Abstract
Respiratory function is the main cause of mortality in patients with Duchenne muscular dystrophy (DMD). Elevated levels of TGF-β play a key role in the pathophysiology of DMD. To determine whether therapeutic attenuation of TGF-β signaling improves respiratory function, mdx mice were treated from 2 weeks of age to 2 months or 9 months of age with either 1D11 (a neutralizing antibody to all three isoforms of TGF-β), losartan (an angiotensin receptor antagonist), or a combination of the two agents. Respiratory function was measured in nonanesthetized mice by plethysmography. The 9-month-old mdx mice had elevated Penh values and decreased breathing frequency, due primarily to decreased inspiratory flow rate. All treatments normalized Penh values and increased peak inspiratory flow, leading to decreased inspiration times and breathing frequency. Additionally, forelimb grip strength was improved after 1D11 treatment at both 2 and 9 months of age, whereas, losartan improved grip strength only at 2 months. Decreased serum creatine kinase levels (significant improvement for all groups), increased diaphragm muscle fiber density, and decreased hydroxyproline levels (significant improvement for 1D11 only) also suggested improved muscle function after treatment. For all endpoints, 1D11 was equivalent or superior to losartan; coadministration of the two agents was not superior to 1D11 alone. In conclusion, TGF-β antagonism may be a useful therapeutic approach for treating DMD patients.
Duchenne muscular dystrophy (DMD) is caused by mutations in the dystrophin gene leading to a loss of the translated protein.1,2 Dystrophin, a large structural protein, is critical for the assembly of the dystrophin-associated complex, a group of proteins that work in concert to link the actin cytoskeleton to the extracellular matrix of the basal lamina.3 The dystrophin-associated protein complex lends structural integrity to the sarcolemma and serves as an important scaffold for signaling entities involved in the modulation of cell survival.4,5 In the absence of dystrophin, the associated proteins are dislocated, membranes are more susceptible to microtears, and various signaling pathways are dysregulated, leading to cycles of myofiber degeneration and regeneration. TGF-β, a profibrotic cytokine, is elevated in DMD and is known to play a central role in the cycles of degeneration and regeneration that ultimate lead to the replacement of skeletal muscle with fat and fibrotic tissue in this progressive disease.6 Several lines of evidence suggest that lowering TGF-β activity in dystrophic muscle may enhance differentiation and fusion of the precursor satellite cells necessary for muscle regeneration and repair.7 Furthermore, TGF-β may promote the differentiation of myogenic cells into fibrotic cells.8 Thus, therapeutic approaches to inhibit TGF-β may address some of the disease manifestations in DMD and other degenerative myopathies.
Respiratory dysfunction is the cause of 80% of the mortality in DMD patients. We studied the effects of administering 1D11 (a neutralizing murine antibody to all three isoforms of TGF-β) on respiratory function, using plethysmography in the mdx mouse, a model of DMD. In addition, we compared antibody treatment to treatment with losartan, an antihypertensive agent that attenuates TGF-β activity by antagonizing angiotensin II receptor type 1 (AT1), and enalapril (an antagonist of the angiotensin-converting enzyme), Short-term studies in which forelimb grip strength was measured in mice dosed from 2 weeks to 2 months of age were used to assess the various treatment modalities. Effective treatment regimes (losartan, 1D11, or a combination of the two agents) were then compared in a long-term study conducted in mice up to 9 months of age, with respiratory function as the key endpoint.
Here we demonstrate, for the first time, that TGF-β antagonism normalized respiratory function in the mdx mouse model. Other measured endpoints were also positively affected by drug treatment. In all cases, 1D11 was equivalent to or superior to losartan, and coadministration of the two agents was not superior to 1D11 alone. Furthermore, these agents were well tolerated, with no changes in body weights in any of the test groups at any time point. These findings demonstrate that TGF-β antagonism can improve respiratory function in mdx mice and support its further evaluation as a potential therapeutic for DMD patients.
Materials and Methods
In Vivo Studies
All animal procedures were approved by our institutional review board and were conducted in our animal facility, which is certified by the Association for Assessment and Accreditation of Laboratory Animal Care International. The mice used in this study were male wild-type C57BL/10SnJ and male mdx C57BL/10ScSn-Dmdmdx/J mice (Jackson Laboratories, Bar Harbor, ME) that were housed and bred in our institutional facilities. BALB/c mice were from Charles River Laboratories (Raleigh, NC). Mice were provided with water and chow ad libitum.
An outline of all studies and endpoints is given in Supplemental Table S1 (available at http://ajp.amjpathol.org) Treatment was initiated at 2 weeks of age and was continued, without pause, until study termination when the mice reached either 2 or 9 months of age. Administration of 1D11 or control antibody (either 13C4,9 a murine anti-Shigella toxin IgG1 antibody produced by Genzyme Corporation, or MOPC 21, an antibody to mineral oil, from Sigma-Aldrich, St. Louis, MO) was by intraperitoneal injection of 5 mg/kg three times per week until 48 hours before the termination of the studies. 1D11 is a murine pan-neutralizing TGF-β IgG1 antibody that neutralizes the active forms of TGF-β1, -β2, and -β3.10 1D11 can also be purchased from ATCC (Manassas, VA) and R&D Systems (Minneapolis, MN). Administration of the AT1 angiotensin receptor antagonist losartan (LKT Laboratories, St. Paul, MN) and the angiotensin-converting enzyme inhibitor enalapril (Sigma-Aldrich) was via drinking water at concentrations of 600 mg/L and 200 mg/L, respectively.
We conducted two studies in the mdx mouse: a short-term study conducted to 2 months of age and a long-term study conducted to 9 months of age. All mice in the 2-month study were treated from 2 weeks of age to 2 months of age, for a total of 6 weeks of consecutive treatment. All mice in the 9-month study were treated from 2 weeks of age to 9 months of age, for a total of 8.5 months of consecutive treatment.
Mice were accessioned into the 2-month study in cohorts, because of limitations on the mouse census in our facilities. Each cohort in the 2-month study included a group of wild-type mice, 1D11-treated mdx mice, and vehicle-treated control mdx mice (n = 8). The first cohort also included a group of mice receiving the control antibody, 13C4 (n = 8). The second cohort included mdx mice treated with either losartan or enalapril (n = 8, each group). The third cohort included a group of mice in which 1D11 (5 mg/kg, three times per week) and losartan (600 mg/L in drinking water) were coadministered, to address whether an additive or synergistic benefit might be observed with combination therapy (n = 9). One group of mice in the third cohort was treated with MOPC 21 containing 1% bovine serum albumin (BSA) in the formulation (n = 10),9 to assess immune responsiveness. Terminal biochemical and histological endpoints for 2-month-old mice were made using samples from mice in either the first or the third cohorts of the 2-month studies. In the 2-month study, forelimb grip strength measurements from different cohorts with identical treatment modalities were pooled for statistical analysis.
In the 9-month study, mdx mice were dosed with vehicle, losartan, 1D11, or a combination of these agents in a single cohort (n = 9, each group). Wild-type mice were included for an age-matched comparison (n = 9). At the end of the treatment period, one animal had died in each of the vehicle control and wild-type groups, leaving eight mice for evaluation in each of those two groups. No mice were lost in the losartan, 1D11, or combination therapy groups.
In both the 2-month and the 9-month studies, body weights were recorded three times per week, and when agents were administered in the drinking water, the water bottles of all groups were weighed to estimate consumption.
TGF-β1 ELISA
Quadriceps muscles were homogenized in cell-lysis buffer (Cell Signaling Technology, Danvers, MA), and the clarified cytosol recovered for assay after centrifugation at 10,000 × g. Protein quantities in the cytosol were determined using a bicinchoninic acid kit (Sigma-Aldrich), and 50 μg was used in the enzyme-linked immunosorbent assay (ELISA). Both total TGF-β1 and activated TGF-β1 concentrations were assessed using a mouse TGF-β1 ELISA kit (MB100B; R&D Systems) according to the manufacturer's protocol (n = 6 per group).
