Abstract
Every year, millions of health care, first responder, and industry workers are exposed to chemical and biological hazards. Disposable nitrile gloves are a common choice as both a chemical and physical barrier to these hazards, especially as an alternative to natural latex gloves. However, glove selection is complicated by the availability of several types or formulations of nitrile gloves, such as low-modulus, medical-grade, low-filler, and cleanroom products. This study evaluated the influence of simulated movement on the physical integrity (i.e., holes) of different nitrile exam glove brands and types. Thirty glove products were evaluated out-of-box and after exposure to simulated whole-glove movement for 2 hr. In lieu of the traditional 1-L water-leak test, a modified water-leak test, standardized to detect a 0.15 ± 0.05 mm hole in different regions of the glove, was developed. A specialized air inflation method simulated bidirectional stretching and whole-glove movement. A worst-case scenario with maximum stretching was evaluated. On average, movement did not have a significant effect on glove integrity (chi-square; p=0.068). The average effect was less than 1% between no movement (1.5%) and movement (2.1%) exposures. However, there was significant variability in glove integrity between different glove types (p ≤ 0.05). Cleanroom gloves, on average, had the highest percentage of leaks, and 50% failed the water-leak test. Low-modulus and medical-grade gloves had the lowest percentages of leaks, and no products failed the water-leak test. Variability in polymer formulation was suspected to account for the observed discrepancies, as well as the inability of the traditional 1-L water-leak test to detect holes in finger/thumb regions. Unexpectedly, greater than 80% of the glove defects were observed in the finger and thumb regions. It is recommended that existing water-leak tests be re-evaluated and standardized to account for product variability.
Keywords: dermal protection, infection control, penetration, personal protective clothing, personal protective equipment, protective gloves
INTRODUCTION
Every year, millions of healthcare, first responder, and general industry workers are exposed to chemical and biological hazards in the workplace.(1,2) Disposable nitrile gloves are a common choice as both a chemical and physical barrier to these hazards, especially as a viable alternative to natural latex exam gloves.(2–4) Nitrile gloves offer a latex-free advantage, thus reducing potential allergy concerns. However, glove selection is complicated by the availability of several types or formulations of nitrile gloves, such as low-modulus (low ratio of stress to applied strain; stretches easier with less resistance), general duty, medical grade, low filler, and cleanroom products. It is anticipated that the abundance or absence of plasticizers, oils, and fillers may have an effect on glove integrity, as well as performance under worker-use conditions. Supporting evidence in the scientific literature is provided below.
Zinner(5) found considerable variability in glove integrity between brands of unused disposable examination gloves; glove defects or failures (water leakage upon testing) ranged from 0 to 5.7%. Three glove products had no leak failures or defects, whereas one glove product was defective. Muto et al.(6) also concluded there were inequalities in integrity between different glove brands, both for surgical and exam gloves. One of the seven exam glove products tested had defects (leakage) above the established criteria of 4.0%. In an investigation of chemical resistance, acrylonitrile content of disposable nitrile gloves varied significantly between glove products and was correlated with chemical resistance to the pesticide captan.(7) Striking disparities between manufacturer formulations were observed. Acrylonitrile content, a functional component of nitrile butadiene rubber (NBR), accounted for about 85% of the observed variability in chemical resistance. Variability in acrylonitrile was likely due to differences in filler and plasticizer content, as well as the NBR formulation (base polymer). The findings of this study support the notion that nitrile glove formulations are variable, even for similar products. Lastly, simulated movement using a robotic hand resulted in consistent glove failures in only one of three disposable nitrile exam glove products.(8) The glove that failed with exposure to movement was reportedly accelerator free. The above studies indicate that nitrile exam gloves are variable in (1) physical integrity, (2) resistance to mechanical stress, (3) chemical resistance, and (4) composition. More research is needed to uncover the underlying causes for these observed disparities with nitrile exam gloves and to narrow the observed gaps in glove integrity and chemical resistance performance.
