Abstract
Background
Although research into the effects of cocaine has focused on the ventral striatum, recent reports have identified a significant role for the dorsal striatum. Given the importance of the dorsal striatum in motor control, the purpose of the present study was to investigate potential sensorimotor deficts among cocaine users and the functional basis of these deficits within the striatum.
Methods
Functional magnetic resonance imaging data were collected from fourteen right-handed, non-treatment seeking chronic cocaine users and fourteen age and gender matched controls during performance of two finger-sequencing paradigms that differentially activate the caudate (internally-guided) and the putamen (externally-guided) interleaved with blocks of rest. The total percent signal change in the dorsal striatum and the contribution of the left and right caudate and putamen were calculated and compared across groups and tasks.
Results
Significant deficits in sensorimotor control were observed in cocaine users for both motor tasks, with the most severe impairments present during internally-guided movements. Cocaine users lacked the typical functional segregation observed in the dorsal striatum of the control subjects. The total percent signal change in the dorsal striatum was not significantly different between the groups, but cocaine users activated significantly less contralateral caudate and putamen for internally-guided versus externally-guided movements, respectively.
Conclusion
These data provide clear evidence that chronic cocaine users have significant motor performance deficits that are accompanied by disrupted processing within the dorsal striatum. These data suggest the effects of cocaine extend beyond the confines of the motivational domains of the ventral striatum.
Keywords: cocaine, motor control, functional MRI, caudate, dorsal striatum, neuroimaging
1. Introduction
Studies of cocaine addiction have historically focused on the ventral striatum (Koob et al., 1994; White and Kalivas, 1998). Many animal studies have demonstrated, for example, that functional activity in the ventral striatum is altered as a result of cocaine exposure (Lyons et al., 1996; Porrino et al., 2002; Porrino et al., 2007). Lesions or other disruptions of the nucleus accumbens decrease cocaine self-administration (Ito et al., 2004; Nestler, 2005; Roberts et al., 1980; Zito et al., 1985). The importance of the ventral striatum-nucleus accumbens is also substantiated in studies of human cocaine users. Functional activity in the ventral striatum has been shown to correlate with cocaine craving (Breiter et al., 1997; Risinger et al., 2005) and the subjective “high” following self-administration in cocaine addicts (Risinger et al., 2005), for example.
The effects of cocaine, however, are not limited to ventral domains of the striatum. The striatum is an anatomically complex and functionally heterogenous brain region. In primates, it can be subdivided anatomically into the nucleus accumbens, the caudate nucleus, and the putamen. Functional subdivisions include the limbic, association, and sensorimotor domains (Haber et al., 2006; Haber et al., 1995; Martin et al., 1991). The ventral striatum is primarily limbic in function, whereas the dorsal striatum is involved in cognitive processing and sensorimotor integration (Balleine et al., 2007). With chronic cocaine exposure dopamine receptor dysregulation extends from the ventral to the dorsal striatum (Porrino et al., 2004). Dopamine release in the dorsal striatum is correlated with cue-induced craving scores in cocaine-dependent subjects (Volkow et al., 2006; Wong et al., 2006). Increased functional activity in the dorsal striatum, as measured by blood-oxygen level dependent signal intensity (BOLD), is also correlated with cue-induced craving (Childress et al., 1999; Risinger et al., 2005). Studies in nonhuman primates have revealed a progressively greater involvement of dorsal striatum with longer durations of cocaine exposure (Letchworth et al., 2001; Porrino et al., 2004). Control over drug use likely evolves from action to habit by shifting from goal-directed processes to more habit-based control (Everitt et al., 2001; Tiffany, 2000). This shift reflects a change in activity from ventromedial, limbic-associated, striatum underlying goal-directed learning processes to the dorsal striatum, necessary for habit formation.
In addition to drug craving and habit formation, the dorsal striatum is involved in sensorimotor control (Brooks, 1995; Jahanshahi et al., 1995; Menon et al., 1998). Internally-guided movement tasks require a network of medial cortical and subcortical brain regions, including the caudate nucleus in the dorsal striatum (Debaere et al., 2003; Elsinger et al., 2006). Externally-guided movement tasks rely on lateral areas of the dorsal striatum, including the dorsolateral putamen (Debaere et al., 2003; Jahanshahi et al., 1995). This dissociation between internally-guided and externally-guided movement is a common theme in movement disorder literature (Delval et al., 2007; Jackson et al., 1995; Jahanshahi et al., 1995). Parkinson’s disease patients, for example, are selectively impaired in internally-guided movements and external cues enhance their motor performance (Jahanshahi et al., 1995).
There is a growing body of evidence that chronic cocaine users have sensorimotor processing deficiencies (Bartzokis et al., 1999; Bauer, 1996a; Cardoso and Jankovic, 1993; Chouinard and Ford, 2000; Pascual-Leone and Dhuna, 1990) which may persist for as long as 15 years after the patient’s last reported drug use (Lundh and Tunving, 1981; Pascual-Leone and Dhuna, 1990; Rylander, 1972). Despite these reports, the neurobiological basis for impaired motor control in chronic cocaine abusers is poorly understood. Given the role of the dorsal striatum in mediating sensorimotor function (Alexander et al., 1986; Robbins, 2007; Romero et al., 2008; Schultz et al., 2000), these motor deficits likely indicate irregularities in dorsal striatal activity.
The aim of this investigation, therefore, was to determine whether chronic cocaine users, similar to Parkinson’s disease patients, are selectively impaired at internally-guided movements and whether motor deficits are associated with dorsal striatal dysfunction. To achieve this aim, we used two finger-sequencing tasks which require participants to use either internal or external cues to guide their movement and are known to differentially activate dorsal striatal nuclei. We sought to determine if sensorimotor deficits in cocaine users were task and nucleus specific, or whether there was a global decrease in both performance and functional specificity within the dorsal striatum of chronic users.