Measurement of Gene Expression
Muscles were frozen in liquid nitrogen cooled isopentane and then stored at −80°C. Total RNA was isolated with TriReagent (Sigma-Aldrich) and homogenized; particulates were removed by centrifugation. The RNA pellet was dissolved in water and 10 μg RNA of each sample was treated with Ambion DNase TurboDNA (Applied Biosystems, Austin, TX). PCR was performed using TaqMan assays (Applied Biosystems, Foster City, CA). Gene expression analysis was performed using the standard curve method with 18S RNA as an endogenous control. Standard curves were run on each plate. Data were normalized to the mean value for the wild-type control group. The following fluorescently tagged primer sequences were used, all from Applied Biosystems: Mm00450111 for periostin, Mm00442754 for CD4, Mm00441724 for TGF-β1, Mm00436952 for TGF-β2, Mm00436960 for TGF-β3, and Mm001182107_g1 for CD8a.
Serum Creatine Kinase
Blood was obtained from 2- and 9-month-old treated mice by retro-orbital collection (n = 8 per group). Serum was shipped on dry ice for creatine kinase determinations (AnaLytics, Gaithersburg, MD).
Pharmacokinetics of 1D11
The pharmacokinetic behavior of 1D11 was determined after a single intraperitoneal dose of 5 mg/kg in BALB/c mice. Serum concentrations of 1D11 were determined using a sandwich ELISA, and noncompartmental modeling was performed using WinNonlin software platform version 5.0.1 (Pharsight Products, Phoenix AZ). Serum samples were taken at 6 hours and at 1, 2, 3, 4, 8, and 14 days after the dose (n = 3 mice per time point). In addition, a single serum sample was taken from each mdx mouse treated with 1D11 in the first cohort of the 2-month study (n = 8). To determine the concentration of 1D11 in serum, an ELISA was used. High protein binding polystyrene 96-well plates were coated overnight at 4°C with TGF-β2 (Sigma-Aldrich). The plates were treated with blocking solution (KPL, Gaithersburg, MD) and washed with PBS. A standard curve was prepared using 1:2 serial dilutions ranging from 0.78 ng/mL to 50 ng/mL. Test samples were diluted in PBS containing 0.2% Tween-20, 0.1% BSA, and 0.05% Triton-X-100. Standards, controls, and samples were added to the blocked, coated plates and incubated at 37°C for 1 hour. Goat-anti-mouse IgG (Fc-specific) horseradish peroxidase conjugate was added to each well at a 1:60,000 dilution, incubated for 1 hour, and detected with 3,3′,5,5′-tetramethylbenzidine.
Respiratory Function
Respiratory function was measured in unrestrained mice by barometric plethysmography using a Buxco plethysmograph (Troy, NY), essentially as described by TREAT-NMD (http://www.treat-nmd.eu/downloads/file/sops/dmd/MDX/DMD_M.2.2.002.pdf) and by others.11,12 Mice were placed in calibrated chambers containing a pneumotachograph that measured pressure differentials within the compartment by a difference in air flow. Mice were allowed to acclimate in chambers for 30 minutes in a dark room before data collection. Data were collected and monitored remotely to minimize variation from environmental stimuli. The inspiration time Ti was defined as the start of inspiration to the end of inspiration and the expiration time Te was defined as the start of expiration to the end of expiration. The relaxation time Tr was defined as the time from the start of expiration to the time when 64% of the total expiratory pressure occurred. Pause and Penh were defined and calculated by the following formulas: Pause = (Te − Tr)/Tr and Penh = (PEP/PIP) × Pause, where PEP is peak expiratory pressure and PIP is peak inspiration pressure. The value of each parameter was collected every minute for 10 minutes and the average was determined. For each test subject, three separate measurements were made on three separate days, and median values were used for statistical analyses.
Forelimb Grip Strength
Forelimb grip strength has been studied as a method to monitor muscle function in vivo in various models of muscular dystrophy.13 Forelimb grip strength was measured using an automated grip-strength meter (Columbus Instruments, Columbus, OH), essentially according to published protocols.14 Measurements were taken once per week from 5 weeks to 2 months of age in the 2-month study. In the 9-month study, measurements were taken at 7 and 9 months of age. The total peak force generated was determined using a force transducer as the mouse was pulled backward gently from the base of the tail. All measurements were performed in a blinded fashion, to minimize operator influence. Five consecutive measurements were made within 1 minute and were averaged to determine the mean forelimb grip strength. All measurements were performed between 9:00 and 11:00 AM, to minimize diurnal variation. The data were normalized to body weight and expressed as kilogram force per kilogram of body weight. Changes in grip strength were determined by analysis of variance followed by Duncan's multiple comparison test.
Immunohistological Detection of Myogenin
Soleus and diaphragm muscles were fixed in 10% neutral buffered formalin (Sigma-Aldrich) for 3 to 7 days. All tissues were embedded in paraffin, and 5 μmol/L cross-sections were cut from the center of each muscle. Myogenin immunostaining was performed using a BondMax immunostaining system (Leica Microsystems, Deerfield, IL) including the Bond polymer refine detection kit (DS9800), which contained peroxide block, polymer, diaminobenzidine, and hematoxylin. Primary antibody [mouse anti-rat myogenin clone F5D, X0931 (Dako, Carpinteria, CA)] was incubated for 30 minutes after blocking with rodent block M (RBM961; Biocare Medical, Concord, CA), followed by rabbit anti-mouse (clone M204-3; Epitomics, Burlingame, CA). The positive controls used were rat hearts injected with rat muscle stem cells. The negative controls used were mouse IgG1 (DAK-GO1; Dako) instead of primary antibody and noninjected rat hearts.
Entire cross-sections of diaphragm and soleus muscle were scanned at ×20 magnification using a Scanscope XT and Imagscope software v10.10.2028 (Aperio Technologies, Vista, CA). Each tissue was analyzed using a nuclear imaging algorithm (Color deconvolution version 9.0, Aperio Technologies) to quantify the number of myogenin-positive nuclei in the viable regions of tissue, excluding artifacts from the analysis. The nuclear algorithm was then used to digitally capture the intensity of diaminobenzidine staining from 0 (negative) to +3 (moderate positivity) and to quantify the number and intensity of myogenin-positive versus total nuclei in the viable muscle tissue section. Computer-identified myogenin-positive nuclei were manually visualized to confirm the accuracy of the digital algorithm.
Morphometric Analysis
Muscle tissue was dissected, pinned to squares of closed-cell extruded polystyrene foam (Styrofoam; Dow Corning, Midland, MI), coated with optimal cutting temperature OCT medium, and immediately frozen in liquid nitrogen-chilled isopentane. Embedded muscles were cross-sectioned (10 μmol/L) with a cryostat and the sections were adhered to glass slides. Mounted sections were fixed with 10% buffered formalin for 10 minutes, and washed. Fixed sections were coated with a mixture of wheat germ agglutinin, Alexa Fluor 488 conjugate (Invitrogen, Carlsbad, CA) diluted 1:100, and DAPI (Invitrogen) diluted 1:1000 and incubated for 1 hour at room temperature. Wheat germ agglutinin-stained sarcolemma was photographed at 450 nmol/L and DAPI-stained nuclei at 650 nmol/L. Three random cross-sections from the diaphragm muscles, containing between 1500 and 2000 fibers, were analyzed using MetaMorph software version 6.1 (Universal Imaging Corp Downington, PA) to determine the fiber area, and fiber breadth. The fibers containing central nuclei were counted manually in a blinded fashion. This protocol is similar to the recently published TREAT-NMD SOP,15 except that OCT was used as the embedding medium and the microtome sections were 10 μm instead of 12 μm in thickness. Selected regions were analyzed with morphometry software. The number of fibers analyzed for diaphragm muscle was between 4000 and 5000 for each group.15 For the soleus muscle, three entire cross-sections were processed. The percentage of muscle area and the total number of fibers per unit area were also quantitated.
Cell Culture and Myosin ELISA
Muscle C2C12 cells (ATCC) were maintained in a humidified incubator at 37°C and 5% CO2 in growth medium consisting of Dulbecco's modified Eagle's medium (DMEM; ATCC #30–2002) supplemented with 10% fetal bovine serum. When cells reached approximately 70% confluency (day 0), the medium was changed to differentiation medium (DMEM plus 2% horse serum; Gibco–Invitrogen, Gaithersburg, MD). TGF-β1 was added on day 0 and day 2. Under control conditions (in the absence of TGF-β), cells were maintained in differentiation medium only. Control and treated cells were collected for RNA, ELISA, or myosin protein expression assays on day 5 after differentiation.