Jackson et al.(4) indicated the need for a scientifically based glove selection criteria for health care workers, with respect to biomechanical performance. In addition, the National Fire Protection Agency and various consensus standards have provided criteria and guidance on the proper selection of emergency medical examination gloves.(9,10) However, the current standards have not addressed the potential influence of movement on glove integrity. Polymer formulation such as plasticizer and filler contents should be considered in the establishment of standards or certifications. For example, the overabundance of a nonpolar (oily) plasticizer is likely to repel water and result in a favorable water resistance but greatly reduce chemical resistance to nonpolar solvents. Conversely, the absence of plasticizers would likely produce a stiffer polymer, which would be affected more by movement. Differences in the NBR formulations used to produce gloves are also likely to affect glove integrity and chemical resistance. It is unlikely that current standards adequately address the abovementioned disparities and questions regarding in-use performance. Most glove standards address watertight barrier properties, physical properties (e.g., thickness and dimensions), and tensile properties. Limitations on plasticizer and filler contents, which may affect in-use performance, are uncommon.
As for glove integrity, the question still remains on how different glove types and formulations perform under simulated in-use stretching and movement. Korniewicz et al.(11) tested the post-use integrity of thicker surgical gloves and found significantly higher failure percentages in nitrile gloves compared with natural latex. Much of this was likely due to cuts and punctures. Nonetheless, there were indications that glove integrity became increasingly compromised with duration of use. Unfortunately, the nitrile gloves in this study were not evaluated for duration of use. To date, information on the in-use performance of disposable nitrile gloves is limited.
Current critical gaps in knowledge include:
Are there significant changes in the protection afforded by protective gloves under worker-use conditions (aside from cuts and punctures) with whole-glove movement?(12)
How do different glove types perform (e.g., low-modulus) under worker-use conditions with whole-glove movement?
The purpose of this study was to evaluate the influence of simulated movement on the physical integrity of different nitrile exam glove brands and types. Glove products were investigated both out-of-box and after exposure to simulated whole-glove movement for 2 hr. Gloves were selected to represent the array of commercially available product formulations, under the broad classifications of general duty, medical grade, low-modulus, and cleanroom (controlled environment) gloves. Glove integrity testing was conducted using a modified water-leak test that was standardized to detect a small hole in various regions of the glove.
MATERIALS AND METHODS
Gloves
Thirty different commercially available glove products were tested and included representatives from each general classification of general duty (10), medical grade (9), cleanroom (6), and low-modulus (5) gloves. All gloves were medium sized with a reported palm thickness of 4 to 5 mil. For each glove product, 30 thickness measurements (9 finger, 3 thumb, 9 knuckle, and 9 palm) were performed using a digital micrometer with constant force ratchet-stop to ensure precision. Glove samples were conditioned overnight in a controlled relative humidity (RH) chamber at 51± 4% RH and 21.1± 0.5°C. The glove manufacturer/brand, type, and thickness information are provided in Table I.
TABLE I.
Glove Brand and Thickness Information
| ID | Manufacturer/Brand | Type | Average Glove Thickness (mm) |
|---|---|---|---|
| 1 | Ammex Xtreme X3 | G | 0.08 ± 0.02 |
| 2 | Ansell Micro-Touch NitraFree | M | 0.103 ± 0.006 |
| 3 | Ansell Nitrilite (low filler) | C | 0.11 ± 0.01 |
| 4 | Ansell Touch N Tuff (low filler) | G | 0.109 ± 0.007 |
| 5 | Best Clean-Dex | C | 0.15 ± 0.03 |
| 6 | Best N-Dex 6005 | M | 0.123 ± 0.007 |
| 7 | Best N-Dex Free (accelerator free) | M | 0.12 ± 0.01 |
| 8 | Cardinal Health Esteem Tru-Blu Stretchy | L | 0.11 ± 0.01 |
| 9 | Fisherbrand Nitrile | M | 0.098 ± 0.009 |
| 10 | Henry Schein Criterion | G | 0.083 ± 0.009 |
| 11 | High Five Cobalt | L | 0.10 ± 0.01 |
| 12 | High Five Onyx | M | 0.12 ± 0.01 |
| 13 | High Five Softwear | L | 0.10 ± 0.02 |
| 14 | Kimberly Clark Kimtech G5 | C | 0.090 ± 0.006 |
| 15 | Kimberly Clark KleenGuard G10 | G | 0.11 ± 0.02 |
| 16 | Medline Sensicare | M | 0.09 ± 0.02 |
| 17 | Microflex CE4 System | C | 0.14 ± 0.03 |
| 18 | Microflex Midknight | G | 0.11 ± 0.01 |
| 19 | Microflex Supreno SE | G | 0.13 ± 0.01 |
| 20 | Microflex Ultrasense | L | 0.095 ± 0.009 |
| 21 | North Chem Soft CE | CA | 0.12 ± 0.02 |
| 22 | North Dexi-Task | G | 0.10 ± 0.02 |
| 23 | Omar Nitrile | G | 0.11 ± 0.02 |
| 24 | PIP Ambi-dex | G | 0.11 ± 0.01 |
| 25 | Prima Pro Gentle Guard | L | 0.11 ± 0.01 |
| 26 | QRP Q095 Qualatrile XC (low filler) | C | 0.12 ± 0.01 |
| 27 | QRP Qualatrile Blue 5 (no plasticizer) | M | 0.11 ± 0.02 |
| 28 | Safety Choice Nitrile | G | 0.11 ± 0.02 |
| 29 | Sempermed SemperSure | M | 0.088 ± 0.005 |
| 30 | Tillotson True Advantage | M | 0.096 ± 0.008 |
Notes: C=cleanroom; G=general-duty, L=low-modulus, M=medical grade.
