Abstract
Inflamed skin contains CD4 T-cell subsets that express chemokine receptors CCR4, CCR6, and/or CCR10. Prior attempts to reveal the distinct role(s) of each receptor in T-cell trafficking to skin have not produced a coherent story. Different conclusions drawn by separate research groups are difficult to reconcile because of the disparate inflammation models used. Here we directly compare CD4 T cells from wild-type, CCR4−/−, CCR6−/−, and CCR10−/− mice in parallel assays of trafficking to skin. Our models require direct competition between wild-type and receptor-deficient populations for access to inflamed cutaneous sites. Major histocompatibility complex-peptide tetramers allowed us to identify antigen-specific endogenous long-term memory CD4 T cells within skin after multiple topical immunizations. We separately analyzed cells from the dermal and epidermal layers, allowing us to assess the involvement of each receptor in trafficking between dermis and epidermis. We found that CCR4 deficiency reduces accumulation of memory CD4 T cells in skin by approximately 20-fold, but neither CCR6 nor CCR10 deficiency yielded any detectable effects. Strikingly, no differences in dermal versus epidermal localization were observed for cells lacking any of these three receptors. Our findings raise the possibility that CCR6 and CCR10 play (as yet) unknown roles in cutaneous T-cell immunology, unrelated to skin-specific trafficking.
Our current understanding of T-lymphocyte trafficking suggests that the circulating T-cell pool contains multiple antigen-experienced subsets bearing distinct tissue tropisms. The two best understood are those associated with skin or intestine. Together, these two populations comprise at least half of all blood-borne antigen-experienced CD4 T cells.1 Each subset is responsible for immunological memory and immunosurveillance of its own target tissue. In both mice and human beings, skin-homing cells express E-selectin ligand (E-lig), whereas intestine-homing cells express integrin α4β7.1 Each of these adhesion molecules is required for normal homing of each cell type to its respective target organ.2 Both regulatory T cells and memory/effector CD4 T cells appear to use these same tissue-specific homing mechanisms.3–6
In concert with adhesion molecules, CCRs also appear to play an important role in tissue-specific T-cell homing. One such receptor, CCR9, is clearly required for homing of α4β7+ T cells to the small intestine.7,8 In the skin, three different chemokine receptors have been put forward as mediators of T-cell homing during inflammation. These are CCR4, CCR6, and CCR10.9–11 T-cell homing to skin can be broken up into steps, including extravasation through dermal venules from blood, and migration from dermis to epidermis.12
CCR6 has been proposed as a skin-associated T-cell homing molecule because of its expression by some E-lig+ T cells in the blood.11 However, although CCR6 is indeed expressed by a large proportion of skin-resident CD4 T cells,13 it also similarly is expressed by those residing in lung,14 liver,15 and intestine.16 Thus, if CCR6 truly does play a role in cutaneous immunology, it is also likely to play similar roles in other tissues. Nonetheless, the potential role of CCR6 in cutaneous homing has not yet been formally confirmed or dismissed in vivo.
Chemokine receptors CCR4 and CCR10 are closely associated with the E-lig+ skin-homing CD4 T-cell population.9,10 However, the specific contribution of each receptor to skin-specific T-cell trafficking is not yet clear and at times has been quite controversial. One study concluded that a function-blocking monoclonal antibody (mAb) against the CCR10 ligand CCL27/CTACK could prevent T-cell homing to skin in CCR4-deficient mice, but not in wild-type (WT) mice.17 This suggested a functional redundancy between CCR4 and CCR10 (ie, no effects were detected unless both CCR4 and CCR10 signaling were disrupted).
In contrast, another study concluded that the same anti-CCL27 mAb used in the earlier-described experiments was (by itself) sufficient to prevent skin-specific T-cell homing in WT mice.10 This suggested that CCR10 alone was required for T-cell trafficking to skin because no redundancy with CCR4 was evident.
Our own recent study shows a nonredundant role for CCR4 in an antigen-dependent skin-specific homing model of T-cell receptor-transgenic CD4 T cells.4 Unlike the first two studies, the latter benefits by its independence from the caveats associated with in vivo mAb injection. The use of CCR4-deficient cells allowed us to directly compare CCR4−/− and WT cells within individual animals. Such competitive assays provide an extremely sensitive assessment of individual homing molecules and their roles in tissue-specific trafficking because WT and receptor-deficient populations must compete with each other to accumulate within the tissue of interest.3,18,19
Although only one study appears to support a nonredundant role for CCR10 in T-cell homing to skin,10 circumstantial evidence continues to implicate CCR10 as an important player at some level in cutaneous trafficking. For instance, CCL27, one of the two known ligands for CCR10, is produced exclusively and constitutively by epidermal keratinocytes.20,21 Furthermore, CCR10 transcription by antigen-specific CD4 T cells is associated with exposure to 1,25-dihydroxy-vitamin-D3, a nutrient generated within sun-exposed skin.22,23
Thus, a division of labor hypothesis has been put forward to explain the evolutionary necessity for two distinct chemokine receptors in cutaneous trafficking, based on the specific anatomical site where each receptor's ligand is localized within skin (ie, the presence of CCR4 ligand on dermal endothelial lumen, and the expression of CCR10 ligand by epidermal keratinocytes1,17,24). This hypothesis proposes that CCR4 facilitates entry of T cells into skin from blood, whereas CCR10 controls their subsequent migration from dermis to epidermis.
To begin resolving inconsistencies among previous skin-homing studies, we have directly compared CD4 T cells from WT, CCR4−/−, CCR6−/−, and CCR10−/− mice in side-by-side assays under identical in vivo conditions. We have taken two complementary in vivo approaches. The first assesses the contribution of each of the 3 receptors to antigen-dependent accumulation of long-term memory endogenous T cells within skin. This was achieved through a recently developed major histocompatibility complex (MHC) class II tetramer system that identifies endogenous antigen-specific CD4 T cells.25 The second approach assessed the requirement for each receptor in blood-to-dermis and dermis-to-epidermis migration, using an OT-II adoptive transfer system and calculating antigen-specific accumulation of T cells separately within dermis versus epidermis.