After 5 days of TGF-β treatment, C2C12 cells were fixed with ice-cold methanol for 20 minutes at 20°C. Cells were then washed with PBS and permeabilized with 0.5% Triton-X-100 in PBS for 10 minutes at room temperature. Cells were blocked with 10% BSA for 1 hour at 20°C. A mouse anti-myosin heavy chain antibody (MF-20 supernatant; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) was incubated with the cells at 20°C for 1 hour (1:100); cells were then counterstained with a rabbit antibody to GAPDH (sc-25778; Santa Cruz Biotechnology, Santa Cruz, CA) (1:100). Cells were washed with PBS, followed by incubation with the secondary antibodies anti-mouse IRDye800 and anti-rabbit IRDye680 (LI-COR Biosciences, Lincoln, NE) for 1 hour at room temperature (1:1000). The LI-COR Odyssey imaging system was used for quantitation of myosin protein expression. The integrated intensity of myosin expression was normalized to that of GAPDH. For images, propidium iodide (1:1000) was used to visualize nuclei and anti-mouse Alexa Fluor 488 (1:1000) was used as secondary antibody in place of anti-mouse IRDye 800, to visualize fused myotubes.
Hydroxyproline Levels
Hydroxyproline assays were performed as previously described.16 Briefly, muscle samples were hydrolyzed in an autoclave at 120°C for 20 minutes. Autoclaved samples were mixed with chloramine T and allowed to incubate for 25 minutes at room temperature. Ehrlich's aldehyde reagent was added to each sample and incubated at 65°C for 20 minutes to develop the chromophore, which was quantitated against a standard curve of hydroxyproline (2 to 20 μg; Sigma-Aldrich) at 550 nmol/L.
Statistical Analysis
Statistical analysis was performed using analysis of variance followed by Dunnett's multiple comparison test against vehicle control or by Tukey's multiple analysis test for comparisons against all combinations of groups, using GraphPad Instat 3.1 software (GraphPad Software, La Jolla, CA).
Results
TGF-β mRNA and Protein Levels Are Elevated in mdx Mouse Muscle
TGF-β1 mRNA levels in the skeletal muscle of mdx mice were elevated threefold to fourfold and that of TGF-β3 1.4-fold to twofold, compared with wild-type mice (Table 1). The steady-state mRNA levels of TGF-β2 were not elevated, as has been reported previously.17 Consistent with the increase in TGF-β1 mRNA levels, total TGF-β1 protein levels were elevated 3.4-fold in quadriceps muscle from mdx mice (221 ± 43 ng/μg protein), compared with wild-type mice (65 ± 10 ng/μg protein). TGF-β exists primarily as the inactive large latent complex that is proteolytically activated in vivo. Levels of active TGF-β1, however, were not detectable in the same samples.
Table 1.
TGF-β Transcript Levels from Skeletal Muscle from mdx Mice
| Muscle | TGF-β1 | TGF-β2 | TGF-β3 |
|---|---|---|---|
| Quadriceps | 4.0 ± 0.4⁎ | 0.95 ± 0.08 | Not done |
| Gastrocnemius | 4.4 ± 0.7⁎ | Not done | 2.1 ± 0.74⁎⁎ |
| Diaphragm | 3.1 ± 0.2⁎⁎ | Not done | 1.4 ± 0.15⁎⁎⁎ |
Data are reported as fold increase over wild type, means ± SE; n = 6 samples per determination from mice 8 weeks of age.
P < 0.05,
P < 0.01, and
P < 0.001, two-way t-test.
Body and Muscle Weights
Body weights of all study mice were determined three times per week. No significant difference in the mean body weight was observed between mice in any treatment group or wild-type controls at any time point. The mean terminal body weights for mice in the short-term and long-term studies are given in Supplemental Table S2 (available at http://ajp.amjpathol.org). No changes in the weight of soleus or tibialis anterior muscles were observed in any groups of 2-month-old mice (data not shown). Terminal body weights at 9 months of age in the treated mice were also not different between groups. However, a 40% increase in the mean weight of the soleus and tibialis anterior muscles from mdx mice was observed, compared with those from wild-type mice at 9 months of age, and this was not affected by treatment.
Dose Selection for 1D11, Enalapril, and Losartan in mdx Mice
The mdx mice were treated with 5 mg/kg 1D11 by intraperitoneal injection three times per week. To elucidate the relationship between the pharmacokinetics and the pharmacodynamic response, the interim plasma levels of 1D11 were sampled in the first cohort of mice studied (n = 8 per group). Interim plasma levels of 1D11 were 163 ± 11 μg/mL and 254 ± 18 μg/mL, 24 hours after the 10th dose at 6 weeks and the 19th dose at 2 months of age, respectively. In a separate study, the plasma half-life of 1D11 in mice BALB/c mice was determined to be 7.9 days. The concentration of 1D11 needed to neutralize TGF-β1 by 50% in vitro, determined using an A549 cell-based assay, was found to be 0.3 μg/mL (data not shown).18 Thus, the concentration of 1D11 in plasma was approximately 500 times the IC50 value for the neutralization of TGF-β1, suggesting that the dose and dosing regimen chosen were adequate to elicit a maximal systemic pharmacodynamic response in mice.
Administration of losartan and enalapril was via drinking water at concentrations of 600 mg/L and 200 mg/L, respectively. These doses have been shown to be efficacious in murine models of kidney disease19 and muscular dystrophy.7,20 Mice drank 3 to 6 mL of water per day, and the mean water intake was not different in any groups. Thus, the dose of losartan was between 60 and 120 mg/kg/day, or roughly four to eight times the maximally effective human dose of 100 mg/day, after accounting for the difference in body surface area between mice and humans. Similarly, the dose of enalapril ingested (24 to 48 mg/kg per day) was roughly 7 to 14 times the maximally effective human dose of 20 mg/day. Thus, the comparisons among losartan, enalapril, and 1D11 made in these studies were most likely performed at maximally effective doses. These doses were well tolerated.
Treatment Decreases Periostin mRNA Transcript Levels in the Skeletal Muscle of mdx Mice
The mRNA transcript level of periostin was elevated 48-fold and 29-fold in quadriceps and diaphragm muscles, respectively, of mdx mice compared with wild-type mice (Figure 1). Treatment with 1D11 significantly decreased muscle periostin transcript by 60% in quadriceps and 40% in diaphragm muscle. Treatment with losartan also significantly decreased periostin mRNA transcript levels by 50% in quadriceps muscle and resulted in a nonsignificant 25% reduction in RNA extracted from diaphragm muscle. Periostin mRNA levels were not different from vehicle control when mice were treated with the nonspecific control antibody 13C4.
Figure 1.
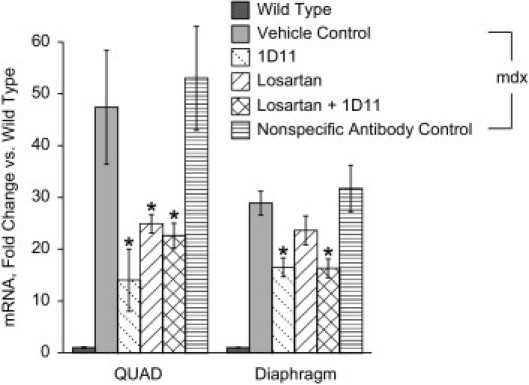
Treatment decreases periostin levels in mdx muscle. Real-time PCR analysis of periostin mRNA levels in quadriceps (QUAD) and diaphragm muscle from mdx mice treated with vehicle, 1D11, losartan, losartan + 1D11, or 13C4 (the nonspecific antibody control). The mdx mice were treated from 2 weeks of age to 2 months of age, when muscles were collected for analysis. Wild-type mice were also assayed as a comparator. Data are reported as means ± SE. Statistical analyses were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.01; n = 8 per group.
Markers of Inflammatory Responsiveness after 1D11 Treatment
CD4 mRNA, a T-cell marker, was elevated approximately 14-fold in quadriceps and fourfold in diaphragm muscles of 2-month-old vehicle-treated mdx mice, compared with their wild-type counterparts (n = 8 per group, P < 0.01; see Supplemental Figure S1 at http://ajp.amjpathol.org). There was no significant change in the level of these markers observed after the suppression of TGF-β activity by treatment with 1D11, losartan, or a combination of the two agents in muscles from 2-month-old mice. CD4 mRNA was significantly reduced in the quadriceps and diaphragm muscle from 9-month-old mdx mice, compared with the levels observed in 2-month-old mdx mice, again with no effect after treatment with 1D11, losartan, or a combination of the two agents. In the diaphragm of 9-month-old treated mice, CD4 mRNA remained elevated in vehicle-treated mdx mice (approximately twofold), compared with wild-type mice; as before, none of the treatments had any effect on the level of this marker. Mice administered the antibody MOPC 21 in a solution containing 1% BSA in its formulation (n = 10) exhibited a dramatic increase in the level of mRNA for CD4 in quadriceps and diaphragm muscle (n = 8), indicating that the mdx mouse is immunocompetent.