The glove was also a low-modulus product but was treated as a cleanroom glove.
Simulated Movement
Air inflation was used to simulate whole-glove movement. Bidirectional stretching of a glove donned on a gloved human hand was measured and then matched by inflating the same glove with air. Each glove was evaluated first on a single human hand and then placed on an air inflation device for evaluation at different gauge pressures. The goal was to closely match the gauge pressure with both (1) the palmar (ventral) stretch during hand extension, and (2) the dorsal stretch during hand flexion or grasping. The steps are outlined below.
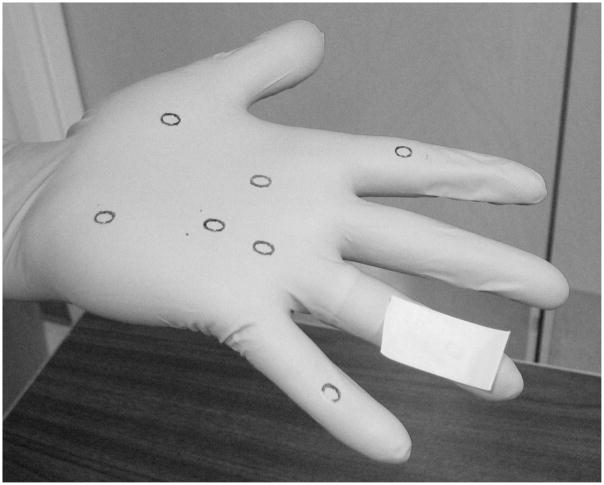
A stratified random selection process was used to select six finger, six knuckle, six palm, and two thumb samples for each glove tested. A 5.5 × 7.3 mm rubber stamp (letter “O”) was used to measure bidirectional stretching on the 10 dorsal and 10 ventral regions of each glove.
A medium-sized glove was donned on a properly sized, as per the manufacturer, human hand. A total of five different human hands from the research staff were evaluated in this study.
The subject first hyperextended his/her hand, and 2 × 3 cm sections of drafting tape were used to capture the stretch of each ventral (palmar) ink stamp (Figure 1). All tape samples were placed on a prelabeled clear plastic sheet.
The subject then lightly grasped a 1.5 inch (3.8 cm) diameter cylinder, and drafting tape was used to capture the stretch of each dorsal ink stamp.
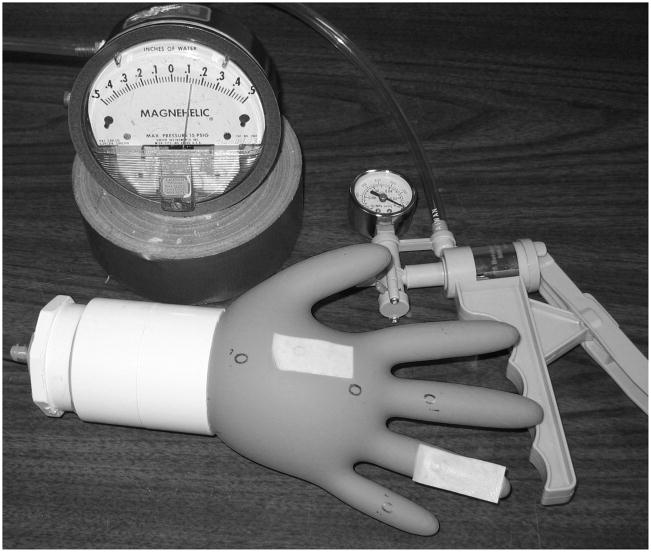
The glove was carefully doffed and then placed on a specialized glove adapter (Figure 2) with attached magnehelic pressure gauge and hand air pump. The adapter consisted of a 2-inch PVC cap with installed ¼-inch hose barb and a 2-inch diameter by 3-inch long PVC coupling. The gloves were positioned between the adapter and coupling, ensuring that the cuff region was isolated within the coupling.