Materials and Methods
Mice
C57Bl/6 mice (CD45.2+) and their CD45.1+ congenic counterparts were purchased from Charles River Labs (Wilmington, MA). CCR4−/−, CCR6−/−, and CCR10−/− mice were highly backcrossed (>12×) onto the C57Bl/6 background. CCR6-deficient mice were a generous gift from Sergio A. Lira (Mt. Sinai Hospital, New York, NY), and CCR10-EGFP function-disrupting knock-in mice were a generous gift from Craig Gerard and Olivier Morteau (Childrens Hospital Boston, Boston, MA). It should be noted that the non-specific EGFP signal found in early examples of mice bearing the CCR10-EGFP construct26 was essentially eliminated after this extensive backcrossing (J.J.C., unpublished data). The OT-II version of each strain described earlier was generated by directly mating homozygous OT-II mice with homozygous CCR knockouts (both already on the C57Bl/6 background), and then mating together the F1 progeny, from which one in eight offspring were homozygous for both loci. Creating the OT-II/CCR10−/− combination was more labor intensive because the OT-II construct apparently was inserted within the chromosome containing the CCR10 locus. Thus, a crossover event was required that occurred in only one in approximately 90 offspring.
Creation of Bone Marrow Chimeras
Bone marrow (BM) chimeras were generated as described by Baekkevold et al.3 Briefly, BM-derived cells from each donor were mixed at a 1:1 ratio in combinations denoted in the text and figures. Recipient mice (F1 CD45.1/CD45.2 heterozygous mice, 6 to 8 weeks) were lethally irradiated with 2 × 600 rads from a cesium-137 source irradiator. Recipient mice were anesthetized with ketamine/xylazine, and received 2 × 106 BM-derived cells (1 × 106 from each donor) via a retro-orbital injection. Mice were given Sulfatrim (Alpharma USDP, Inc., Baltimore, MD) in drinking water for 3 weeks after irradiation. Bone marrow chimerism was confirmed via flow cytometric analysis10 of peripheral blood 6 weeks after reconstitution.
OT-II Adoptive Transfer
Splenocytes from OT-II or CCR−/−/OT-II were adoptively transferred i.v. as described by Campbell et al,4 except that fewer cells were transferred (5 × 106 total splenocytes) because this lower number gave similar results but required sacrifice of fewer donor mice (N.J.T., unpublished observations).
Topical Ear Skin Immunization
Topical immunization of ear skin after tape stripping was performed exactly as described by Baekkevold et al3 and Campbell et al.4 The peptide antigen was either 2W1S (AWGALANWAVDS) or OVA323-339 (ISQAVHAAHAEINEAGR), both purchased form Biomatik, Inc. (Markham, ON, Canada). The adjuvant (cholera toxin) was purchased from List Biological Labs, Inc. (Campbell, CA).
Isolation of Cells from Intact Skin
Ears were harvested from three to five mice per condition. Before ear removal, euthanized animals were flushed with 5 mL PBS via cardiac puncture, to flush blood from peripheral vessels. Ears then were excised, and dorsal and ventral sides were separated and minced using scalpel blades. Pooled skin pieces then were put into a 50-mL Erlenmeyer flask containing 20 mL HBSS + 10 mmol/L HEPES + 2 mmol/L EDTA (Gibco/Invitrogen, Grand Island, NY) and gently agitated with a stir bar at 4°C for 4 hours. The resulting cell solution was passed through a 40-μm filter (to remove any excess hair particles), washed twice with PBS + 10% bovine serum (Gibco/Invitrogen), and stained for flow cytometry.
Separation of Dermis and Epidermis
Ears were harvested from three to five mice per condition, and mice were flushed with 5 mL PBS as described earlier. Dorsal and ventral ear sides were separated with forceps. Dorsal ear halves were floated dermal side down in 2.0 U/mL solution of Dispase II (Roche Diagnostics, Indianapolis, IN) in PBS for 40 minutes at 37°C. Dorsal halves then were rinsed in PBS to eliminate residual enzyme. Epidermal sheets were lifted off of dorsal dermis of ears using forceps. Ventral sides were not used for dermal/epidermal separation. We found that epidermis lifted easily off of dermis whether or not the skin was inflamed, suggesting that our topical inflammation model did not disrupt the dermal/epidermal structure. Epidermal and dermal sheets were minced separately, and placed into 50-mL Erlenmeyer flasks containing 20 mL HBSS + 10 mmol/L HEPES + 2 mmol/L EDTA, and cell suspensions were prepared as for whole skin, as described earlier.
2W1S-Specific MHC Class II Tetramer
2W1S-specific MHC class II tetramer (2W1S:I-Ab) was prepared by J.B.M. with Marc Jenkins (University of Minnesota, Minneapolis, MN) as described.25
Flow Cytometry
Directly conjugated mAbs were purchased from eBioscience (La Jolla, CA) or BD Pharmingen (San Jose, CA). The 2W1S:I-Ab tetramer was a generous gift from Marc Jenkins (University of Minnesota). Staining with 2W1S:I-Ab was performed as described by Moon et al,25 that is, at room temperature for 1 hour, followed by standard mAb staining procedure on ice. Flow cytometry was performed on a BD FACS Canto (Becton Dickinson, San Jose, CA), and analyzed by FlowJo software version 8.8.6 (Treestar, Inc., Stanford, CA).
Statistics
All statistics were performed using the one-tailed or two-tailed Mann-Whitney U-tests using Prism software version 5.0a (GraphPad, Inc., La Jolla, CA).
