice, whereas enzymatic activities in the pancreas of the other 3 transgenic lines showed variable phospholipase A2 activities that were not different from that observed with non-transgenic mouse pancreas (Fig. 1D).
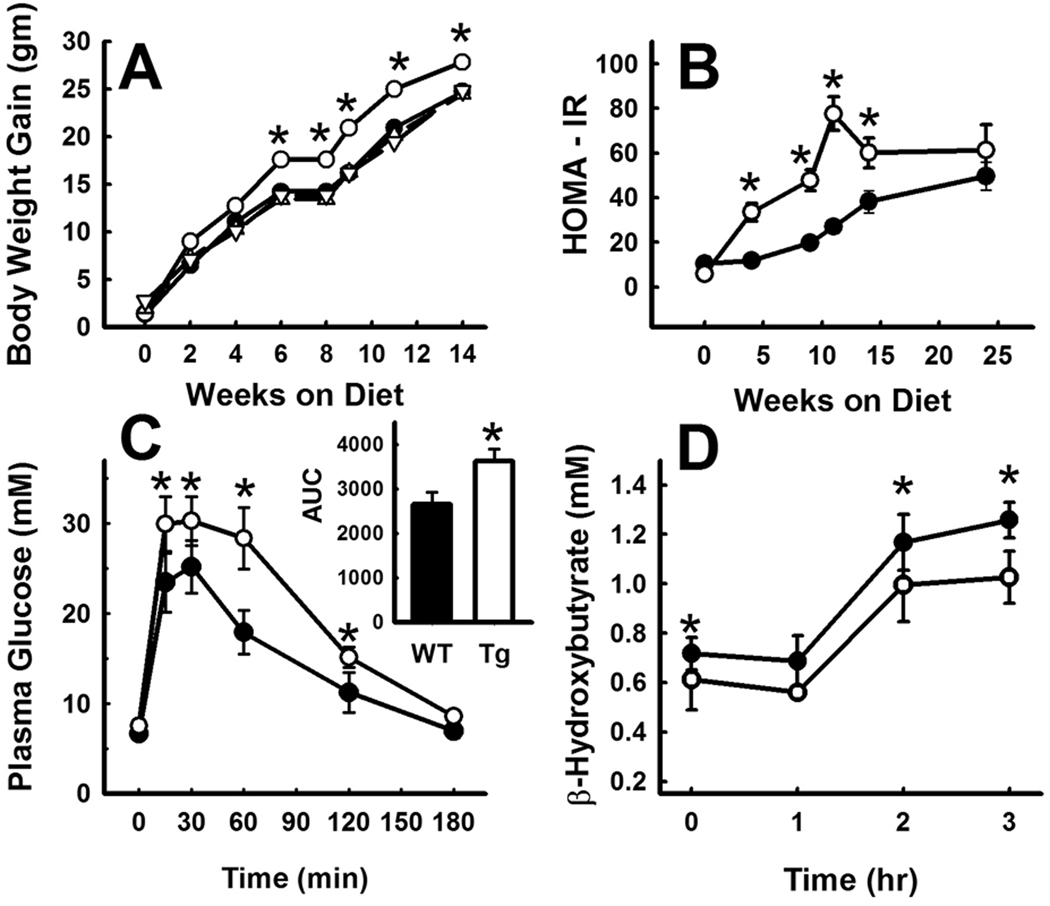
Initial characterization of the 4 transgenic mouse lines showed that the animals were healthy and breed normally with no obvious signs of distress. Plasma lipid levels and body weights were also similar between wild type and PLA2G1B-transgenic mice when they were maintained on regular chow diet. In contrast, the Tg-2 PLA2G1B transgenic mice were significantly larger and gained more weight compared to wild type and other lines of PLA2G1B transgenic mice when the animals were fed the hypercaloric high fat/high carbohydrate diet (Fig. 2A). Homeostasis model assessment of fasting plasma glucose and insulin levels revealed an accelerated rate of diet-induced insulin resistance in the Tg-2 PLA2G1B transgenic mice (Fig. 2B). Glucose tolerance test administered to these animals 19 weeks after feeding the hypercaloric diet also revealed delayed glucose excursion in the PLAG1B transgenic mice (Fig. 2C).
Figure 2.
Diet-induced obesity and diabetes in wild type and PLA2G1B transgenic mice. Panel A shows body weight gain of wild type (filled circles) and PLA2G1B transgenic mouse lines Tg-1 (filled triangles), Tg-2 (open circles), Tg-3 (open triangles), Tg-4 (open inverted triangles) in response to high fat/carbohydrate diet. Data represent mean ± s.e. from 8 mice in each group. Note that the lines for WT, Tg-1, Tg-3, and Tg-4 are superimposable. Panel B shows homeostasis assessment – insulin resistance HOMA-IR) index of high fat/carbohydrate-fed wild type mice (filled circles) and Tg-2 PLA2G1B-transgenic mice (open circles) (n=8 in each group). Panels C and D show plasma glucose and β-hydroxybutyrate levels in wild type (filled circles) and Tg-2 PLA2G1B-transgenic mice (open circles) after overnight fast (time = 0) and after feeding a bolus lipid meal (n=4 mice in each group). The inset to panel C shows areas under the curve determinations of the glucose tolerance tests. * denotes statistical significant differences from the wild type group at P<0.05.