The glove was inflated to 0.06, 0.08, 0.10, and 0.12 inches of water (gauge pressure), and at each stage, masking tape was used to capture the stretch of the ink stamp.
Each tape sample with captured ink marks was matched with the original mark from Step 3 or 4 and then placed on the clear plastic sheet.
The plastic sheets with tape samples were attached to the glass of a well-lighted fume hood. A digital caliper was used to measure the width and height of the “O” ink stamp for each tape sample.
A paired t-test was used to compare bidirectional stretching (width and height) for each glove region and hand posture. The criteria for proper simulation was no significant difference (p>0.05) between paired samples (same ink stamp) for each direction, region, and the combined regions.
FIGURE 1.
Tape lift (ring finger) of an ink stamp on the ventral region of an extended gloved hand. Drafting tape was used to capture the bidirectional stretch of an ink mark.
FIGURE 2.
Glove inflation apparatus with hand pump, pressure gauge, and specialized cuff adaptor
Variation existed between the five subjects (research staff), with the optimal inflation gauge pressure varying from 0.08 to 0.10 inches water. The truncated results are presented in Table II. An inflation gauge pressure of 0.08 inches water was most favorable; however, 0.10 inches of water was a limitation of the available pneumatic controller (described later). Preliminary testing with one glove, not included in this study, indicated no significant change in glove integrity after exposure to repeated inflation at 0.10 inches water gauge pressure for up to 8 hr. In contrast, a measurable effect was observed in concurrent chemical permeation testing at 0.10 inches of water gauge pressure. Thus, the inflation pressure in this study was increased in an attempt to uncover any measureable effects of movement on glove integrity. The exposure in this study was a worst-case scenario, comparable to an improperly fitted glove. Inflation was increased to 0.20 inches water gauge pressure, which was approximately equivalent to an extra-large size hand (research staff) using a medium-sized glove. Such a scenario can occur in the event an organization purchases only one or two glove sizes for a diverse work force.
TABLE II.
Optimal Inflation Pressure
| Pressure (inches H20) | Number of Test Subjects
|
||
|---|---|---|---|
| Underinflation | No Significant Difference (p > 0.05) | Overinflation | |
| 0.06 | 5 | — | — |
| 0.08 | 1 | 3 | 1 |
| 0.10 | — | — | 5 |
| 0.12 | — | — | 5 |
Notes: A total of five test subjects (research staff) were evaluated against each of the inflation pressures. Each row totals to exactly five and represents how the test subject data compared with the inflation pressure data. Underinflation indicated that the bidirectional stretching was significantly less than the test subject (p ≤ 0.05), whereas overinflation indicated stretching was significantly greater (p ≤ 0.05).
A pneumatic controller (Geocontrol Pro, Geotech, Denver, Colo.) was used to inflate and deflate up to eight gloves at a time for a total of 2 hr. Gloves were attached to the airline manifold using -inch nylon tubing and the specialized glove adapter described earlier. The inflation and deflation cycle time was 30 sec, which included a 5 sec-static time between stages to ensure complete deflation. The gloves were in continual movement more than 80% of the time. A total of 240 inflation and deflation actions occurred during the 2-hr exposure.
Glove Integrity Test Procedure
A modified water-leak test (Figure 3) was used in this study. It was previously determined that the American Society of Testing and Materials (ASTM) Method D5151(13) and the Food and Drug Administration (FDA) Medical Glove Guidance Manual(14) water-leak tests did not reliably detect a needle puncture (21-gauge) in either the thumb or pinky of the gloves used in this study. This possibility was anticipated based on the reported disparities from previous studies,(5,6) as well as on a report that indicated the inability of the water-leak test to identify holes in the index, middle, and ring fingers.(15) Most notably, the thumb and pinky regions were not evaluated in the previous studies, which was an area on concern. Thus, a standardized method was developed to detect holes in the finger, thumb, and palm regions. The cuff region was precluded, because stretching was determined to be greatest in the palm, knuckle, and finger regions.