Serum monocyte chemoattractant protein 1 (MCP-1) levels were also elevated 4.3-fold in serum from vehicle control, compared with wild-type mice; however, treatment with 1D11 led to a significant decreases in the serum level of MCP-1 in 2-month-old mdx mice, compared with vehicle-treated mdx mice (Figure 2A; n = 8).
Figure 2.
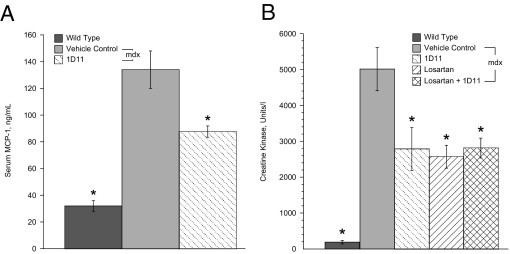
Biomarkers of inflammation and muscle damage were reduced by treatment. A: Circulating levels of monocyte chemoattractant protein (MCP-1) in 2-month-old mdx mice with 1D11 were significantly lower than those of vehicle-treated mdx mice. B: Levels of serum creatine kinase were significantly decreased in 9-month-old mdx mice treated with 1D11, losartan, or a combination of the two agents. Data are reported as means ± SE. Statistical analyses were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.01; n = 8 per group.
Treatment Decreases Serum Creatine Kinase Levels
Damaged muscle fibers release the cytosolic enzyme creatine kinase.21 Treatment with losartan, 1D11, or a combination of the two agents decreased serum creatine kinase levels by approximately 50% in 9-month-old mice, suggesting that there was less muscle damage in treated mdx mice (Figure 2B; n = 8). Serum from the treated 2-month-old mice also showed a reduced creatine kinase levels, similar to that observed at 9 months of age, but this trend did not reach statistical significance (data not shown; n = 8).
Hydroxyproline Levels Are Decreased by 1D11 Treatment
Tissue levels of hydroxyproline, a major component of collagen, are correlated with the degree of fibrosis in muscle tissue.16 Hydroxyproline levels were measured in the quadriceps, diaphragm, and soleus muscles from mdx and wild-type mice. Hydroxyproline levels were elevated approximately eightfold in muscle homogenates from the diaphragms of mdx mice, compared with wild-type mice. Treatment with 1D11 resulted in a 20% decrease in hydroxyproline levels in diaphragm muscle from 9-month-old mdx mice (data not shown, P < 0.05). No changes were observed in hydroxyproline levels after losartan treatment. There was only a slight elevation in hydroxyproline levels in quadriceps (15%) and soleus (23%) muscles, and these were not significantly affected by either treatment.
Treatment Improves Respiratory Function in mdx Mice
Respiratory function was measured in conscious, unrestrained mdx mice using whole-body plethysmography (Table 2). A number of respiratory parameters were altered in the vehicle-treated mdx mice, compared with wild-type controls. These changes were detectable in mdx mice as early as 3 months of age, although they were more pronounced at 9 months of age, when the effect of anti-TGF-β treatment was evaluated. At 9 months of age, the frequency of breathing (F) was decreased in mdx mice, compared with wild-type mice, because of longer inspiration times (Ti) and an increase in the pause between breaths (EIP). In addition, the peak inspiratory flow rate (PIP) was lower in mdx mice. Both tidal volume (TV) and relaxation time (Tr) did not different between mdx and wild-type mice. As expected, the inspiratory parameters were those most affected in mdx mice.
Table 2.
Parameters of Respiratory Function in Treated mdx Mice Compared with Vehicle Control mdx Mice
| Variable† | Wild type | Vehicle | 1D11 | Losartan | 1D11 + Losartan |
|---|---|---|---|---|---|
| Penh | 0.54 ± 0.02⁎⁎ | 1.3 ± 0.16 | 0.68 ± 0.04⁎⁎ | 0.76 ± 0.07⁎⁎ | 0.67 ± 0.05⁎⁎ |
| F, breaths/m | 323 ± 15⁎⁎ | 238 ± 17 | 286 ± 13⁎ | 312 ± 15⁎ | 261 ± 13⁎ |
| PIP, mL/s | 6.9 ± 0.51⁎⁎ | 4.78 ± 0.5 | 6.56 ± 0.4⁎ | 6.53 ± 0.3⁎ | 5.08 ± 0.3 |
| Ti, s | 0.063 ± 0.06⁎⁎⁎ | 0.168 ± 0.21 | 0.078 ± 0.004⁎ | 0.067 ± 0.05⁎⁎ | 0.088 ± 0.067 |
| EIP, ms | 4.45 ± 0.05⁎⁎ | 4.7 ± 0.02 | 4.53 ± 0.11⁎ | 4.6 ± 0.06⁎ | 4.63 ± 0.08⁎ |
| MV, ± mL/m | 77.8 ± 6.4⁎⁎ | 50.8 ± 5.9 | 70.9 ± 4.90⁎ | 74.4 ± 2.6⁎⁎ | 63.8 ± 3.7 |
| PEP, mL/m | 3.89 ± 0.3 | 3.04 ± 0.28 | 3.7 ± 0.23 | 3.74 ± 0.13 | 2.91 ± 0.17 |
| Te, s | 0.17⁎⁎ | 0.25 | 0.24 | 0.18⁎⁎ | 0.24 |
| TV, mL | 0.246 ± 0.01 | 0.218 ± 0.01 | 0.247 ± 0.03 | 0.242 ± 0.007 | 0.232 ± 0.008 |
| Tr, s | 0.101 ± .007 | 0.101 ± .004 | 0.115 ± .006 | 0.082 ± 0.01 | 0.111 ± 0.02 |
| EEP, ms | 35.5 ± 4 | 55.7 ± 8 | 43.1 ± 5 | 55.1 ± 6 | 43.8 ± 5 |
Respiratory function was measured by plethysmography in 9-month-old mdx mice treated with vehicle, 1D11, losartan, or a combination of 1D11 and losartan. Data are reported as means ± SE, with n = 8 for the vehicle-treated mdx and wild-type groups at the end of the study and n = 9 for the other treatment groups.
P < 0.05,
P < 0.01, and
P < 0.001, by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control.
EEP, end inspiratory pause; EIP, end inspiratory pause; f, frequency of breaths; MV, minute volume; Pause = (Te minus Tr)/Tr, calculated with Buxco plethysmography software (version 0.0; Wilmington, NC) for each recorded breath; Penh, enhanced pause [calculated for each recorded breath, using Buxco plethysmography software, version 0.0, as Penh = (PEP/PIP) × Pause]; PEP, peak expiratory flow; PIP, peak inspiratory flow; Te, expiration time (from start of expiration to end of expiration); Ti, inspiration time (from start of inspiration to end of inspiration); Tr, relaxation time (from start of expiration to the time-point at which 64% of the total expiratory pressure occurred); TV, tidal volume.
Enhanced pause (Penh) is commonly used as a means to noninvasively assess respiratory function in mice.11,22 Penh was significantly elevated (144%, P < 0.05) in vehicle-treated, 9-month-old, mdx mice compared with wild-type mice, indicating impaired respiratory function (Table 2). Treatment of mdx with 1D11, losartan, or a combination of the two agents normalized Penh to values similar to those observed in wild-type mice. In addition, the F, MV, PIP, and Ti parameters all improved in treated mdx mice, compared with vehicle control mice.