2. Methods
2.1 Subjects
Participants in this study included 14 chronic cocaine abusers (8 men, 6 women; age = 38.1 ± 10.4 years; education = 13.8 ± 2.6 years; race = 10 African-American, 4 Caucasian, mean ± S.D.), and 14 healthy control subjects matched for age, gender and education (8 men, 6 women; age = 34.6 ± 7.9 years, p= 0.31; education = 15.1 ± 2.3 years, p= 0.11; race = 4 African-American, 9 Caucasian, 1 Pacific Islander). The cocaine users had a used crack cocaine for 16.3 ± 7.6 years (range: 11–25 years) and reported smoking as their primary route of administration. All participants were recruited from local television advertisements and by word-of-mouth. Each participant provided a written informed consent approved by the Wake Forest University Institutional Review Board. All participants were carefully screened to ensure that they fulfilled study criteria. Exclusion criteria included: a history of dependence on illegal drugs (other than cocaine, for the User group), a history or current diagnosis of neurologic disease, head trauma, DSM-IV Axis 1 psychiatric disorder, an episode of being unconscious for more than 3 minutes, metallic objects in the body, claustrophobia, and a score of less than 75 on either the verbal or performance IQ scale of the Wechsler Adult Intelligence Scale-Revised. The average IQ score in the cocaine users was 87.1± 10.5 and 100.7 ± 8.2 in the controls (t= 2.31, p < .05).
All individuals were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (Ventura et al., 1998). Controls were excluded if they met criteria for dependence or abuse of any substance including alcohol or if they had a positive urine drug screen on the day of testing. All included cocaine abusers met DSM-IV criteria for cocaine dependence and at least a 12-month history of cocaine or crack use. Cocaine users were excluded if they met criteria for lifetime abuse or dependence on opioids, stimulants other than cocaine, hallucinogens, or inhalants. Additionally, they were excluded if they had a positive urine drug screen for amphetamines, benzodiazepines, or opiates on the day of the study. All cocaine subjects had a positive urine drug screen for cocaine on the morning of the study and reported an average of 13.1 (+/− 4.0) hours since their last use (range: 9–30h). The functional MRI scan was intentionally given three hours after the urine drug screen to eliminate any effects of acute cocaine on the functional data. Sixty six percent of the subjects also had a positive urine toxicology screen for marijuana though they reported no use of marijuana within the last 24 hours. Ten cocaine subjects were current cigarette smokers and four cocaine subjects were non-smokers. Eleven control subjects were non-smokers and three control subjects were current cigarette smokers. In order to avoid any confounding effects of nicotine withdrawal, all regular smokers were given the opportunity to smoke approximately an hour prior to the MRI scanning session. None of the participants were dependent on alcohol as assessed by psychiatric evaluation as well as the Alcohol Use Disorders Identification Test (AUDIT). The average AUDIT score was not significantly different between the groups (controls = 3.5 ± 3.9, cocaine users= 8.1 ± 5.4, p > 0.05).
2.2 Finger Sequencing Task
All subjects performed two versions of a sequential finger tapping task (externally-guided sequencing (EG) and internally-guided sequencing (IG)). The tasks required the subject to fixate on an animated movie of a hand performing a series of finger tapping sequences (0.5 Hz) on a 4-button box. For both tasks, the subjects were instructed to mimic the movie in real-time by tapping on a button box with their right hand. While in the scanner the subjects were not able to see their hands at any time. For the EG condition the finger tapping sequences were pseudorandomized and unpredictable. This required that the participants rely on external visual cues to perform accurately. For the IG task the sequences repeated in blocks of four taps. Consequently, the finger tapping movements were predictable and could be performed based on internal cues alone. The 30s EG and IG movement blocks alternated with 30s rest blocks, and there was a 15s interval before each task block during which the participants were told that the upcoming sequence was either predictable or unpredictable. Each functional run lasted 7.2 min and all subjects performed two runs. The task order was counter-balanced across runs.
Subjects practiced the task prior to the scanning session to be sure they achieved a 90% accuracy rate for both the IG and EG condition. A maximum of 3, one minute practice blocks were presented. All participants had to achieve 90% accuracy (as determined by number of correct button presses) during the practice session to continue with the study. After this accuracy rate was achieved, all participants performed another 1 minute practice block to minimize differences in time spent at maximal performance between the groups. All fourteen control participants reached criterion performance during the first minute block. Seven of the fourteen cocaine users reached criterion in the first minute block, whereas five required two practice blocks, and two users required three blocks.
During the scanning session the stimuli were presented to the subjects on MRI-compatible LCD goggles (Resonance technology, Inc.) and eye-tracking software was used to be sure that the subjects remained awake throughout the study. Performance was recorded directly through an MRI-compatible response box via Eprime software (Psychology Software Tools). Reaction times and error rates were calculated for each task.
2.3 FMRI Acquisition and Preprocessing
Images were acquired on a 1.5 Tesla General Electric scanner with a birdcage type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. The participant’s head was positioned along the canthomeatal line. Foam padding limited head motion within the coil. High-resolution T1 weighted anatomical images (3D SPGR, TR=14ms, TE=7700ms, flip angle=25°, voxel dimensions 1.0×1.0×1.0mm, 256×256 voxels, 160 slices) were acquired for coregistration and normalization of functional images. Forty-nine co-planar T2 weighted functional images were acquired using a gradient echoplanar sequence (TR=3000ms, TE=40ms, flip angle=90°, NEX=1, voxel dimensions 3.0×3.0×3.0mm, imaging matrix 64×64 voxels). Each functional run consisted of 144 time points. Two radio frequency excitations were performed prior to image acquisition to achieve steady-state transverse relaxation.