FIGURE 3.
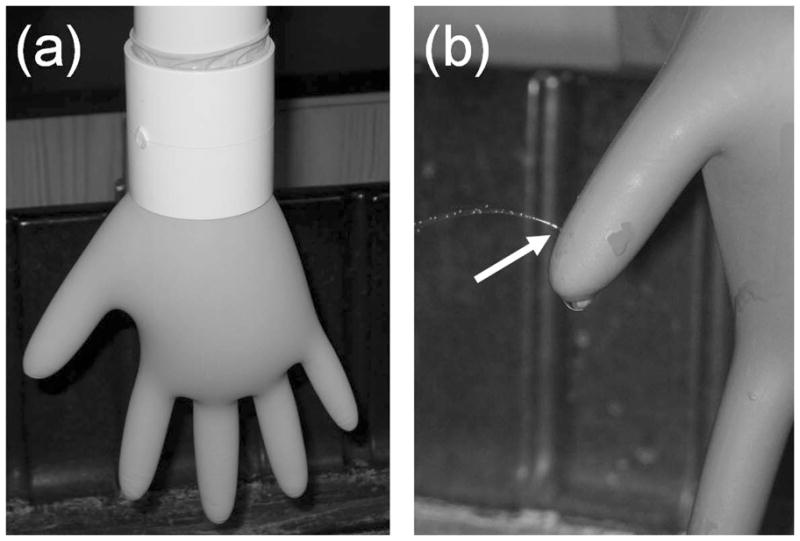
Modified water-leak test apparatus: (a) a specialized adapter was used to restrict glove expansion in the cuff region, and (b) leaks (indicated by the arrow) were detected visually
Stretching in the cuff region was restricted using a 3 inch (7.6 cm) length coupling between the glove and adjoining 2 inch (5.1 cm) diameter and 24 inch (61 cm) length water column. This increased the water pressure in the rest of the glove and helped increase the sensitivity of the water-leak test in the thickest regions of the glove, which were the fingers and thumb. The water-leak test was standardized to detect a small 30-gauge needle hole (BD Ultra-Fine II Lancets; Fisher Safety, Hanover Park, Ill.) in various regions of the glove. For each glove product, holes were made in the finger, thumb and palm regions of 10 gloves via insertion of the 30-gauge needle through an inflated glove. Each glove was lightly inflated with compressed air prior to puncture with the needle. The holes were crescent-shaped with an average length of 0.15 ± 0.05 mm. A crescent-shaped hole was selected over a round hole because it best mimicked observed glove defects/holes in preliminary tests. Glove defects were most often long, slender slits in the polymer. Glove holes, as identified by water-leak test, were either crescent-shaped or linear slits in the glove material. A total of 27 holes (20 finger, 5 thumb, and 2 palm) were evaluated for each glove product. Initially, 1 L of water was added to the water-leak test device. The glove was immediately inspected for leaks in each of the locations with known holes. If a readily observable water leak (water jet or steady dripping) was not observed, then an additional 0.5 L of water was added. The process was repeated until water leaks were easily and reliably detected for all holes. It was previously determined that the addition of up to 2.5 L of water did not increase the number of holes detected beyond the 30-gauge hole. For each glove product, all subsequent water-leak testing was performed using the maximum required water volume. The water volumes ranged between 1 to 2 L. It must be noted that the modified water-leak test was standardized for a defined hole size and not a water volume.
The standardized water-leak results are presented in Table III. The low-modulus gloves required no excess water to detect known holes, whereas 50% of the cleanroom gloves required extra water. About 22% of the medical grade gloves required extra water, which indicated that the 1 L water-leak test should be re-evaluated for medical grade approvals. These results showed that disparities in glove properties and formulation existed between glove products of the same general type (e.g., medical grade).
TABLE III.
Summary of Standardized Water-Leak Results
| Glove Type | Percent Requiring Extra WaterA |
|---|---|
| Cleanroom | 50% |
| Medical grade | 22% |
| General duty | 30% |
| Low-modulus | 0% |
Percentage of glove products that required greater than 1 L water to detect known 30-gauge needle holes in the finger, thumb, and palm regions of 10 glove samples.