Results
The 2W1S:I-Ab Tetramer Specifically Recognizes T Cells Bearing Cognate T-Cell Receptors within the Cutaneous CD4 Population
The MHC class II tetramer 2W1S:I-Ab was developed to identify CD4 T cells whose T-cell receptor recognizes the 2W1S peptide (AWGALANWAVDS) in the context of I-Ab.25 This population is very small yet polyclonal in unimmunized C57Bl/6 mice, and expands prodigiously after i.v. immunization with lipopolysaccharide + 2W1S.25 We tested this reagent for its ability to detect antigen-specific cells generated in our established murine model of topical ear skin immunization.3 This type of topical inflammation mimics the cytokine environment of human psoriasis, by inducing a mixed Th1 and Th17 cutaneous response.4 Ear skin was immunized three times with peptide plus adjuvant (cholera toxin) at ≥15-day intervals, and harvested for analysis 3 days after the final immunization. Some mice were immunized with cholera toxin plus a negative control peptide, OVA323-339, to generate cells responsive to an irrelevant antigen and not expected to bind the 2W1S:I-Ab tetramer.
Tetramer (TET)+ CD4 T cells (ie, 2W1S:I-Ab tetramer-binding) were rare within the CD44lo (naive) peripheral blood population from 2W1S-immunized mice (Figure 1, A and B). This is consistent with the previous finding that a total of only approximately 200 TET+ naive cells exist within a typical C57Bl/6 mouse.25 TET+ cells were approximately 25-fold more abundant in the circulating CD44hi (antigen-experienced) population, but were most abundant in the immunized skin, reaching approximately 1% of total ear-infiltrating CD4 T cells (Figure 1, A and B). TET+ CD4 T cells were exceedingly rare in mice that were immunized with the irrelevant OVA323-339 peptide in all populations tested (Figure 1B). [Note: naive (CD44lo) CD4 T cells, which are abundant in the blood, were not found among cells isolated from the ear skin, suggesting minimal blood contamination in these cell preparations (data not shown).] These data show that the 2W1S:I-Ab tetramer is sufficiently robust and specific to use as a flow cytometry reagent that reliably detects 2W1S-responsive endogenous cells in skin (Figure 1B).
Figure 1.
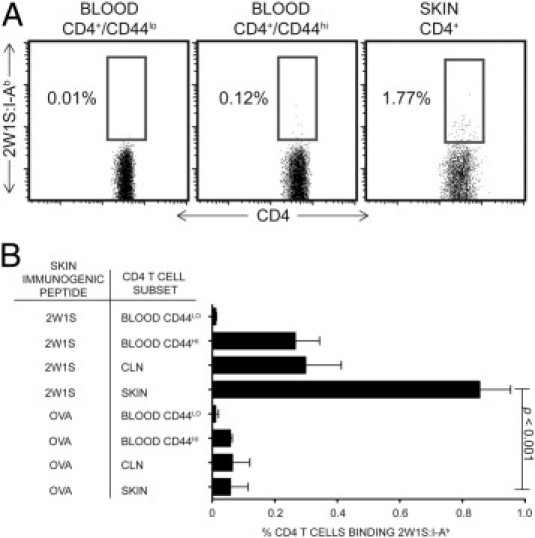
The 2W1S:I-Ab tetramer specifically recognizes T cells bearing cognate T-cell receptor within the cutaneous CD4 population by flow cytometry. A: Identification of 2W1S:I-Ab–positive CD4 T cells in CD44lo (naive) peripheral blood lymphocytes (PBL), CD44hi PBL (antigen-experienced), and skin resident populations from mice immunized topically with 2W1S peptide. Blood and cutaneous lymphocytes shown from a representative experiment. B: Quantification of 2W1S:I-Ab–expressing populations in the same CD4 T-cell populations represented in A, plus in total CD4 T cells from skin-draining (cervical) lymph node (LN) of mice immunized topically with 2W1S or OVA323-339 peptide as indicated. One-tailed Mann-Whitney test P values shown. 2W1S immunization data from 16 independent experimental groups, and OVA323-339–immunized skin data from 3 independent experimental groups (three to five mice per group).
The 2W1S:I-Ab Tetramer System Shows that CCR4, but Not CCR6 or CCR10, Plays A Vital Role in Antigen-Specific Accumulation of Memory CD4 T Cells in Skin
Competitive BM chimeras were constructed as previously described.3,18,19 Briefly, lethally irradiated F1 (CD45.1+/CD45.2+) WT recipient mice were reconstituted with equal numbers of BM cells from a WT CD45.1+ donor and a CCR-deficient CD45.2+ donor. CD45.2+ BM was derived from WT (control), CCR4−/−, CCR6−/−, CCR10+/−, or CCR10−/− donor mice. We began topical immunization with 2W1S peptide + adjuvant (as described earlier) 6 to 8 weeks after BM transfer, to allow time for full reconstitution of the immune system. The animals were sacrificed 3 days after the third immunization. Peripheral blood and skin were harvested and stained with fluorescent mAbs to determine the CD45.1:CD45.2 ratio of donor-derived cells within various CD4 T-cell populations (rare cells expressing both CD45.1 and CD45.2 were excluded from the calculations because they represented radio-resistant host-derived cells).
We adjusted for potential differences in thymic maturation and/or engraftment efficiency between donor strains by normalizing the raw CD45.1:CD45.2 ratio for each population to that of a reference population from each mouse (providing the R value described by Baekkevold et al3). The ratio within the naive CD4 T-cell population was considered an appropriate reference for this normalization because skin homing cells ultimately arise from naive (CD44lo) CD4 T cells. As described by Baekkevold et al,3 the CD45.1:CD45.2 ratio for the reference population varied only between 0.50 and 1.53 in comparison with the ideal reconstitution value of 1.00. However, we believe the R value constitutes a more useful number than the raw CD45.1:CD45.2 ratio because it eliminates this random variability from mouse to mouse. Figure 2 shows the R value for TET+ and TET− CD4 T-cell populations from 2W1S-immunized ear skin. Any ratio >1 indicates the extent to which WT CD4 T cells were more efficient than their CCR-deficient counterparts at accumulating within the inflamed skin.
Figure 2.