Treatment Improves Forelimb Grip strength
The effect of 1D11 on forelimb grip strength (n = 25) was compared with other treatment modalities in mice treated from 2 weeks up to 2 months of age: losartan (n = 16), enalapril (n = 8), 1D11 coadministered with losartan (n = 9), and the nonspecific antibody treatment control, 13C4 (n = 8). In addition the grip strength of mice in the 9-month study was measured at 7 and 9 months of age (n = 8 or 9). The results from both studies are presented in Figure 3A (normalized to body weight) and Figure 3B (absolute force in kilograms). 1D11 significantly improved grip strength relative to mdx mice treated with vehicle, losartan, or the control antibody 13C4 at all time points. Grip strength in mice treated with the control antibody 13C4 was not different from vehicle control mdx mice. The grip strength of losartan-treated mdx mice was significantly different from that of vehicle control only in 2-month-old treated mice, and no improvement in grip strength was observed in mdx mice treated with enalapril. The coadministration of 1D11 and losartan did not improve grip strength more than 1D11 alone.
Figure 3.
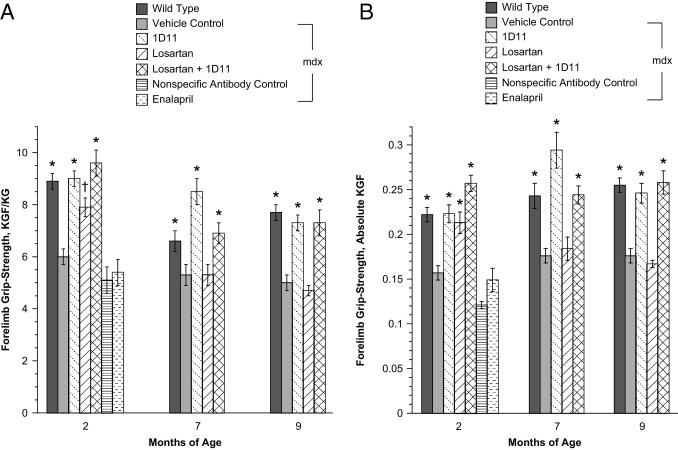
Forelimb grip strength was improved by treatment with 1D11. A: Grip-strength normalized to body weight values improved after treatment in 2-month-old mdx mice that had been treated from 2 weeks to 9 months of age with 1D11 (n = 24), losartan (n = 16), 1D11 + losartan (n = 8), compared with vehicle (n = 24) or nonspecific control antibody 13C4 (n = 8). Enalapril-treated mdx mice (n = 8) were not different from vehicle control at the 2-month time point. A wild-type group (n = 26) was also included. Forelimb grip-strength data were similarly affected in 7-month-old mice treated for 6.5 months, and 9-month-old mice treated for 8.5 months with 1D11 and a combination of 1D11 + losartan (n = 9). Losartan had no effect on grip strength at the 7-month or 9-month time points (n = 9). For wild-type and vehicle control groups at 7 and 9 months there were 8 mice per group. B: The data shown in A of this figure depicted as absolute kilogram force with no correction for body weight. Statistical analyses were by analysis of variance followed by Tukey's multiple comparison test. *P < 0.0001 versus vehicle control; †P < 0.02 versus 1D11.
Myogenin Expression Is Increased in Skeletal Muscle from Drug-Treated mdx Mice
TGF-β is known to inhibit satellite cell differentiation and fusion in vitro23,24 and the addition of TGF-β1 to C2C12 cell culture media decreased expression of myosin heavy chain and reduced expression of myogenin mRNA in a dose-dependent manner (see Supplemental Figure S2, A and B, at http://ajp.amjpathol.org). Addition of 1D11 or angiotensin II to the culture at concentrations of 450 ng/mL or 100 ng/mL, respectively, did not affect the kinetics of myotube formation under the same conditions (data not shown).
The number of myogenin-positive nuclei in soleus muscle of treated mdx mice was tallied, to quantitate the process of myofiber regeneration. Representative images of soleus muscle from wild-type, 1D11-treated, and vehicle control mdx mice are shown in Figure 4, A–C. Negative controls (rat heart alone) and positive controls (sections of rat heart injected with skeletal muscle myoblasts) demonstrated the specificity of this method for activated skeletal muscle myoblasts (Figure 4, D and E). Additionally, the number of myogenin-positive satellite cells increased 6.9-fold in diaphragm and 2.1-fold in soleus muscles from mdx mice, compared with those from wild-type mice (Figure 5). Mice treated with 1D11 had more myogenin-positive nuclei in diaphragm and soleus muscle than did vehicle-treated mdx mice (2.1-fold and 2.8-fold, respectively). A smaller increase in the number of activated satellite cells was observed in losartan-treated mdx mice, compared with those treated with 1D11 (1.6-fold in both muscles). Similar findings were observed in the 2-month study (data not shown).
Figure 4.
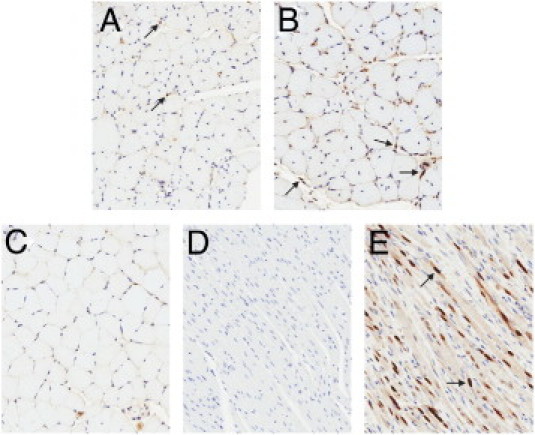
Myogenin immunostained skeletal muscle. Representative digital scans of myogenin immunostained soleus muscle sections from mdx mice treated from 2 weeks to 9 months of age with vehicle (A), 1D11 (B), or vehicle-treated wild-type mice (C), along with negative controls (rat heart, D) and positive controls (rat heart injected with rat skeletal muscle myocytes, E). Arrows indicate myogenin positive nuclei. Original magnification, ×20.
Figure 5.
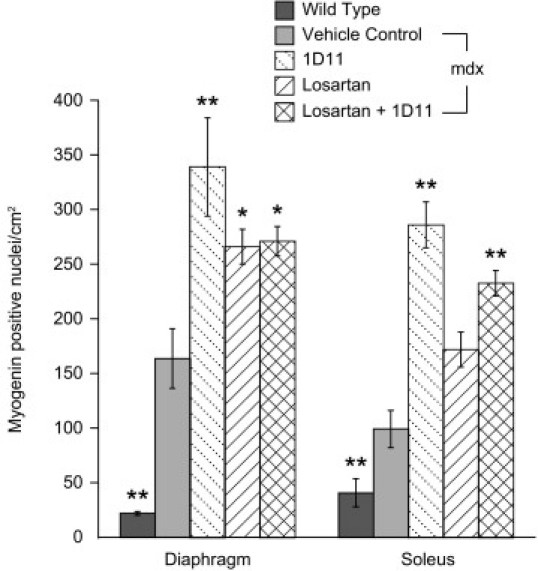
1D11 treatment increases the number of activated satellite cells in mdx mice. The numbers of myogenin-positive nuclei in diaphragm and soleus muscles treated from 2 weeks to 9 months of age with 1D11 (n = 9) were significantly greater than those from vehicle-treated mdx mice (n = 8). In mice treated with losartan alone or in combination with 1D11 (n = 9, each group), the number of myogenin-positive satellite cells was also increased, but not as much as with 1D11 alone. Data are reported as means ± SE. Statistical analyses were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.001 and **P < 0.01 versus vehicle control.
Muscle Fiber Density Is Increased in Diaphragm of Drug-Treated mdx Mice
Morphometric analysis was performed on cryosections of diaphragm muscle, stained with wheat germ agglutinin, from 9-month-old mice. The cross-sectional area, fiber diameter, fiber breadth, and the number of centrally nucleated fibers were determined in diaphragm muscle. Histograms of the fiber size distribution illustrate the increase in the total number of fibers of all sizes in drug-treated mice (Figure 6). The total cross-sectional area made up of muscle fibers was significantly increased by 43% after 1D11 treatment (P < 0.05), suggesting that there were more muscle fibers, less fibrosis, and less interstitial space, compared with the vehicle control groups (see Supplemental Figures S3 and S4 at http://ajp.amjpathol.org). There was also an increase in the total number of centrally nucleated fibers with 1D11 and combination treatment per unit area (data not shown).
Figure 6.