The sampling protocol was conducted using FDA guidance (Patient Examination Gloves and Surgeons’ Gloves; Sample Plans and Test Method for Leakage Defects; Adulteration 20CFR800.20).(16) An adulteration level of 4.0% was used to detect defective glove products on a pass or fail basis. If the total number of leaks or defects exceeded 4.0%, then the glove product failed the water-leak test. The newer adulteration level of 2.5 was not used in this study because testing was initiated prior to the recent changes, and most glove products were not medical grade or FDA approved. The goal of this study was to detect a significant increase in failures after exposure to movement, on a pass or fail basis. Initial sample size was 80. Each glove was closely observed for leaks immediately after the introduction of water and then again after 2 min. The locations of leaks were also recorded. If more than 2 defective gloves were detected, then a second sample of 80 gloves was leak tested. If more than 7 defective gloves were detected, then a third sample of 80 gloves was leak tested. Sample sizes were restricted to a total 240 gloves for each glove product. The glove product failed if the percentage of defective gloves was greater than 4.0%.
Statistical Analyses
Statistical analyses included Student t, analysis of variance (ANOVA), correlation, regression, and, when appropriate, nonparametric methods. Results were deemed significant if the p value was not larger than 0.05. At least 10 samples were used to define arithmetic means and standard deviations (SD). Multiple/logistic regression, correlation, and ANOVA analyses were performed using Stata version 11 (StataCorp, College Station, Texas). Tests for the normal distribution of individual variables were conducted with Shapiro-Wilks, Shapiro-Francia, and skewness/kurtosis normality tests. Comparisons of failure percentages among glove types and simulated movement vs. nonmovement exposures were conducted using chi-square (χ2) analysis.
RESULTS
Simulated Movement
Glove failure percentages by glove type and simulated movement are presented in Table IV. Simulated movement does not appear to influence product failure, defined as a failure percentage above 4.0%. Gloves 5 and 23 passed initially (out-of-box) and then failed after exposure to simulated movement. However, the opposite occurred for Gloves 21 and 28. Glove 3 failed both before and after exposure the movement. Therefore, the pass/fail data were inconclusive regarding the influence of whole-glove movement on glove integrity.
TABLE IV.
Glove Failure Percentages by Glove Type and Simulated Movement
| Type | ID | Total Gloves Tested | Failure Percent (%)
|
||
|---|---|---|---|---|---|
| No Movement (out-of-box) | Simulated Movement | Combined Results | |||
| Cleanroom | 3 | 320 | 6.25 | 5.8 | 5.9 |
| 5 | 320 | 1.25 | 4.6 | 3.7 | |
| 14 | 160 | 0 | 0 | 0 | |
| 17 | 160 | 1.25 | 0 | 0.6 | |
| 21 | 340* | 6.25 | 2.2 | 4.7 | |
| 26 | 160 | 0 | 1.25 | 0.6 | |
| Cleanroom totals | 2.5 ± 3.0 | 2.3 ± 2.4 | 2.6 ± 2.5 | ||
| Medical grade | 2 | 160 | 1.25 | 2.5 | 1.9 |
| 6 | 160 | 1.25 | 0 | 0.6 | |
| 7 | 160 | 0 | 0 | 0 | |
| 9 | 240 | 0 | 1.9 | 1.2 | |
| 12 | 160 | 0 | 1.25 | 0.6 | |
| 16 | 160 | 0 | 1.25 | 0.6 | |
| 27 | 160 | 0 | 1.25 | 0.6 | |
| 29 | 160 | 0 | 0 | 0 | |
| 30 | 160 | 0 | 0 | 0 | |
| Medical grade totals | 0.3 ± 0.6 | 0.9 ± 0.9 | 0.6 ± 0.6 | ||
| General duty | 1 | 160 | 1.25 | 1.25 | 1.25 |
| 4 | 160 | 3.75 | 1.25 | 3.1 | |
| 10 | 160 | 0 | 2.5 | 1.25 | |
| 15 | 160 | 1.25 | 2.5 | 1.9 | |
| 18 | 160 | 1.25 | 0 | 0.6 | |
| 19 | 160 | 0 | 0 | 0 | |
| 22 | 160 | 1.25 | 0 | 0.6 | |
| 23 | 320 | 2.5 | 6.25 | 5.0 | |
| 24 | 160 | 0 | 0 | 0 | |
| 28 | 310 | 6.25 | 3.5 | 4.2 | |
| General duty totals | 1.8 ± 2.0 | 1.7 ± 2.0 | 1.8 ± 1.8 | ||
| Low-modulus | 8 | 160 | 0 | 1.25 | 0.6 |
| 11 | 160 | 1.25 | 0 | 0.6 | |
| 13 | 160 | 1.25 | 1.25 | 1.25 | |
| 20 | 180A | 1.25 | 1.0 | 1.1 | |
| 25 | 240 | 1.9 | 0 | 1.2 | |
| Low-modulus totals | 1.4 ± 0.3 | 0.6 ± 0.6 | 1.0 ± 0.3 | ||
These gloves were used to establish the feasibility of the modified water-leak test and sampling protocol. Initial sample sizes were n=100.