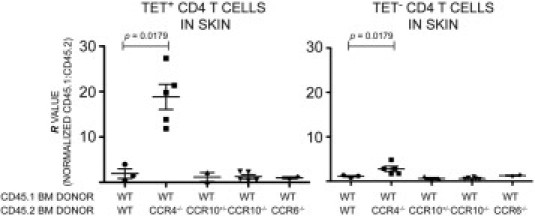
The 2W1S:I-Ab tetramer system shows that CCR4 (but not CCR6 or CCR10) plays a vital role in antigen-specific CD4 T-cell accumulation in skin. Comparison of skin-homing efficiency between donor populations in competitive BM chimeras. Normalized CD45.1:CD45.2 ratios (R) are shown for TET+ (left panel) or TET− (right panel) CD4 T cells isolated from 2W1S-immunized ear skin. (Note: R values >1 indicate disadvantage in homing for cells from receptor-deficient donors.) The CCR4−/− populations display a dramatic disadvantage when competing with WT cells in these experiments. The approximately 20-fold disadvantage in the TET+ population indicates that 95% of all ear skin CD4 T cells were derived from the WT donor. This is in contrast to the donor population, which started with a 1:1 ratio, or the CD44lo naive CD4 T population that had an R value of 1 (by definition). None of the other knockout populations showed an appreciable homing disadvantage. One-tailed Mann-Whitney P values shown. (Note: P values are identical in the Mann-Whitney U-test in both panels because there was no overlap between WT and CCR4−/− in either population, and the N values were the same.) Each data point represents pooled cells from three to five mice. N for each type of chimera: WT/WT = 3, WT/CCR4−/− = 5, WT/CCR10+/− = 2, WT/CCR10−/− = 5, WT/CCR6−/− = 2.
CCR4 deficiency caused notable effects: CCR4−/− cells were approximately 20-fold less abundant than WT cells within the TET+ population from inflamed ear skin, and approximately threefold less abundant within the TET− population. Neither CCR6−/− nor CCR10−/− donor cells displayed any significant competitive disadvantage within the TET+ or TET− populations (Figure 2).
CCR10 Is Highly Expressed by Skin-Homing CD4 T Cells despite the Absence of Sunlight
Sunlight exposure and the resulting 1,25-dihydroxy-vitamin-D3 production in skin are reported to enhance CCR10 expression by human CD4 T cells.22,23 Because of the relatively dark conditions of a typical research vivarium, we considered the possibility that WT CD4 T cells might not be induced to express CCR10 in our system. To resolve this potentially confounding issue, we used the EGFP reporter capability of the CCR10–knock-in-construct27 to assess transcriptional activity at the CCR10 locus. By design, CCR10–EGFP hemizygotes (CCR10+/−) express both the EGFP reporter and CCR10 protein. We confirmed this by observing that CD4+/EGFP+/E-lig+ cells from CCR10+/− but not CCR10−/− lymph nodes could migrate to CCL27 in standard in vitro Transwell (Corning, Inc., Corning, NY) chemotaxis assays (J.J.C., unpublished data).
We found that approximately 50% of the CCR10+/− CD4 T cells within inflamed ear skin transcribed the CCR10 locus (Figure 3), implying the presence of a functional receptor. The naive CCR10+/− CD4 T-cell population contained very few EGFP-expressing cells (Figure 3A). Exposure to antigen or to the inflamed cutaneous environment itself is likely to have induced CCR10 transcription because skin homing cells ultimately derive from naive cells that lack CCR10. EGFP expression did not vary appreciably between CCR10+/− and CCR10−/− CD4 T-cell populations. The WT CD4 T-cell population in skin lacked EGFP expression because these cells do not bear the EGFP construct (Figure 3). These data strongly suggest that CCR10 is expressed at high levels by cutaneous CD4 T cells in mice in our model, despite the finding that this receptor apparently plays no role in homing to skin.
Figure 3.
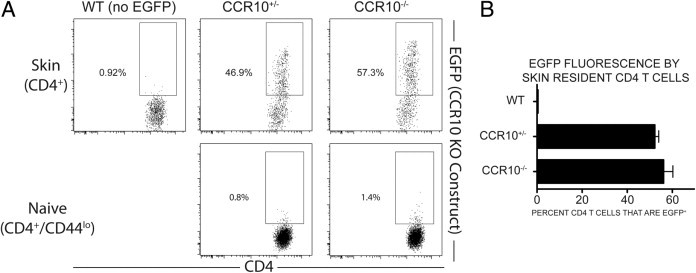
CCR10 is highly expressed by skin-homing CD4 T cells, despite the absence of sunlight. Flow cytometric identification and comparison of CCR10-promoter–dependent EGFP transcription in the BM chimeras. A: Representative examples of EGFP expression (under the CCR10 promoter) in the skin (upper dot plots) or naive LN CD4 T cells (lower dot plots). B: Quantification of CCR10-promoter–dependent EGFP fluorescence in skin resident CD4 T cells. A total of 52.25% of CCR10+/− CD4 T cells were EGFP+, compared with 56.12% of CCR10−/− CD4 T cells. N for each cell type: WT = 6; CCR10+/− = 2; CCR10−/− = 4.
CCR6 and CCR10 Do Not Provide a Competitive Advantage for Epidermal versus Dermal Accumulation
CCL27 is one of the two known ligands for CCR10, and is expressed exclusively by epidermal keratinocytes.20,21,28 It is therefore possible that a CCL27 protein gradient might emanate from the epidermis and attract CCR10+ T cells from the dermis. We tested this notion by harvesting inflamed ear skin from OT-II adoptive-transfer experiments, and separating it into its constituent layers. This was achieved by gentle digestion of the skin with Dispase II, which preferentially digests the basement membrane between epidermis and dermis.29 We then isolated T cells separately from dermis and epidermis.
We chose the OT-II adoptive transfer model for these experiments because the 2W1S:I-Ab approach could not provide sufficient numbers of TET+ cells from the separated layers to analyze reliably. We bred the CCR10–EGFP and CCR6 knockout constructs onto the OT-II background as previously accomplished for CCR4.4 We injected splenocytes from young OT-II donors i.v. into CD45-disparate recipients, and immunized ear skin topically with OVA323-339 + cholera toxin as described.4 Lymphocytes were isolated from inflamed ear skin 6 days after topical immunization.