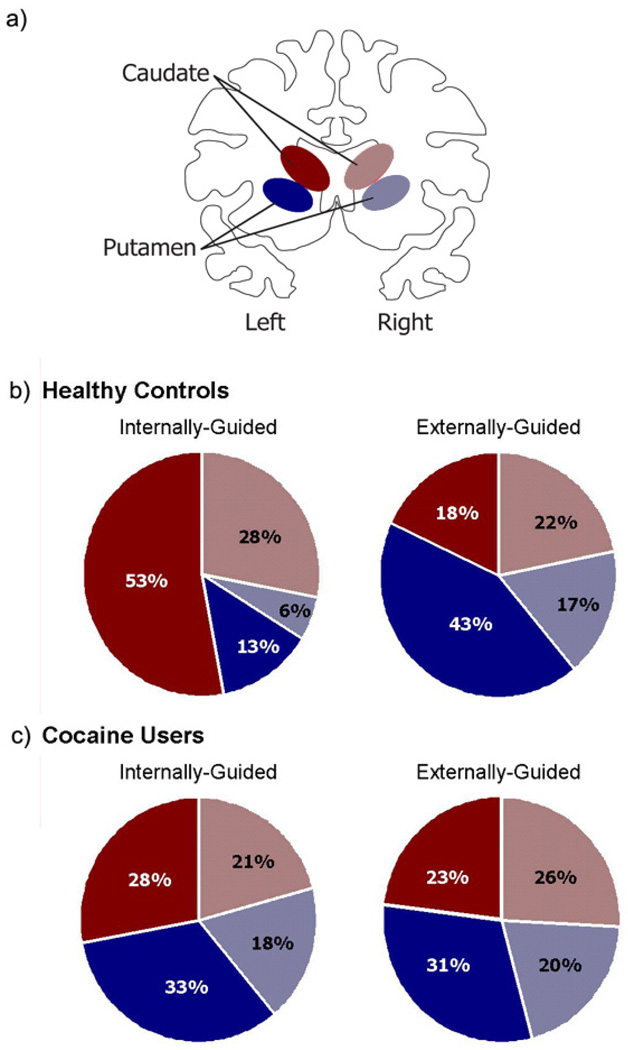
The functional MRI data were preprocessed for each participant independently using standard parametric mapping techniques (SPM5, Wellcome Department of Imaging Neuroscience, London, UK (Ashburner and Friston, 2005)). The time series of functional images were aligned for each slice in order to minimize the signal changes related to small motion of the subject during the acquisition. Head motion was less than 1-mm translation and 1°-rotation for all scans. Spatial filtering of functional time series was performed by convolution of each EPI image with a two-dimensional Gaussian smoothing kernel with full width at half maximum (FWHM) = 2.8 mm2. The data were corrected for acquisition time (slice timing), realigned to the first volume (motion correction), normalized into a standardized neuroanatomical space (Montreal Neurological Institute brain template). Analyses of time data series were performed individually using a boxcar model convolved with the canonical hemodynamic response function. Temporal filtering of functional time series included removal of the linear drifts of the signal with respect to time from each voxel's time-course, and low-pass filtering of each voxel's time-course with a one-dimensional Gaussian filter with FWHM=6s. As the hypothesis of this study was focused on changes in the dorsal striatum, analysis was restricted to the dorsal striatum, as defined by an explicit mask of the left caudate, right caudate, left putamen and right putamen (WFU PickAtlas (Maldjian et al., 2003)) shown schematically in Figure 3a. These regions of interest contained 619, 620, 1035, and 1036 voxels respectively. The voxels were weighted uniformly as there was no a priori assumption of spatial distributions of activity within the ROI.
Figure 3. Dorsal striatal activity during right-hand movement.
The dorsal striatum was divided into four subregions: left (contralateral) and right (ipsilateral) caudate, left and right putamen. A schematic representation of the caudate (red) and putamen (blue) on the contralateral (solid) and ipsilateral (dotted) sides is displayed (a). The distribution of dorsal striatal activity is shown for controls (b) and cocaine users (c) during both internally-guided and externally-guided movement. In controls, activity during internally-guided movement was weighted towards the contralateral caudate. Activity during externally-guided movement was weighted towards the contralateral putamen. In contrast, dorsal striatal activity in cocaine users was evenly distributed among the four regions for both tasks (as revealed by Chi Square goodness of fit tests (p > 0.05)).