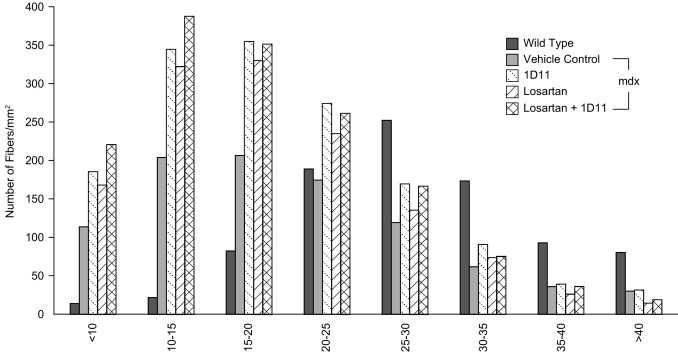
The density of myofibers is increased in diaphragm muscle from mdx mice treated with 1D11 or losartan. Cryosections from diaphragm muscle were stained with Alexa Fluor 488-labeled wheat germ agglutinin to visualize the sarcolemma and with DAPI to visualize the nuclei. Fibers were quantitated by their size distribution in microns (horizontal axis) using image analysis software. Data are reported as means. Statistical analyses were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.01; n = 8 per group.
Discussion
TGF-β1 and TGF-β3 mRNA levels in the skeletal muscle of mdx mice were elevated, compared with their wild-type counterparts. Consistent with the increase in TGF-β1 mRNA levels, total TGF-β1 protein levels were also elevated in quadriceps muscle homogenates from mdx mice, compared with wild-type mice. Levels of activated TGF-β1 were below the limit of detection of the ELISA. This was not surprising, because TGF-β is secreted from cells in its latent form.25 For this reason, we measured the levels of periostin, a well-characterized downstream marker of TGF-β activity. Periostin levels are transcriptionally elevated in response to TGF-β in cells from bone,26 kidney,27 lung,28 and heart.29 We also observed significant elevations in periostin mRNA in skeletal muscle and diaphragm from mdx mice, compared with wild-type mice, that were attenuated in 2-month-old mice treated with 1D11, losartan, or a combination of the two agents, suggesting that a dose sufficient for reducing TGF-β in skeletal muscle was used.
Weakening of the diaphragm and intercostal muscles leads to impaired respiratory function in DMD patients and is the cause of more than 70% of patient fatalities. In DMD patients, the skeletal muscles also weaken progressively, leading to a loss of ambulation by 8 years of age. In mdx mice, however, most of the skeletal muscle pathology is comparatively mild and appears to plateau after 3 months of age. In contrast, the diaphragm muscle is more severely and progressively affected in mdx mice and thus is more like the muscles in DMD patients.30 Whole-body plethysmography has been used to demonstrate impaired respiratory function in mdx mice, and this impairment was related to the fibrotic condition of the diaphragm muscle.31 We also observed increased fibrosis in diaphragm muscle of mdx mice relative to wild-type mice, which decreased significantly after treatment with 1D11.
Using whole-body plethysmography, we demonstrated that neutralizing all three isoforms of TGF-β with 1D11 or through use of losartan normalizes respiratory function in 9-month-old mdx mice. Respiratory dysfunction in mdx mice was related primarily to changes in the inhalation phase of the respiratory cycle, as evidenced by decreased peak inspiratory flow and increased inspiration time, and is consistent with pathology seen in the diaphragm. The respiratory parameters Penh, breathing frequency, peak inspiratory flow, inspiration time, and minute volume were significantly improved in 9-month-old mdx mice treated with either 1D11 or losartan. In other studies, response to hypercapnia was not different in 5-month-old mdx mice,32 nor were basal Penh values in 7-month-old mdx mice measurably different from wild-type mice.31,33 By 16 months of age, however, mdx mice showed failed respiratory compensation under hypercapnia.32 Thus, it is likely that the worsening basal respiratory dysfunction becomes sufficiently severe by 9 months of age (when we conducted our studies) for detection by plethysmography.
TGF-β has been linked to failed regeneration of skeletal muscle and an exacerbation of the disease phenotype in models of muscular dystrophy.7 TGF-β inhibits skeletal muscle myoblast differentiation23,34–36 by altering the signaling of SMAD3,37 a signal transduction protein that directly inhibits the transcription of the myogenic regulators myogenin and MyoD.38 In addition, myoblasts transfected with decorin, an inhibitor of TGF-β, show enhanced myotube formation, as well as an up-regulated expression of MyoD and myogenin.39 Myogenin is specifically expressed in skeletal muscle myoblasts that are differentiated and ready to fuse to form muscle fibers.40,41 Thus, myogenin is a good marker of the regenerative activity of skeletal muscle. A twofold increase in the number of myogenin-positive nuclei and an increase in the number of centrally nucleated fibers were noted in diaphragm muscle from 1D11-treated mdx mice, compared with vehicle-treated mdx mice. This suggests that there were more activated myoblasts in muscle when TGF-β signaling was attenuated with 1D11 treatment. We also observed an increase in the number of fibers per unit area and a decrease in fibrosis in treated mdx mice, findings that are consistent with improved regeneration.
Losartan, an antagonist of the angiotensin II type I receptor is a vasodilator used to treat hypertension; it is also known to decrease TGF-β signaling. TGF-β is secreted from muscle, fibroblasts, and inflammatory cells as a latent complex that is proteolytically activated by thrombospondin-1, which is in turn is transcriptionally up-regulated by angiotensin II.42–44 Recently, Cohn et al7 demonstrated that losartan treatment of mdx mice reduced muscle fibrosis and increased hindlimb grip strength, attributing these benefits to TGF-β antagonism through a decrease in thrombospondin-1 levels. Losartan may also improve muscle function by improving blood flow by a direct effect on the vascular smooth muscle. A neutralizing antibody to TGF-β would be expected to antagonize all sources of active TGF-β, including that activated by fibrillin-1 fragments released during muscle degeneration6 and those not in the proximity of AT1 receptors, and thus may be more efficacious than AT1 antagonists in degenerative diseases. Measurement of grip strength, fibrosis, myogenin-positive nuclei, and periostin levels all suggested that 1D11 was more effective than losartan. In a recent study, Spurney et al20 showed, using the same dose of losartan reported here, a significantly decreased cardiac muscle fibrosis after 6 months of treatment with no change in forelimb grip strength, rotarod, or behavioral measurements. We also saw only transient improvements in grip strength in losartan-treated 2-month-old mice and no improvement of grip strength after further treatment to an age of 7 or 9 months old. However, both 1D11 and losartan were equally effective at correcting respiratory function and improving muscle integrity, as indicated by a 50% decrease in the levels of serum creatine kinase, although others have not seen changes in serum creatine kinase levels after losartan treatment.7 The apparent discrepancy could be due to the smaller number of mice per group used in that other study,7 coupled with the high degree of variability in creatine kinase measurements. Taken together, the data suggest that neutralization of all three isoforms of TGF-β may be more effective than antagonism of AT1 in treating some aspects of the pathology observed in the mdx mouse.
TGF-β is a pleiotrophic cytokine that has both inflammatory and anti-inflammatory effects, and so the effect of decreasing its bioavailability in a clinical context might be complex. Complete elimination of TGF-β1, as seen in knockout mice, leads to excessive inflammatory responses that lead to wasting and death by the 4 weeks of age.45 However, one would expect treatment of mdx mice with a TGF-β-neutralizing antibody to result in a partial reduction of the active cytokine, in part because certain locations may be inaccessible, such as the intracellular space or that bound in latent form to the inner reaches of extracellular matrix. Indeed, 1D11 neutralizes only the active form of TGF-β, and all treatments were well tolerated for 8.5 months of continuous dosing, with no evidence of increased cellular infiltrates in the mdx groups. Andreeta et al46 observed a modest twofold increase in the number of CD4-positive T-cells by immunohistochemistry (IHC) in diaphragm muscle of 3-month-old mdx mice that had been treated with 1D11 for 6 weeks, and it was suggested that long-term neutralization of TGF-β may lead to increased skeletal muscle inflammation. In the present study, CD4 mRNA was elevated in all mdx mice by 2 months of age, compared with wild-type mice, regardless of treatment: approximately 13-fold elevation in the quadriceps and approximately fourfold in the diaphragm muscles. By 9 months of age, CD4 mRNA levels in the quadriceps were dramatically reduced in all mdx groups to levels below that found in wild-type mice, but continued to be elevated in mdx diaphragm, compared with nondiseased mice (threefold to fourfold), again not affected by 1D11 treatment. We can only speculate that the discrepancy in our CD4 mRNA levels versus the cellular infiltrates observed by Andreeta et al46 might be due to tissue transcript levels not directly reflecting the number of cells in the tissue. Furthermore, we have no information as to the phenotype of the CD4 cells in the Andreeta et al study,46 nor how the quantities compare to that which might have been found in mice treated with a positive control, because no such positive control was reported. A fuller understanding of any potential immunological consequence resulting from chronic TGF-β reduction in the setting of muscular dystrophy must await analyses performed in severe animal models as part of studies designed to evaluate immunological function.