OT-II cells isolated separately from dermis (Figure 4, middle panel) or epidermis (Figure 4, right panel) showed no appreciable differences among WT, CCR6−/−, and CCR10−/− OT-II cells in either of the two skin layers. If CCR6 or CCR10 had been involved in dermal-to-epidermal migration, one would have expected a decrease in OT-II proportion within epidermis and/or an increase in dermis because cells unable to access the epidermis might have become trapped in the dermis.
Figure 4.
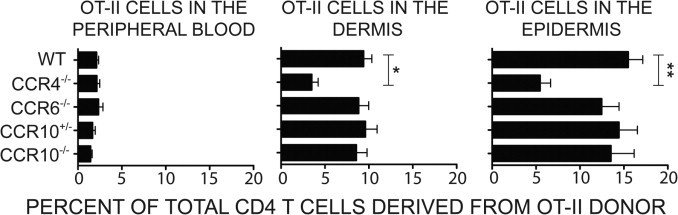
CCR6 and CCR10 do not provide a competitive advantage for epidermal versus dermal accumulation. Left panel: presence of adoptively transferred OT-II CD4 T cells in the peripheral blood after OVA323-339 ear immunization. There was no significant difference in the percentage of blood resident CD4 T cells that were OT-II donor derived between CCR4−/− OT-II (2.1%), CCR6−/− OT-II (2.3%), CCR10+/− (1.7%), or CCR10−/− (1.4%) adoptive transfer compared with WT OT-II (2.1%) adoptive transfer. N for each OT-II cell type: WT = 18, CCR4−/− = 9, CCR10+/− = 5, CCR10+/− = 5, CCR6−/− = 8, where each data point (N) is an experiment with three to five mice per group. Middle panel: localization of OT-II cells within the dermis after Dispase II–mediated dermis-epidermis separation. In the dermis, 9.4% of CD4 T cells were OT-II donor derived after WT OT-II cell adoptive transfer, compared with 3.5% after CCR4−/− OT-II cell adoptive transfer, a highly significant difference (*P < 0.01). There was no significant change in the percentage of CD4 T cells that were OT-II compared with WT in the CCR6−/− OT-II (8.8%), CCR10+/− OT-II (9.6%), or CCR10−/− OT-II (8.6%) adoptive transfer data sets. N = 16 for WT OT-II, N = 4 for CCR4−/− OT-II, N = 8 for CCR6−/− OT-II, N = 6 for CCR10+/− OT-II, and N = 7 for CCR10−/− OT-II, where each data point (N) is an experiment with a pool of three to five mice. Right panel: localization of OT-II cells within the epidermis after Dispase II–mediated dermis-epidermis separation. In the epidermis, 15.5% of CD4 T cells were OT-II donor derived when WT OT-II cells were adoptively transferred, compared with 5.5% when CCR4−/− OT-II cells were adoptively transferred, a highly significant difference (**P < 0.01). There was no significant change in the percentage of CD4 T cells that were OT-II compared with WT in the CCR6−/− OT-II (12.5%), CCR10+/− OT-II (14.4%), or CCR10−/− OT-II (13.5%) data sets. N = 16 for WT OT-II, N = 4 for CCR4−/− OT-II, N = 8 for CCR6−/− OT-II, N = 6 for CCR10+/− OT-II, and N = 7 for CCR10−/− OT-II, where each data point (N) is from a pool of three to five mice.
CCR4−/− OT-II cell accumulation was reduced significantly in both layers, and the amount of reduction was similar in both dermis and epidermis. This suggests that, similar to CCR6 and CCR10, CCR4 is not involved in the mechanism by which T cells migrate from dermis to epidermis. As noted previously, however, CCR4 is important for the mechanism by which CD4 T cells migrate into the dermis from blood. Similar results were obtained when these experiments were performed on the skin of the torso rather than the ear (see Supplemental Figure S1 at http://ajp.amjpathol.org).
The left panel of Figure 4 shows the proportion of donor-derived OT-II cells occurring within the circulating CD4 population in peripheral blood. When compared with the middle and right panels, this control shows that OT-II cells are greatly enriched in dermis and epidermis with respect to blood, and establishes that OT-II cells isolated from the skin layers are truly tissue-resident rather than contaminating cells from vessels within skin. In parallel to the CCR10 expression observed via EGFP brightness, CCR10 also was highly expressed by skin-infiltrating OT-II cells. EGFP was expressed by approximately 20% of CCR10+/−–EGFP OT-II cells from inflamed ear skin in the earlier-described assays (data not shown).
Discussion
Direct Comparison of Skin-Associated Chemokine Receptors
The availability of 2W1S:I-Ab tetramer25 has allowed us for the first time to ascertain the CCR requirements for antigen-dependent accumulation of antigen-specific endogenous long-term memory CD4 T cells within inflamed skin. We have also for the first time directly compared CD4 T cells from WT, CCR4−/−, CCR6−/−, and CCR10−/− mice side-by-side under identical conditions in vivo. This exercise has allowed us to resolve several inconsistencies in the literature regarding the contribution of each receptor to skin-specific T-cell homing. We found that CCR6 or CCR10 deficiency caused no appreciable defect as determined by these highly sensitive in vivo competitive assays. Because previous studies did not assess all three receptors in parallel within a consistent model, we raise the possibility that neither CCR6 nor CCR10 play a role at any point in the generation of a skin-homing T-cell population, or in the processes by which CD4 T cells enter or exit the skin during inflammation. There is of course a finite possibility that these receptors would show different roles in skin inflammation models other than the two used here. However, this first parallel comparison of all three receptors in a consistent assay has yielded robust conclusions.