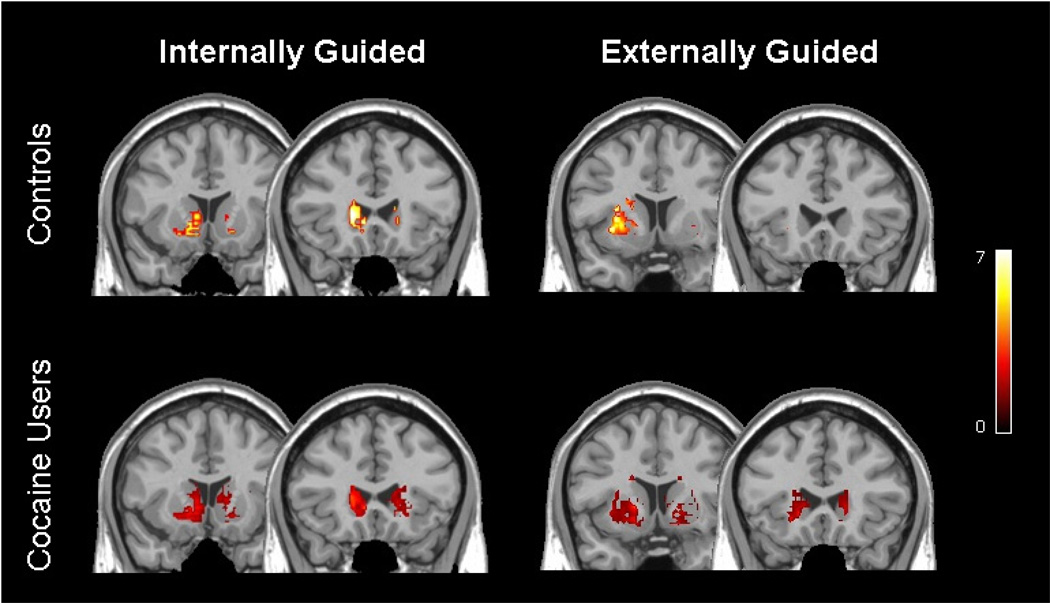
Statistical parametric maps were generated using the general linear model within SPM5. The data for all rest and task blocks was modeled together for all cocaine users and controls. Statistical contrast maps (IG vs Rest, EG vs. Rest) were then created for each group. To determine the areas significantly activated during the task relative to rest for each subject a minimum cluster size was set at 25 voxels with a threshold of statistical significance was set at p < 0.001 for uncorrected values. The data are presented in figure 2 to illustrate the distribution of BOLD signal change in the populations. These data were not quantified further, however, as the primary objective of this study was to assess the fractional contribution of the caudate and the putamen to IG and EG movement.
Figure 2. Distribution of striatal activity during right-hand finger tapping.
Voxel-based maps of significant brain activity for controls (top) and chronic cocaine users (bottom) during internally-guided (left) and externally-guided (right) finger-tapping relative to rest. These data were modeled across all subjects in each group in order to get a voxel-based representation of the distribution of activity within the striatum. The colorbar indicates z-scores from voxels that met criteria of a minimum cluster size of 25 voxels with a threshold of statistical significance at p < 0.001 for uncorrected values.
2.4 Region-of-interest (ROI) analysis
2.4.1 Percent Signal Change
The total percent signal change from rest was calculated for both IG and EG movement based on previously established techniques (Sadato et al 1997). The percent signal change from rest ((BOLD signal during task blocks-BOLD signal during rest blocks)/ BOLD signal during rest blocks *100) was calculated for each voxel contained in the dorsal striatum (left and right caudate and putamen). The first 3 data points from each task and rest block were discarded based on prior research demonstrating that the BOLD signal peaks 8–12 seconds following movement (Rao et al. 1996, DeYoe et al. 1992).
2.4.2 Fractional Distribution
In order to investigate the relative contribution of each dorsal striatal nucleus to the IG and EG movement tasks, a region of interest analysis was performed on each of the dorsal striatal nuclei that contributed to the explicit mask: the left and right caudate and the left and right putamen. The relative contribution of each ROI to the total percent signal change was calculated by adding the percent signal change from rest for each voxel in the ROI and dividing it by total percent signal change. The final output for each individual was one total percent signal change and four relative contribution values for both IG and EG movement tasks. The relative contribution values for each ROI were reported as a percent contribution to the total percent signal change and averaged across each group. This was performed for both the IG and EG tasks.
2.5 Statistical Analysis
Motor performance (reaction time and error rate) for each task was compared within and between groups using analyses of variance (group × task) followed by appropriate tests for multiple comparisons (p<0.05 as the threshold for statistical differences). Total percent signal change in the dorsal striatum was compared across groups and tasks using an analysis of variance (group × task). To determine if the distribution of activity within the four ROIs within the dorsal striatum (left and right caudate and putamen) differed from chance, chi-square goodness of fit tests were performed for the IG and EG task data for each group. The observed distribution of activity in users was further compared to the distribution found in controls for both IG and EG tasks using the chi-square test for identity. Additionally, potential correlations between activity within the dorsal striatum and both drug use variables and motor performance data were conducted using Pearson product-moment correlations.
3. Results
3.1 Behavioral Findings
3.1.1 Reaction Time
Reaction times in controls and users differed significantly for IG and EG tasks (F(3,50)= 100.57, p<0.001). Controls responded significantly faster on the IG task than the EG task (t(13) = 29.01, p<0.001). Users were also significantly faster during the IG than the EG task (t(13) = 13.48, p<0.001). During both tasks the users were significantly slower than the controls (IG: t(26) = 36.82, p<0.0001, EG: t(26) = 75.63, p<0.001; Fig. 1a).
Figure 1. Task Performance.