Treatment of rag2 knockout mice with 1D11 has been associated with the formation of esophageal lesions,47 leading to rapid weight loss and morbidity after 3 months of dosing. In the present studies, 1D11 was well tolerated at the same dose and dosing regimen used in the rag2 study after 8.5 months of continuous dosing, with no clinical manifestations or weight loss observed. It is possible that neutralizing antibodies to TGF-β are better tolerated when there is an excess of TGF-β present to complex and clear the antibody, as would be the case for the mdx but not the rag2 mouse. Additionally, levels of activated TGF-β may vary in different genetic backgrounds, which may influence the relevance of 1D11 treatment in any particular mouse strain.48 In conclusion, these studies support further investigation of therapeutic interventions to decrease TGF-β signaling in degenerative muscular dystrophies.
Acknowledgments
We thank John M. McPherson, Steven R. Ledbetter, Katherine W. Klinger, Beth L. Thurberg, Robert H. Barker, and Scott M. Lonning for their valuable guidance during the conduct of these studies; Leah M. Curtin, for diligent management of the breeding colonies of mdx mice; Brian P. Delgiudice, for graphically rendering the figure and photograph files; and John Lydon for refining and conducting the myogenin immunostaining procedure.
Footnotes
Supported by Genzyme Corporation.
Disclosures: All authors except for L.C. have stock options, and all are employed by Genzyme.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.02.024.
Supplementary data
Transcripts of mRNA for CD4. The amount of CD4 mRNA from quadriceps or diaphragm was measured and normalized to wild-type levels. The mdx mice were treated from 2 weeks to 2 months of age or from 2 weeks of age to 9 months of age with 1D11, losartan, or a combination of the two agents. Positive controls were mice treated with MOPC 21 containing 1% BSA. mRNA for CD4 from the muscles of these mice was elevated significantly in both quadriceps and diaphragm relative to vehicle control mdx (P < 0.01). Data are presented as means ± SE. Statistical comparisons were by analysis of variance followed by Dunnett's multiple comparison test against vehicle-treated mdx mice.
TGF-β inhibits myofiber formation in C2C12 cells. TGF-β was added to cultures 70% confluent C2C12 cells with differentiation medium and again 2 days later. Measurements of myosin heavy chain and myogenin mRNA were made 5 days after the initiation of differentiation. A: TGF-β inhibited myofiber formation as assessed by myosin heavy chain expression. B: TGF-β inhibited the expression of myogenin mRNA expression in C2C12 cells in a dose-dependent manner. Data are reported as means ± SE. Statistical analyses were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.05. C and D: Representative images of myotubes stained for myosin heavy chain in the absence (C) and presence (D) of TGF-β (2 ng/mL). Original magnification ×10.
Cryosections from diaphragm muscle from mice treated for 8.5 months were stained with Alexa Fluor 488-labeled wheat germ agglutinin to visualize the sarcolemma and with DAPI to visualize the nuclei. Representative images are shown for wild-type mice (A), vehicle-treated mdx mice (B), and 1D11-treated mdx mice (C). Original magnification ×10.
The number of myofibers per unit area increased in treated mice compared with vehicle control mdx mice. Cryosections were stained as described for Figure 4 and were quantitated using imaging software. Data are reported as means ± SE. Statistical comparisons were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.05.
References
- 1.Koenig M., Hoffman E.P., Bertelson C.J., Monaco A.P., Feener C., Kunkel L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman E.P., Brown R.H., Jr, Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Ibraghimov-Beskrovnaya O., Ervasti J.M., Leveille C.J., Slaughter C.A., Sernett S.W., Campbell K.P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 4.Rando T.A. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24:1575–1594. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- 5.Niebroj-Dobosz I., Fidziańska A., Hausmanowa-Petrusewicz I. Controversies about the function of dystrophin in muscle. Folia Neuropathol. 2001;39:253–258. [PubMed] [Google Scholar]
- 6.Chaudhry S.S., Cain S.A., Morgan A., Dallas S.L., Shuttleworth C.A., Kielty C.M. Fibrillin-1 regulates the bioavailability of TGFbeta1. J Cell Biol. 2007;176:355–367. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn R.D., van Erp C., Habashi J.P., Soleimani A.A., Klein E.C., Lisi M.T., Gamradt M., ap Rhys C.M., Holm T.M., Loeys B.L., Ramirez F., Judge D.P., Ward C.W., Dietz H.C. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [Erratum appeared in Nat Med 2007, 13:511] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Foster W., Deasy B.M., Chan Y., Prisk V., Tang Y., Cummins J., Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strockbine N.A., Marques L.R., Holmes R.K., O'Brien A.D. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985;50:695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasch J.R., Pace D.R., Waegell W., Inenaga D., Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta: Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989;142:1536–1541. [PubMed] [Google Scholar]
- 11.Lin C.C. Noninvasive method to measure airway obstruction in nonanesthetized allergen-sensitized and challenged mice. Respiration. 2001;68:178–185. doi: 10.1159/000050489. [DOI] [PubMed] [Google Scholar]
- 12.Khurana T.S. TREAT-NMD Neuromuscular Network SOP M.2.2_002. Wellstone Muscular Dystrophy Center; Washington, DC: 2008. Respiratory System Evaluation. [Google Scholar]
- 13.Connolly A.M., Keeling R.M., Mehta S., Pestronk A., Sanes J.R. Three mouse models of muscular dystrophy: the natural history of strength and fatigue in dystrophin-, dystrophin/utrophin-, and laminin alpha2-deficient mice. Neuromuscular Disord. 2001;11:703–712. doi: 10.1016/s0960-8966(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 14.Luca A.D. TREAT-NMD Neuromuscular Network SOP M.2.2_001. Wellstone Muscular Dystrophy Center; Washington, DC: 2008. Use of Grip Strength Meter to Assess the Limb Strength of mdx Mice. [Google Scholar]
- 15.Dubache-Powell J. TREAT-NMD Neuromuscular Network SOP M.2.2_001. Wellstone Muscular Dystrophy Center; Washington, DC: 2008. Quantitative Determination of Muscle Fiber Diameter (Minimal Feret's Diameter) and Percentage of Centralized Nuclei. [Google Scholar]
- 16.Reddy G.K., Enwemeka C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L., Porter J.D., Cheng G., Gong B., Hatala D.A., Merriam A.P., Zhou X., Rafael J.A., Kaminski H.J. Temporal and spatial mRNA expression patterns of TGF-beta1, 2, 3 and TbetaRI, II, III in skeletal muscles of mdx mice. Neuromuscul Disord. 2006;16:32–38. doi: 10.1016/j.nmd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Rapoza M.L., Fu D., Sendak R.A. Development of an in vitro potency assay for therapeutic TGFbeta antagonists: the A549 cell bioassay. J Immunol Methods. 2006;316:18–26. doi: 10.1016/j.jim.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Yu L., Border W.A., Anderson I., McCourt M., Huang Y., Noble N.A. Combining TGF-beta inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int. 2004;66:1774–1784. doi: 10.1111/j.1523-1755.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 20.Spurney C.F., Sali A., Guerron A.D., Iantorno M., Yu Q., Gordish-Dressman H., Rayavarapu S., van der Meulen J., Hoffman E.P., Nagarju K. Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin deficient mdx mice. J Cardiovasc Pharmacol Ther. 2011;16:87–95. doi: 10.1177/1074248410381757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu A.H., Perryman M.B. Clinical applications of muscle enzymes and proteins. Curr Opin Rheumatol. 1992;4:815–820. [PubMed] [Google Scholar]
- 22.Pauluhn J. Comparative assessment of early acute lung injury in mice and rats exposed to 1,6-hexamethylene diisocyanate-polyisocyanate aerosols. Toxicology. 2008;247:33–45. doi: 10.1016/j.tox.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Allen R.E., Boxhorn L. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol. 1987;133:567–572. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- 24.Stewart J.D., Masi T.L., Cumming A.E., Molnar G.M., Wentworth B.M., Sampath K., McPherson J.M., Yaeger P.C. Characterization of proliferating human skeletal muscle-derived cells in vitro: differential modulation of myoblast markers by TGF-beta2. J Cell Physiol. 2003;196:70–78. doi: 10.1002/jcp.10322. [DOI] [PubMed] [Google Scholar]