In contrast to CCR6 and CCR10, we show that CCR4 deficiency renders endogenous antigen-specific memory CD4 T cells approximately 20-fold less efficient at accumulating within inflamed skin than WT cells. If one were to consider this finding in isolation, one could not determine the specific step at which CCR4 function is required during the cutaneous immune response. The observed competitive advantage theoretically could occur during the following: i) expansion of antigen-specific clones; ii) imprinting of antigen-specific cells for E-lig expression; iii) homing of CD4 T cells to skin from peripheral blood; or iv) retention of antigen-specific CD4 cells within the inflamed cutaneous site.
However, previous work with OT-II donor cells in vivo showed that CCR4−/− naive cells are unimpaired in their ability to differentiate into circulating E-lig+ cells.4 Furthermore, the CCR4 ligand CCL17/TARC is present on the lumen of cutaneous post capillary venules,9,30 and signaling through CCR4 can trigger adhesion under shear.9 Considered cumulatively, these new findings suggest that the CCR4-mediated competitive advantage is manifested at the moment when circulating CD4 T cells enter skin from the blood. Although CCR4+ lymphocytes occur in organs other than skin,14 the combination of E-lig and CCR4 together on a given cell appears to constitute the unique postal code for homing to skin.1
Antigen-Specific T Cells versus Other Skin-Resident CD4 T Cells
Although both TET+ and TET− CD4 cells were strongly disadvantaged by CCR4 deficiency, it is interesting to note that the effect was much more prominent within the TET+ population. This difference may reflect the likelihood that TET+ cells comprise a purely memory response. In contrast, the TET− population is likely a more diverse population containing bystander cells and long-term skin-resident cells. As such, the TET+ cells most likely have gone through at least three rounds of skin entry (one for each immunization), and may have received survival or antigen-specific retention signals within the skin to which other cells were not subjected. Despite the difference in magnitude, the effect of CCR4 deficiency on the TET− population nonetheless remained highly significant.
Migration from Dermis to Epidermis
To our own surprise, we did not find a role for any of these three receptors in T-cell migration from dermis to epidermis after extravasation from the peripheral blood during inflammation. Ample circumstantial evidence, including the exclusive expression of CCL27 by epidermal keratinocytes, pointed to a role for CCR10 in this process. Nonetheless, our competitive assays do not support this notion.
Dermal-to-epidermal migration is clearly a directed process, as evinced by the requirement for integrin α1β1.12 Our data now bring into question the notion that a chemokine receptor (or any other chemoattractant receptor) is required for dermal-to-epidermal migration. Pharmacological blocking of dermal-to-epidermal migration has been considered a promising target for clinical intervention in psoriasis.12 Our data suggest that CCR4, CCR6, and CCR10 are unlikely to provide useful targets for this particular approach, at least individually. Thus, extravasation from the blood is currently the only component of cutaneous trafficking associated with a consistent and clearly shown chemokine receptor function (ie, CCR4).
Expression of CCR6 and CCR10 by Skin-Homing Lymphocytes
As discussed earlier, CCR6 is expressed by many skin-infiltrating cells but also is abundant in several other tissues.11,13–15 If we had found a role for CCR6 in cutaneous localization, it likely would have been a generic role applicable to many organs. At present, it remains unclear what role this receptor might play in skin.
The expression pattern of CCR10 is very different from that of CCR6. CCR10 appears restricted to a subset of skin-homing/E-lig+/CCR4+ CD4 T cells.24 However, CCR10 is not expressed by the majority of skin-homing T cells, including many found within the skin itself (Figure 3 and Soler et al24). This restriction of CCR10 to a subset of the skin-homing CD4 population implies an important function. Nonetheless, we were not able to show a role for CCR10 in skin-specific homing. CCR10 likely plays an as-yet unknown role in cutaneous T-cell immunology, probably unrelated to skin-specific T-cell trafficking.
Sunlight, Vitamin D, and CCR10
The mouse is a nocturnal burrowing animal covered with fur, similar to the proposed common ancestor of rodents and human beings. Very little sunlight is likely to ever reach the skin of such animals. Nocturnal mammals obtain their vitamin D as a nutrient or through alternative metabolic pathways independent of sunlight.31 Unlike human beings, one would not expect the highest concentrations of vitamin D in these animals to occur in the skin.
Nonetheless, evolution has maintained the keratinocyte/CCL27 association in skin and E-lig+/CCR10 association in cutaneous T cells.10,24 It is therefore likely that a more ancient, sunlight-independent regulatory mechanism controls CCR10 expression in many mammals. The recently discovered vitamin D–controlled mechanism of CCR10 induction in human beings may supplement this original mechanism.
We further speculate that CCR10 is induced at some point after naive CD4 T cells are first imprinted to differentiate into skin-homing cells: the two homing receptors most clearly shown to function in normal cutaneous T-cell trafficking (ie, E-lig and CCR4) are not up-regulated by 1,25-dihydroxy-vitamin-D3.23 In fact, one study suggested that this nutrient actually down-regulates E-lig expression.32 Because both E-lig and CCR4 are thought be induced by antigen within skin-draining lymph nodes, CCR10 actually might be induced after nascent E-lig+/CCR4+ skin-homing T cells enter inflamed skin.
Roles for Chemokine Receptors Unrelated to Lymphocyte Trafficking
Although chemokines are best known for their roles in leukocyte trafficking and migration, the notion that they may have other immunological functions is not unprecedented.33 In fact, two recent reports have described a population of CD4 T cells in human blood that uniquely produce IL-22, but not IL-17, and simultaneously express CCR4, CCR6, and CCR10.34,35 These cells were tentatively dubbed “Th22” T cells. It was further reported that T-cell culture with 1,25-dihydroxy-vitamin-D3 facilitates development of this newly identified Th22 subset, along with CCR10 expression.34 Thus, although CCR6 and CCR10 do not appear to influence cutaneous trafficking, one might imagine a role for such receptors in shaping the class of immune response generated within the skin, be it Th1, Th2, Th17, Th22, regulatory T cells, or others not yet discovered. As such, pharmacological inhibitors of CCR6 and CCR10 may be more likely to affect the polarization of skin-homing molecules rather than their trafficking per se.