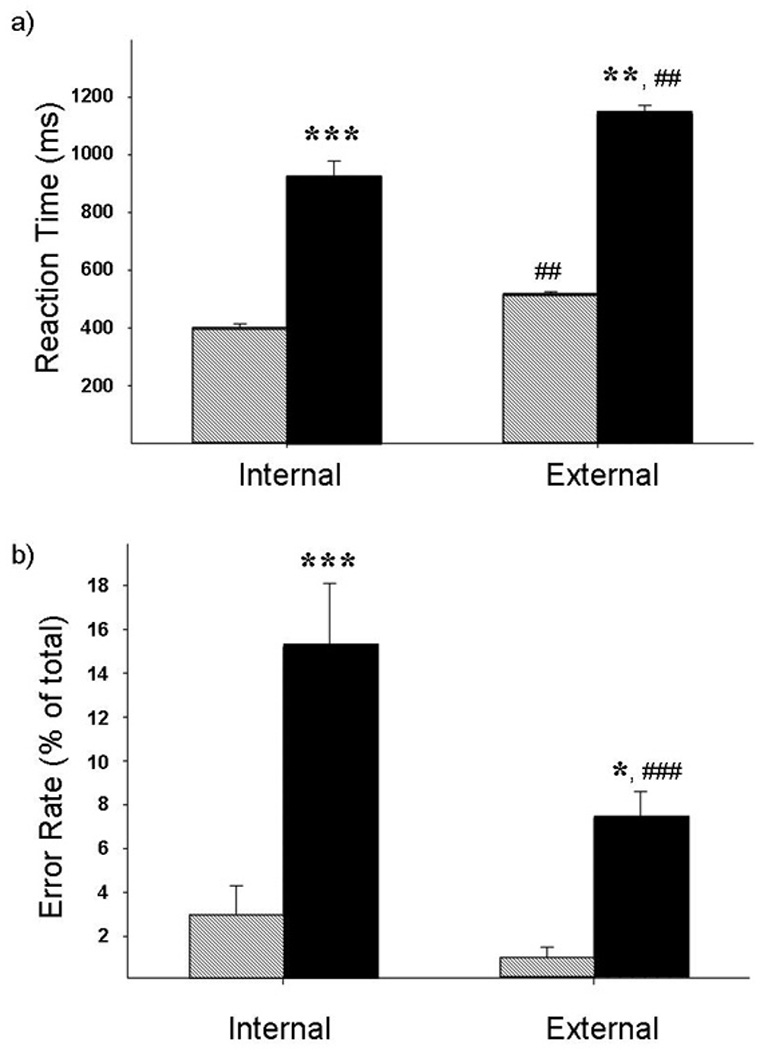
Mean (with standard error) reaction time (a) and error rate (b) for the non-drug using control group (dashed) and the cocaine users (solid). The cocaine users had significantly longer reaction times and higher error rates for both the internally-guided and externally-guided tasks. * p<0.01, ** p<0.001, ***p<0.0001
3.1.1 Error Rate
The error rate was also significantly different between the groups (F(3,50)= 5.978, p=0.001). The users made significantly more errors than controls on both the IG (t(26) = 19.067, p<0.0001) and EG (t(26) = 2.88, p= 0.008) tasks (Fig. 1b). In addition, the error rate in users was significantly higher during IG movement than EG movement (t(13) = 9.625, p<0.0001).
To determine whether any group differences in motor performance may be explained by differences in demographics or current drug use (other than cocaine), we performed a multivariate analysis of covariance (MANCOVA) with reaction time and error rate as the dependent variables and gender, age, race, education, IQ, AUDIT score, cigarette smoking status, and marijuana smoking status as independent variables. The results of this analysis indicated that the none of these variables were statistically significant with regard to their impact on motor performance (gender, F = 0.378 ; age, F = 0.256; race, F = 0.153; education, F = 0.61; IQ, F = 0.85; AUDIT, F = 1.45; cigarette smoking, F = 3.12; marijuana smoking, F = 3.00; NS). Furthermore, Pearson product-moment correlations modeling the parametric variables independently (age, IQ, AUDIT score) also revealed no significant relationship between these measures and either reaction time or error rate among the participants.
3. 2 Functional Magnetic Resonance Imaging Findings
3.2.1 Total percent signal change
The total percent signal change did not differ by group (controls vs users) or by task (IG vs EG) (F(3,50) = 2.193).
3.2.2 Distribution of Activity within the Dorsal Striatum: Controls
Figure 3 shows the relative distribution of task-related BOLD signal in the dorsal striatum (the left and right caudate and putamen ROIs shown schematically in Fig. 3a). During performance of IG movements there was a significant difference in the relative contribution of each ROI to the total percent signal change (X2= 97.59, df = 3, p < 0.0001). The largest contribution was from the left caudate (53%; Fig. 3b). During performance of EG movements there was also a significant difference in the relative contribution of each ROI to the total percent signal change (X2= 182.41, df = 3, p < 0.0001). The left putamen accounted for the largest amount of activity during this task (43%; Fig. 3b).
3.2.3 Distribution of Activity within the Dorsal Striatum: Cocaine Users
The relative distribution of activity in the dorsal striatum of users during performance of the IG and EG tasks is shown in Fig. 3c. During performance of IG movements by users, there were no significant differences in the relative contribution of each ROI to the total percent signal change (X2= 7.44, df = 3, p = 0.06; Fig. 3c). Similarly, during EG movements there were no differences in the relative contributions of the caudate and putamen (X2= 2.72, df = 3, p = 0.43; Fig. 3c).
During the performance of IG movements, users had a significantly different pattern of activity in the dorsal striatum than controls (X2=72.85, df = 3, p <0.0001). During EG movement however, there was no significant difference in the distribution of activity between groups (X2= 5.95, df = 3, p = 0.135). Correlations between motor performance measures (reaction time and error rate) and percent signal change in the caudate and the putamen for both IG and EG movement revealed no significant relationships. Furthermore, there were no significant correlations between length of cocaine use and total percent signal change in the dorsal striatum for IG (r = 0.31, NS) or EG movement (r = 0.16, NS).
To determine whether alterations in dorsal striatal activity were influenced by demographic or drug use variables (other than cocaine use), we performed a MANCOVA for both EG movement and IG movement wherein percent signal change in the four dorsal striatal nuclei were the dependent variables and the aforementioned demographic and drug use indicators were the dependent variables. As with the motor performance data, there was no significant effect of any of the dependent variables on BOLD signal change for either EG or IG movement.