- 25.Rifkin D.B., Dabovic B. TGF-beta bioavailability, latency, targeting, and activation. In: Derynck R., Miyazono K., editors. The TGF-beta Family. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. pp. 179–202. [Google Scholar]
- 26.Horiuchi K., Amizuka N., Takeshita S., Takamatsu S., Katsuura M., Ozawa H., Toyama Y., Bonewald L., Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 27.Wallace D.P., Quante M.T., Reif G.A., Nivens E., Ahmed F., Hempson S.J., Blanco G., Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol. 2008;295:F1463–F1471. doi: 10.1152/ajprenal.90266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard C., Mingler M.K., McBride M., Putnam P.E., Collins M.H., Chang G., Stringer K., Abonia J.P., Molkentin J.D., Rothenberg M.E. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y.F., Feng J.A., Li P., Xing D., Zhang Y., Serra R., Ambalavanan N., Majid-Hassan E., Oparil S. Dominant negative mutation of the TGF-beta receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol. 2006;100:564–571. doi: 10.1152/japplphysiol.00595.2005. [DOI] [PubMed] [Google Scholar]
- 30.Stedman H.H., Sweeney H.L., Shrager J.B., Maguire H.C., Panettieri R.A., Petrof B., Narusawa M., Leferovich J.M., Sladky J.T., Kelly A.M. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 31.Ishizaki M., Suga T., Kimura E., Shiota T., Kawano R., Uchida Y., Uchino K., Yamashita S., Maeda Y., Uchino M. Mdx respiratory impairment following fibrosis of the diaphragm. Neuromuscul Disord. 2008;18:342–348. doi: 10.1016/j.nmd.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Gayraud J., Matecki S., Hnia K., Mornet D., Prefaut C., Mercier J., Michel A., Ramonatxo M. Ventilation during air breathing and in response to hypercapnia in 5 and 16 month-old mdx and C57 mice. J Muscle Res Cell Motil. 2007;28:29–37. doi: 10.1007/s10974-007-9101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosselin L.E., Barkley J.E., Spencer M.J., McCormick K.M., Farkas G.A. Ventilatory dysfunction in mdx mice: impact of tumor necrosis factor-alpha deletion. Muscle Nerve. 2003;28:336–343. doi: 10.1002/mus.10431. [DOI] [PubMed] [Google Scholar]
- 34.Schabort E.J., van der Merwe M., Loos B., Moore F.P., Niesler C.U. TGF-beta's delay skeletal muscle progenitor cell differentiation in an isoform-independent manner. Exp Cell Res. 2009;315:373–384. doi: 10.1016/j.yexcr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Li X., McFarland D.C., Velleman S.G. Effect of Smad3-mediated transforming growth factor-beta1 signaling on satellite cell proliferation and differentiation in chickens. Poult Sci. 2008;87:1823–1833. doi: 10.3382/ps.2008-00133. [DOI] [PubMed] [Google Scholar]
- 36.Vaidya T., Rhodes S., Taparowsky E., Konieczny S. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989;9:3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D., Black B., Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–2966. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin J.F., Li L., Olson E.N. Repression of myogenin function by TGF-beta 1 is targeted at the basic helix-loop-helix motif and is independent of E2A products. J Biol Chem. 1992;267:10956–10960. [PubMed] [Google Scholar]
- 39.Li Y., Li J., Zhu J., Sun B., Branca M., Tang Y., Foster W., Xiao X., Huard J. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther. 2007;15:1616–1622. doi: 10.1038/sj.mt.6300250. [DOI] [PubMed] [Google Scholar]
- 40.Heron-Milhavet L., Mamaeva D., LeRoith D., Lamb N.J., Fernandez A. Impaired muscle regeneration and myoblast differentiation in mice with a muscle-specific KO of IGF-IR. J Cell Physiol. 2010;225:1–6. doi: 10.1002/jcp.22218. [DOI] [PubMed] [Google Scholar]
- 41.Yen Y.P., Tsai K.S., Chen Y.W., Huang C.F., Yang R.S., Liu S.H. Arsenic inhibits myogenic differentiation and muscle regeneration. Environ Health Perspect. 2010;118:949–956. doi: 10.1289/ehp.0901525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer J.W., Stoll M., Hahn A.W., Unger T. Differential regulation of thrombospondin-1 and fibronectin by angiotensin II receptor subtypes in cultured endothelial cells. Cardiovasc Res. 2001;51:784–791. doi: 10.1016/s0008-6363(01)00345-5. [DOI] [PubMed] [Google Scholar]
- 43.Chua C.C., Hamdy R.C., Chua B.H. Regulation of thrombospondin-1 production by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta. 1997;1357:209–214. doi: 10.1016/s0167-4889(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 44.Scott-Burden T., Resink T.J., Hahn A.W., Bühler F.R. Induction of thrombospondin expression in vascular smooth muscle cells by angiotensin II. J Cardiovasc Pharmacol. 1990;16(Suppl 7):S17–S20. [PubMed] [Google Scholar]
- 45.Kulkarni A.B., Huh C.G., Becker D., Geiser A., Lyght M., Flanders K.C., Roberts A.B., Sporn M.B., Ward J.M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andreetta F., Bernasconi P., Baggi F., Ferro P., Oliva L., Arnoldi E., Cornelio F., Mantegazza R., Confalonieri P. Immunomodulation of TGF-beta1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. J Neuroimmunol. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Vitsky A., Waire J., Pawliuk R., Bond A., Matthews D., LaCasse E., Hawes M.L., Nelson C., Richards S., Piepenhagen P.A., Garman R.D., Andrews L., Thurberg B.L., Lonning S., Ledbetter S., Ruzek M.C. Homeostatic role of transforming growth factor-beta in the oral cavity and esophagus of mice and its expression by mast cells in these tissues. Am J Pathol. 2009;174:2137–2149. doi: 10.2353/ajpath.2009.080723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heydemann A., Ceco E., Lim J.E., Hadhazy M., Ryder P., Moran J.L., Beier D.R., Palmer A.A., McNally E.M. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice [Erratum appeared in J Clin Invest 2010, 120:645] J Clin Invest. 2009;119:3703–3712. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transcripts of mRNA for CD4. The amount of CD4 mRNA from quadriceps or diaphragm was measured and normalized to wild-type levels. The mdx mice were treated from 2 weeks to 2 months of age or from 2 weeks of age to 9 months of age with 1D11, losartan, or a combination of the two agents. Positive controls were mice treated with MOPC 21 containing 1% BSA. mRNA for CD4 from the muscles of these mice was elevated significantly in both quadriceps and diaphragm relative to vehicle control mdx (P < 0.01). Data are presented as means ± SE. Statistical comparisons were by analysis of variance followed by Dunnett's multiple comparison test against vehicle-treated mdx mice.
TGF-β inhibits myofiber formation in C2C12 cells. TGF-β was added to cultures 70% confluent C2C12 cells with differentiation medium and again 2 days later. Measurements of myosin heavy chain and myogenin mRNA were made 5 days after the initiation of differentiation. A: TGF-β inhibited myofiber formation as assessed by myosin heavy chain expression. B: TGF-β inhibited the expression of myogenin mRNA expression in C2C12 cells in a dose-dependent manner. Data are reported as means ± SE. Statistical analyses were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.05. C and D: Representative images of myotubes stained for myosin heavy chain in the absence (C) and presence (D) of TGF-β (2 ng/mL). Original magnification ×10.
Cryosections from diaphragm muscle from mice treated for 8.5 months were stained with Alexa Fluor 488-labeled wheat germ agglutinin to visualize the sarcolemma and with DAPI to visualize the nuclei. Representative images are shown for wild-type mice (A), vehicle-treated mdx mice (B), and 1D11-treated mdx mice (C). Original magnification ×10.
The number of myofibers per unit area increased in treated mice compared with vehicle control mdx mice. Cryosections were stained as described for Figure 4 and were quantitated using imaging software. Data are reported as means ± SE. Statistical comparisons were by analysis of variance followed by Dunnett's multiple comparison test relative to vehicle control. *P < 0.05.