Acknowledgments
We thank Dr. Thomas S. Kupper and the Brigham and Women’s Hospital Department of Dermatology for intellectual and financial support; Suzanne T. Nizza, Bryan Vander Lugt, Zachary T. Beck, and Thomas S. Kupper for critical reading of the manuscript; and Craig Gerard and Olivier Morteau for CCR10−/− mice, Sergio Lira for CCR6−/− mice, and Marc Jenkins25 for 2W1S:I-Ab tetramer.
Footnotes
Supported by National Institutes of Health grants R01-AI046784 and R03-AI083796 (J.J.C.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi:10.1016/j.ajpath.2011.02.031
Supplementary data
Roles of CCR4 and CCR10 in epidermal CD4 T-cell homing in torso (back) skin consistent with that seen for ear skin in Figure 4. CCR10 previously has been suggested to have a role in homing of CD4 T cells to the epidermis. However, the results of our ear skin inflammation model did not support this hypothesis. We therefore considered the possibility that ear skin may not be representative of normal skin in mice, that is, the skin that covers most of the mouse's body. To investigate this, we looked at trafficking of OT-II CD4 T cells to the epidermis of the skin on the upper back of the mouse. Briefly, animals were injected with 5e6 WT OT-II, CCR4−/− OT-II, or CCR10−/− OT-II cells, as described in the Materials and Methods section. WT OT-I I cells were used as a positive homing control. CCR4−/− OT-II cells were used as a negative homing control. One day later, animals were shaved on the back with electric shears, and Nair was applied to a quarter (coin)-sized area on the mouse's back, then removed after approximately 1 minute. The area then was washed with cotton balls soaked in warm water to remove residual Nair is distributed by Church and Dwight Co, Princeton, NJ and dried with fresh cotton balls. The denuded area was gently tape-stripped and treated with acetone (as described for ear skin protocol). A total of 50 μL of a 1.0 mg/mL cholera toxin solution (50 μg/mouse) and 50 μL of a 3.33 mg/mL OVA323-339 solution (111 μg/mouse) was applied to the skin, and both were distributed evenly with a small artist paintbrush. After 6 days, the animals were sacrificed, and blood and back skin were harvested. Fat mechanically was removed from the skin, and epidermis was separated from other skin components as described in the Materials and Methods section. Cell suspensions for flow cytometry analysis were prepared as described in the Materials and Methods section. Some WT OT-II recipients were treated with adjuvant [cholera toxin (CT)] only, as a negative control for those that received antigen plus adjuvant (indicated in red on the figure). The peripheral blood of CCR4−/− OT-II recipients contained an appreciably higher proportion of OT-II cells than blood from WT OT-II or CCR10−/− OT-II recipients. This is consistent with the relative paucity of CCR4−/− OT-II cells from skin. The results in skin were consistent with those observed in the ear skin inflammation model. WT OT-II and CCR10−/− OT-II cells accumulated similarly well in the epidermis, whereas CCR4−/− OT-II cells accumulated poorly. Thus, we conclude that our observation of CCR10 as an unnecessary component of antigen-specific CD4 T-cell homing to CT inflamed skin is consistent between ear and torso skin. N was at least 4 individual mouse experiments for all data described in this Figure.
References
- 1.Campbell J.J., Butcher E.C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 2.Butcher E.C., Williams M., Youngman K., Rott L., Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 3.Baekkevold E.S., Wurbel M.A., Kivisakk P., Wain C.M., Power C.A., Haraldsen G., Campbell J.J. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J Exp Med. 2005;201:1045–1051. doi: 10.1084/jem.20041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell J.J., O'Connell D.J., Wurbel M.A. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudda J.C., Perdue N., Bachtanian E., Campbell D.J. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sather B.D., Treuting P., Perdue N., Miazgowicz M., Fontenot J.D., Rudensky A.Y., Campbell D.J. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wurbel M.A., Malissen M., Guy-Grand D., Malissen B., Campbell J.J. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J Immunol. 2007;178:7598–7606. doi: 10.4049/jimmunol.178.12.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zabel B.A., Agace W.W., Campbell J.J., Heath H.M., Parent D., Roberts A.I., Ebert E.C., Kassam N., Qin S., Zovko M., LaRosa G.J., Yang L.L., Soler D., Butcher E.C., Ponath P.D., Parker C.M., Andrew D.P. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P., Andrew D.P., Warnke R., Ruffing N., Kassam N., Wu L., Butcher E.C. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 10.Homey B., Alenius H., Muller A., Soto H., Bowman E.P., Yuan W., McEvoy L., Lauerma A.I., Assmann T., Bunemann E., Lehto M., Wolff H., Yen D., Marxhausen H., To W., Sedgwick J., Ruzicka T., Lehmann P., Zlotnik A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 11.Homey B., Dieu-Nosjean M.C., Wiesenborn A., Massacrier C., Pin J.J., Oldham E., Catron D., Buchanan M.E., Muller A., DeWaal Malefyt R., Deng G., Orozco R., Ruzicka T., Lehmann P., Lebecque S., Caux C., Zlotnik A. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 12.Conrad C., Boyman O., Tonel G., Tun-Kyi A., Laggner U., de Fougerolles A., Kotelianski V., Gardner H., Nestle F.O. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 13.Clark R.A., Chong B., Mirchandani N., Brinster N.K., Yamanaka K., Dowgiert R.K., Kupper T.S. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 14.Campbell J.J., Brightling C.E., Symon F.A., Qin S., Murphy K.E., Hodge M., Andrew D.P., Wu L., Butcher E.C., Wardlaw A.J. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 15.Boisvert J., Kunkel E.J., Campbell J.J., Keeffe E.B., Butcher E.C., Greenberg H.B. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 16.Kunkel E.J., Campbell D.J., Butcher E.C. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 17.Reiss Y., Proudfoot A.E., Power C.A., Campbell J.J., Butcher E.C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uehara S., Grinberg A., Farber J.M., Love P.E. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 19.Wurbel M.A., Malissen B., Campbell J.J. Complex regulation of CCR9 at multiple discrete stages of T cell development. Eur J Immunol. 2006;36:73–81. doi: 10.1002/eji.200535203. [DOI] [PubMed] [Google Scholar]