4. Discussion
Previous reports have shown that the performance of simple motor tasks can require the recruitment of activity in different portions of the striatum, depending on the cognitive demands of the task (Crosson et al., 2001; Debaere et al., 2003; Elsinger et al., 2006; Nakai et al., 2003). The present investigation confirms these findings by showing that, within the striatum of controls, internally-guided movement is primarily associated with activity in the contralateral caudate and externally-guided movement is primarily associated with activity in the contralateral putamen. This pattern of functional segregation, however, was not present in chronic cocaine users. On both tasks, cocaine users had consistently higher error rates and significantly longer response latencies than controls. Dorsal striatal activity in cocaine users was more diffuse, less segregated and less lateralized than controls regardless of the task demands. These data demonstrate that in chronic users the behavioral and functional effects of cocaine may extend well beyond the ventral striatum into the sensorimotor domains of the striatum.
The longer reaction times and higher error rates of cocaine users while performing both the internally-guided and externally-guided motor tasks as seen here are consistent with several studies documenting slower response times (Bolla et al., 2004; Bolla et al., 1999; Roberts and Bauer, 1993) and decreased visuomotor accuracy in cocaine users (Kubler et al., 2005). Beyond changes in these basic indicators of sensorimotor function, cocaine use has been associated with a variety of movement disorders including tics, dystonia, and dyskinesias (Bauer, 1996b; Cardoso and Jankovic, 1993; Chouinard and Ford, 2000; Pascual-Leone and Dhuna, 1990). Unlike many previous reports, however, the current study was designed to systematically investigate motor deficits and related dorsal striatal dysfunction in chronic cocaine users. Although cocaine users were impaired on both tasks, the accuracy of internally-guided movements was significantly more affected than externally-guided movement. This may be due to a decreased ability to maintain the sequence in working memory (Kubler et al., 2005; Kubler et al., 2003) or an inability to learn the short, predictable sequences (Fillmore and Rush, 2006). These data demonstrate that, while both tasks are negatively impacted by chronic cocaine use, internally-guided movement is more affected than externally-guided performance.
The inability to perform internally and externally-guided movements is a symptom of various dorsal striatal diseases. For example, Parkinson’s disease patients are specifically impaired on the performance of internally-guided (or memory cued) movements, when compared to their performance on externally-guided (visually cued) tasks (Jackson et al., 1995; Jahanshahi et al., 1995). Externally-guided movements remain largely intact in these patients and, in fact, the availability of external cues can improve performance on internally-guided tasks (Brown and Marsden, 1988). The sparing of externally-guided motor performance has been hypothesized to be due to preservation of activity in cerebellar-thalamo-cortical loops (Lewis et al., 2007). Activity in these additional motor areas may be sufficient to maintain performance despite dorsal striatal dysfunction (Lewis et al., 2007). In contrast, the performance of Huntington’s disease patients is impaired on both internally and externally-guided movements (Johnson et al., 2000), with greater impairment generally observed on internally-guided tasks (Delval et al., 2007). Although the motor deficits observed in cocaine users were not as severe as the bradykinesia associated with Parkinson’s disease or the dyskinesias present in Huntington’s disease, the performance of cocaine users resembled the performance of Huntington’s patients, as they were unable to use external cues to perform these tasks more accurately. This may indicate an inability to recruit other circuits to compensate for striatal dysfunction.
Corresponding to the impairments in performance of the internally-guided and externally-guided tasks, cocaine users exhibited disrupted patterns of activation within both the caudate and putamen. When total activation across the dorsal striatum was considered without regard to the specific subdivision (left and right caudate and putamen), there were no differences between cocaine users and controls. The distribution of activity among these regions, however, was significantly different between the groups. Consistent with prior studies, within the striatum controls relied largely on the contralateral caudate for internally-guided movement, and the contralateral putamen for externally-guided movement (Debaere et al., 2003; Jahanshahi et al., 1995; Jueptner and Weiller, 1998; Kawashima et al., 2000). In contrast, there was little differentiation between the patterns of activation of cocaine users regardless of the motor task demands. Performance of both the internally-guided and externally-guided tasks by cocaine users resulted in non-specific activation patterns across the dorsal striatum. As with the behavior, these diffuse patterns of activation suggest global deficits involving all dorsal striatal subdivisions.
One potential explanation for the disparities in dorsal striatal activity is the difference in task proficiency between these two groups. In the early stages of motor learning, diffuse functional activity occurs in motor-related neural networks (Karni et al., 1998; Ungerleider et al., 2002; Wu et al., 2004). As task proficiency improves, activity becomes less diffuse and more efficient. This functional consolidation has been observed in both cortical (Karni, 1996; Karni et al., 1998) and subcortical areas (Wu et al., 2004). As both caudate and putamen are involved in skill learning (Haruno et al., 2004; Tricomi and Fiez, 2008), functional consolidation likely occurs in these areas as well. The poor performance of cocaine users manifested by longer reaction times and higher error rates may reflect early-stage motor learning deficits (Karni et al., 1995; Karni et al., 1998). While this was not a primary outcome measure of this investigation it is valuable to note that during the practice session the cocaine users required more practice than the controls to acquire 90% proficiency (see Methods), further supporting the interpretation that there may be differences in motor learning between the groups. Furthermore, while cocaine users readily achieved 90% proficiency during the practice session, proficiency in the MRI scanner was 84.5% for internally-guided movement. This decrement in proficiency in the MRI scanner, wherein the participants were engaged in the task for approximately 15 minutes, suggests that there may be an interaction between sustained attention and sensorimotor deficits in these users. Further studies are needed to determine whether the functional segregation observed in control participants would emerge in cocaine users following extended practice and improved performance.