Previous studies have shown improved glucose tolerance and obesity resistance in Pla2g1b-null mice due to elevated hepatic fatty acid oxidation as a consequence of reduced lysophospholipid absorption.3 In the current study, plasma β-hydroxybutyrate levels, a surrogate measurement of hepatic fatty acid oxidation,3 were significantly lower in the Tg-2 PLA2G1B transgenic mice compared to wild type mice under both fasting conditions as well as throughout a 3 hr postprandial period after a fatty meal (Fig. 2D). Thus, reduced hepatic fatty acid oxidation may also account for the increase in diet-induced obesity and glucose intolerance in the transgenic mice.
Discussion
Polymorphisms in the PLA2G1B gene associated with central obesity in humans are either polymorphisms in non-coding introns or synonymous base substitutions in exons that do not result in sequence alterations of the PLA2G1B protein.1 Therefore, the mechanism by which these PLA2G1B polymorphisms contribute to central obesity remains an enigma. The current study shows over-expression of PLA2G1B in the pancreas reduces hepatic fatty acid oxidation and promotes diet-induced body weight gain and glucose intolerance. These results, together with our previous studies showing decreased Pla2g1b activity is protective against diet-induced obesity and diabetes in mice,3–5,13 indicate that varying levels of PLA2G1B expression and activity in the digestive tract is an important determinant of susceptibility to diet-induced obesity and glucose intolerance. Thus, the association between PLA2G1B polymorphisms with obesity may be related to PLA2G1B expression level in the pancreas.
The contributory role of PLA2G1B in promoting diet-induced obesity and glucose intolerance suggests that pharmacologic inhibition of its enzyme activity may be a viable strategy to prevent these metabolic disorders. In fact, we have shown in a proof of concept study that oral administration of a generic phospholipase A2 inhibitor methyl indoxam effectively reduces plasma lysophospholipid levels and protects against diet-induced obesity and glucose intolerance in mice.13 The bioavailability of orally fed methyl indoxam is ~13%13 and is capable of inhibiting other phospholipases.14 Thus, methyl indoxam may incur undesirable adverse effects in the systemic that limits its utility as a pharmacologic inhibitor of PLA2G1B for obesity and diabetes intervention. The current study showing that specific elevation of PLA2G1B expression in pancreatic acinar cells is sufficient to reduce hepatic fatty acid oxidation and promotes diet-induced obesity and diabetes. These results suggest that development of non-absorbable inhibitors that specifically target PLA2G1B in the digestive tract may be a safer and effective option for obesity and diabetes intervention.
Acknowledgements
We thank Drs. Galvin Swift and Raymond MacDonald, University of Texas Southwestern Medical Center (Dallas, TX) for providing a plasmid vector containing the rat pancreatic elastase-1 promoter sequence. This work was supported by NIH grant DK69967.
Footnotes
Conflict of interest - None.
References
- 1.Wilson SG, Adam G, Langdown M, Reneland R, Braun A, Andrew T, et al. Linkage and potential association of obesity-related phenotypes with two genes on chromosome 12q24 in a female dizygous twin cohort. Eur J Hum Genet. 2006;14:340–348. doi: 10.1038/sj.ejhg.5201551. [DOI] [PubMed] [Google Scholar]
- 2.Pietilainen KH, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, et al. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects - a monozygotic twin study. PLoS ONE. 2007;2:e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labonté ED, Pfluger PT, Cash JG, Kuhel DG, Roja JC, Magness DP, et al. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: the molecular link between group 1B phospholipase A2 and diet-induced obesity. FASEB J. 2010;24:2516–2524. doi: 10.1096/fj.09-144436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huggins KW, Boileau AC, Hui DY. Protection against diet-induced obesity and obesity-related insulin resistance in Group 1B PLA2-deficient mice. Am J Physiol. 2002;283:E994–E1001. doi: 10.1152/ajpendo.00110.2002. [DOI] [PubMed] [Google Scholar]
- 5.Labonté ED, Kirby RJ, Schildmeyer NM, Cannon AM, Huggins KW, Hui DY. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes. 2006;55:935–941. doi: 10.2337/diabetes.55.04.06.db05-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richmond BL, Hui DY. Molecular structure and tissue-specific expression of the mouse pancreatic phospholipase A2 gene. Gene. 2000;244:65–72. doi: 10.1016/s0378-1119(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 7.Seilhamer JJ, Randall TL, Yamanaka M, Johnson LK. Pancreatic phospholipase A2: Isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA. 1986;5:519–527. doi: 10.1089/dna.1.1986.5.519. [DOI] [PubMed] [Google Scholar]
- 8.Ramanadham S, Ma Z, Arita H, Zhang S, Turk J. Type IB secretory phospholipase A2 is contained in insulin secretory granules of pancreatic islet beta-cells and is co-secreted with insulin from glucose stimulated islets. Biochim Biophys Acta. 1998;1390:301–312. doi: 10.1016/s0005-2760(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 9.Hammer RE, Swift GH, Ornitz DM, Quaife CJ, Palmiter RD, Brinster RL, et al. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol Cell Biol. 1987;7:2956–2967. doi: 10.1128/mcb.7.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodvawala A, Ghering AB, Davidson WS, Hui DY. Carboxyl ester lipase expression in macrophages increases cholesteryl ester accumulation and promotes atherosclerosis. J Biol Chem. 2005;280:38592–38598. doi: 10.1074/jbc.M502266200. [DOI] [PubMed] [Google Scholar]
- 11.Richmond BL, Boileau AC, Zheng S, Huggins KW, Granholm NA, Tso P, et al. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A2 deficient mice. Gastroenterology. 2001;120:1193–1202. doi: 10.1053/gast.2001.23254. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Hui DY, Cope MJ, Labonte ED, Chang H-T, Shao J, Goka E, et al. The Phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br J Pharmacol. 2009;157:1263–1269. doi: 10.1111/j.1476-5381.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, et al. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]