- 20.Homey B., Wang W., Soto H., Buchanan M.E., Wiesenborn A., Catron D., Muller A., McClanahan T.K., Dieu-Nosjean M.C., Orozco R., Ruzicka T., Lehmann P., Oldham E., Zlotnik A. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 21.Morales J., Homey B., Vicari A.P., Hudak S., Oldham E., Hedrick J., Orozco R., Copeland N.G., Jenkins N.A., McEvoy L.M., Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigmundsdottir H., Butcher E.C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigmundsdottir H., Pan J., Debes G.F., Alt C., Habtezion A., Soler D., Butcher E.C. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 24.Soler D., Humphreys T.L., Spinola S.M., Campbell J.J. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 2003;101:1677–1682. doi: 10.1182/blood-2002-07-2348. [DOI] [PubMed] [Google Scholar]
- 25.Moon J.J., Chu H.H., Pepper M., McSorley S.J., Jameson S.C., Kedl R.M., Jenkins M.K. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson E., Butcher E.C. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200:805–809. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morteau O., Gerard C., Lu B., Ghiran S., Rits M., Fujiwara Y., Law Y., Distelhorst K., Nielsen E.M., Hill E.D., Kwan R., Lazarus N.H., Butcher E.C., Wilson E. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol. 2008;181:6309–6315. doi: 10.4049/jimmunol.181.9.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moed H., Boorsma D.M., Tensen C.P., Flier J., Jonker M.J., Stoof T.J., von Blomberg B.M., Bruynzeel D.P., Scheper R.J., Rustemeyer T., Gibbs S. Increased CCL27-CCR10 expression in allergic contact dermatitis: implications for local skin memory. J Pathol. 2004;204:39–46. doi: 10.1002/path.1619. [DOI] [PubMed] [Google Scholar]
- 29.Stenn K.S., Link R., Moellmann G., Madri J., Kuklinska E. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93:287–290. doi: 10.1111/1523-1747.ep12277593. [DOI] [PubMed] [Google Scholar]
- 30.Chong B.F., Murphy J.E., Kupper T.S., Fuhlbrigge R.C. E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: a foundation for a cutaneous immunosurveillance system. J Immunol. 2004;172:1575–1581. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- 31.Cavaleros M., Buffenstein R., Ross F.P., Pettifor J.M. Vitamin D metabolism in a frugivorous nocturnal mammal, the Egyptian fruit bat (Rousettus aegyptiacus) Gen Comp Endocrinol. 2003;133:109–117. doi: 10.1016/s0016-6480(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka K., Dimitroff C.J., Fuhlbrigge R.C., Kakeda M., Kurokawa I., Mizutani H., Kupper T.S. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. J Allergy Clin Immunol. 2008;121:148–157. doi: 10.1016/j.jaci.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; e143
- 33.Broxmeyer H.E., Kim C.H. Regulation of hematopoiesis in a sea of chemokine family members with a plethora of redundant activities. Exp Hematol. 1999;27:1113–1123. doi: 10.1016/s0301-472x(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 34.Duhen T., Geiger R., Jarrossay D., Lanzavecchia A., Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 35.Trifari S., Kaplan C.D., Tran E.H., Crellin N.K., Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17. T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Roles of CCR4 and CCR10 in epidermal CD4 T-cell homing in torso (back) skin consistent with that seen for ear skin in Figure 4. CCR10 previously has been suggested to have a role in homing of CD4 T cells to the epidermis. However, the results of our ear skin inflammation model did not support this hypothesis. We therefore considered the possibility that ear skin may not be representative of normal skin in mice, that is, the skin that covers most of the mouse's body. To investigate this, we looked at trafficking of OT-II CD4 T cells to the epidermis of the skin on the upper back of the mouse. Briefly, animals were injected with 5e6 WT OT-II, CCR4−/− OT-II, or CCR10−/− OT-II cells, as described in the Materials and Methods section. WT OT-I I cells were used as a positive homing control. CCR4−/− OT-II cells were used as a negative homing control. One day later, animals were shaved on the back with electric shears, and Nair was applied to a quarter (coin)-sized area on the mouse's back, then removed after approximately 1 minute. The area then was washed with cotton balls soaked in warm water to remove residual Nair is distributed by Church and Dwight Co, Princeton, NJ and dried with fresh cotton balls. The denuded area was gently tape-stripped and treated with acetone (as described for ear skin protocol). A total of 50 μL of a 1.0 mg/mL cholera toxin solution (50 μg/mouse) and 50 μL of a 3.33 mg/mL OVA323-339 solution (111 μg/mouse) was applied to the skin, and both were distributed evenly with a small artist paintbrush. After 6 days, the animals were sacrificed, and blood and back skin were harvested. Fat mechanically was removed from the skin, and epidermis was separated from other skin components as described in the Materials and Methods section. Cell suspensions for flow cytometry analysis were prepared as described in the Materials and Methods section. Some WT OT-II recipients were treated with adjuvant [cholera toxin (CT)] only, as a negative control for those that received antigen plus adjuvant (indicated in red on the figure). The peripheral blood of CCR4−/− OT-II recipients contained an appreciably higher proportion of OT-II cells than blood from WT OT-II or CCR10−/− OT-II recipients. This is consistent with the relative paucity of CCR4−/− OT-II cells from skin. The results in skin were consistent with those observed in the ear skin inflammation model. WT OT-II and CCR10−/− OT-II cells accumulated similarly well in the epidermis, whereas CCR4−/− OT-II cells accumulated poorly. Thus, we conclude that our observation of CCR10 as an unnecessary component of antigen-specific CD4 T-cell homing to CT inflamed skin is consistent between ear and torso skin. N was at least 4 individual mouse experiments for all data described in this Figure.