Alternatively, the diffuse striatal activity in cocaine users may reflect an underlying inefficiency or disorganization of neural activity during the performance of each of the motor tasks. Inefficiency may be due to alterations in brain structure (Pulipparacharuvil et al., 2008; Ujike et al., 2002), neurochemistry (Letchworth et al., 2001; Porrino et al., 2004), and/or vascularization (Browndyke et al., 2004; Kosten et al., 1998) that accrue with chronic cocaine use. Such alterations may lead to substantial functional reorganization (Graybiel, 2004; Nestler, 2001). There may also be adaptive plasticity that occurs alongside progressive pathology, as is seen in stroke patients and in animal models of ischemia (Hallett, 2001; Nudo, 1999; Nudo and Milliken, 1996). Whether local plasticity is adaptive or counteradaptive, striatal remodeling may interrupt neural architecture within the caudate and putamen contributing to a loss of functional organization associated with internally-guided and externally-guided movements.
There are some limitations of the current study that should be considered. First, the relatively small sample size (14/group) and the relatively long durations of cocaine use among the participants (11–25 years) limit the ability to address the relationship between drug history, motor performance and striatal function. Second, the rate of both cigarette smoking and recent marijuana use among cocaine users was higher than among controls. To avoid any deleterious effects of nicotine withdrawal on motor performance, all subjects were given the opportunity for a “smoke break” up to an hour prior to image acquisition. Given that nicotine has been shown to have performance enhancing effects on visuomotor task performance (Foulds et al., 1996), however, smokers would be expected to perform better than non-smokers which was not evident in the present study. The higher error rates and slower reaction times in cocaine users are not likely due to concomitant marijuana use, as marijuana use does not appear to impair finger-tapping performance, nor dorsal striatal activity associated with finger-tapping (Pillay et al., 2004). The cocaine users also had a lower average IQ than the control group. Although there is some evidence that IQ affects motor performance (Sen et al., 1983), the application of an analysis of covariance did not alter the robust differences between cocaine users and controls on motor performance. A further analysis did not reveal significant correlations between IQ score and ROI signal change. This further diminishes the likelihood of IQ as a confound in our results. Finally, there are always difficulties in the interpretation of data from substance abusers. It is not possible to distinguish between the effects of substance abuse and the existence of psychiatric conditions that may have pre-dated drug dependence. The lack of significant correlations between length of cocaine use and either motor performance or dorsal striatal activity is likely due to the exclusive presence of long-term cocaine users specifically recruited for this study. Given that all of the participants had used cocaine for at least 11 years, we are not able to address the question of whether these movement and dorsal striatal abnormalities are present during the early phases of cocaine dependence. Despite these limitations, these data reveal clear deficits in the performance of simple motor tasks by long-term cocaine users.
In summary, this investigation demonstrates that chronic cocaine users perform poorly on both internally-guided and externally-guided motor tasks. Furthermore, cocaine users exhibited significant disruption in the activation of the dorsal striatal regions that subserve performance on these tasks. These behavioral and neurofunctional abnormalities are more prominent when cocaine users rely on internal, rather than external, cues to guide their movements, suggesting the cognitive demands of the internally-guided task may play an important role in this sensorimotor impairment. The dysfunction of sensorimotor domains of the dorsal striatum exhibited in this study are buttressed by animal models that have shown progressive involvement of the cognitive and sensorimotor domains of the dorsal striatum with increasing durations of cocaine exposure (Porrino et al., 2004). This dysfunction may reflect considerable adaptive and counteradaptive neuroplasticity that occurs within the striatum following chronic cocaine use (Koob, 1996; Nestler, 2001). While the movement disruptions of cocaine users observed in the present study may not significantly impair activities of daily living, they provide a behavioral marker of dorsal striatal dysfunction and lend insight into the extent of neural disruption in these chronic cocaine users.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Wirshing DA, Lu PH, Foster JA, Mintz J. Choreoathetoid movements in cocaine dependence. Biol Psychiatry. 1999;45:1630–1635. doi: 10.1016/s0006-3223(98)00238-8. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Psychomotor and electroencephalographic sequelae of cocaine dependence. NIDA Res Monogr. 1996a;163:66–93. [PubMed] [Google Scholar]
- Bauer LO. Resting hand tremor in abstinent cocaine-dependent, alcohol-dependent, and polydrug-dependent patients. Alcohol Clin Exp Res. 1996b;20:1196–1201. doi: 10.1111/j.1530-0277.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. The role of the basal ganglia in motor control: contributions from PET. J Neurol Sci. 1995;128:1–13. doi: 10.1016/0022-510x(94)00206-4. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. An investigation of the phenomenon of "set" in Parkinson's disease. Mov Disord. 1988;3:152–161. doi: 10.1002/mds.870030207. [DOI] [PubMed] [Google Scholar]
- Browndyke JN, Tucker KA, Woods SP, Beauvals J, Cohen RA, Gottschalk PC, Kosten TR. Examining the effect of cerebral perfusion abnormality magnitude on cognitive performance in recently abstinent chronic cocaine abusers. J Neuroimaging. 2004;14:162–169. [PubMed] [Google Scholar]
- Cardoso FE, Jankovic J. Cocaine-related movement disorders. Mov Disord. 1993;8:175–178. doi: 10.1002/mds.870080210. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard S, Ford B. Adult onset tic disorders. J Neurol Neurosurg Psychiatry. 2000;68:738–743. doi: 10.1136/jnnp.68.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gokcay D, Mohr CM, Auerbach EJ, Freeman AJ, Leonard CM, Briggs RW. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. J Cogn Neurosci. 2001;13:272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. 2003;19:764–776. doi: 10.1016/s1053-8119(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Delval A, Krystkowiak P, Blatt JL, Labyt E, Bourriez JL, Dujardin K, Destee A, Derambure P, Defebvre L. A biomechanical study of gait initiation in Huntington's disease. Gait Posture. 2007;25:279–288. doi: 10.1016/j.gaitpost.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Elsinger CL, Harrington DL, Rao SM. From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006;31:1177–1187. doi: 10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Network-level neuroplasticity in cortico-basal ganglia pathways. Parkinsonism Relat Disord. 2004;10:293–296. doi: 10.1016/j.parkreldis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol. 1995;362:400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res Brain Res Rev. 2001;36:169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, Imamizu H, Kawato M. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. J Neurosci. 2004;24:1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Jackson GM, Harrison J, Henderson L, Kennard C. The internal control of action and Parkinson's disease: a kinematic analysis of visually-guided and memory-guided prehension movements. Exp Brain Res. 1995;105:147–162. doi: 10.1007/BF00242190. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118(Pt 4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Bennett JE, Georgiou N, Bradshaw JL, Chiu E, Cunnington R, Iansek R. Bimanual co-ordination in Huntington's disease. Exp Brain Res. 2000;134:483–489. doi: 10.1007/s002210000485. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121(Pt 8):1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- Karni A. The acquisition of perceptual and motor skills: a memory system in the adult human cortex. Brain Res Cogn Brain Res. 1996;5:39–48. doi: 10.1016/s0926-6410(96)00039-0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Tajima N, Yoshida H, Okita K, Sasaki T, Schormann T, Ogawa A, Fukuda H, Zilles K. The effect of verbal feedback on motor learning--a PET study. Positron emission tomography. Neuroimage. 2000;12:698–706. doi: 10.1006/nimg.2000.0643. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr. 1994;145:1–18. [PubMed] [Google Scholar]
- Kosten TR, Cheeves C, Palumbo J, Seibyl JP, Price LH, Woods SW. Regional cerebral blood flow during acute and chronic abstinence from combined cocaine-alcohol abuse. Drug Alcohol Depend. 1998;50:187–195. doi: 10.1016/s0376-8716(98)00038-6. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Kaufman J, Stein EA, Garavan H. Co-ordination within and between verbal and visuospatial working memory: network modulation and anterior frontal recruitment. Neuroimage. 2003;20:1298–1308. doi: 10.1016/S1053-8119(03)00400-2. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, McKeown MJ, Mailman RB, Belger A, Huang X. Task specific influences of Parkinson's disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience. 2007;147:224–235. doi: 10.1016/j.neuroscience.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H, Tunving K. An extrapyramidal choreiform syndrome caused by amphetamine addiction. J Neurol Neurosurg Psychiatry. 1981;44:728–730. doi: 10.1136/jnnp.44.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Hadfield MG, Dellovade TL, Price DL. The striatal mosaic in primates: patterns of neuropeptide immunoreactivity differentiate the ventral striatum from the dorsal striatum. Neuroscience. 1991;43:397–417. doi: 10.1016/0306-4522(91)90303-6. [DOI] [PubMed] [Google Scholar]
- Menon V, Glover GH, Pfefferbaum A. Differential activation of dorsal basal ganglia during externally and self paced sequences of arm movements. Neuroreport. 1998;9:1567–1573. doi: 10.1097/00001756-199805110-00058. [DOI] [PubMed] [Google Scholar]
- Nakai T, Kato C, Glover GH, Toma K, Moriya T, Matsuo K. A functional magnetic resonance imaging study of internal modulation of an external visual cue for motor execution. Brain Res. 2003;968:238–247. doi: 10.1016/s0006-8993(03)02249-2. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol. 1999;9:740–747. doi: 10.1016/s0959-4388(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Dhuna A. Cocaine-associated multifocal tics. Neurology. 1990;40:999–1000. doi: 10.1212/wnl.40.6.999. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G, Jon DI, Gruber S, Simpson N, Cherayil M, Pope HG, Yurgelun-Todd DA. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: an fMRI study. Drug Alcohol Depend. 2004;76:261–271. doi: 10.1016/j.drugalcdep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Bauer LO. Reaction time during cocaine versus alcohol withdrawal: longitudinal measures of visual and auditory suppression. Psychiatry Res. 1993;46:229–237. doi: 10.1016/0165-1781(93)90091-t. [DOI] [PubMed] [Google Scholar]
- Romero MC, Bermudez MA, Vicente AF, Perez R, Gonzalez F. Activity of neurons in the caudate and putamen during a visuomotor task. Neuroreport. 2008;19:1141–1145. doi: 10.1097/WNR.0b013e328307c3fc. [DOI] [PubMed] [Google Scholar]
- Rylander G. Psychoses and the punding and choreiform syndromes in addiction to central stimulant drugs. Psychiatr Neurol Neurochir. 1972;75:203–212. [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sen A, Jensen AR, Sen AK, Arora I. Correlation between reaction time and intelligence in psychometrically similar groups in America and India. Appl Res Ment Retard. 1983;4:139–152. doi: 10.1016/0270-3092(83)90006-1. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Evaluating relationships between craving and drug use. Addiction. 2000;95:1106–1107. doi: 10.1046/j.1360-0443.2000.957110613.x. author reply 1107. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Fiez JA. Feedback signals in the caudate reflect goal achievement on a declarative memory task. Neuroimage. 2008;8:1154–1167. doi: 10.1016/j.neuroimage.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensitization to psychostimulants. Ann N Y Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol. 2004;91:1690–1698. doi: 10.1152/jn.01052.2003. [DOI] [PubMed] [Google Scholar]
- Zito KA, Vickers G, Roberts DC. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1985;23:1029–1036. doi: 10.1016/0091-3057(85)90110-8. [DOI] [PubMed] [Google Scholar]