Of the 30 glove products tested, three cleanroom gloves and two general duty gloves failed either out-of-box or with simulated movement. The two general duty gloves were lower cost alternatives. No medical grade or low-modulus glove products had failure percentages greater than 2.5%. These results indicate the importance of glove formulation as a factor in glove integrity. The presence of additional plasticizers or formulation changes used to soften a glove product, as with the low-modulus gloves, does not appear to lessen glove integrity.
Table V shows the results of the chi-square analysis of simulated movement vs. observed leaks for an aggregate of all glove products combined. Based on the results of 5790 water-leak tests, movement was not found to have a significant effect on glove integrity (p = 0.068). In addition, the overall effect was small and less than 1% between movement (2.1%) and no movement (1.5%) exposures.
TABLE V.
Chi-square Movement vs. Glove Leaks (with Row Percentages) for an Aggregate of All Glove Products Combined
| Exposure | No Leaks | Leaks | Total |
|---|---|---|---|
| No Movement | 2520 (98.5%) | 38 (1.5%) | 2558 (100%) |
| Movement | 3163 (97.9%) | 69 (2.1%) | 3232 (100%) |
|
| |||
| Total | 5683 (98.2%) | 107 (1.8%) | 5790 (100%) |
Pearson chi-square=3.3195 (p=0.068)
Glove Type
Table VI shows the results of the chi-square analysis of glove type versus observed leaks. A significant difference in leaks was observed between the different glove types (p < 0.001). On average, the cleanroom gloves had the highest number of leaks (3.1%), followed by the general-duty gloves (2.25%). The medical-grade gloves had the lowest number of leaks (0.66%), on average, followed by the low-modulus gloves (1.0%). Glove integrity was significantly affected by glove type, which was also in agreement with the results presented in Table IV.
TABLE VI.
Chi-Square Glove Type vs. Glove Leaks (with Row Percentages)
| Glove Type | No leaks | Leaks | Total |
|---|---|---|---|
| Cleanroom | 1415 (96.92%) | 45 (3.08%) | 1460 (100%) |
| Medical grade | 1,510 (99.34%) | 10 (0.66%) | 1,520 (100%) |
| General duty | 1867 (97.75%) | 43 (2.25%) | 1,910 (100%) |
| Low-modulus | 891 (99.00%) | 9 (1.00%) | 900 (100%) |
|
| |||
| Total | 5683 (98.15%) | 107 (1.85%) | 5790 (100%) |
Pearson chi-square = 29.4104 (p < 0.001)
Glove failure percentages for each glove and glove type are summarized in Table IV. Variation was observed between glove products within each glove type. The coefficients of variation ranged from 64 to 200%. Significant differences in glove formulation between manufacturers are expected to account for this observed variation. Notwithstanding, the overall ranking of failure percentages between the four glove types was consistent for both out-of-box and simulated movement exposures. Cleanroom gloves had the highest average failure percentages, followed in descending order by the general duty, low-modulus, and medical grade gloves. These results and rankings are consistent with those presented in Table VI.
Table VII shows the location of glove leaks by glove type. Significant variation was observed between glove products within each glove type; however, a majority of the leaks (81.7%) occurred in the fingers and thumb. This indicated that the modified water-leak test reliably detected holes in the fingers and thumbs, which may otherwise go undetected without standardization.
TABLE VII.
Average Number of Glove Leaks by Location and Glove Type
| Glove Type | Thumb | Fingers | Palm |
|---|---|---|---|
| Cleanroom | 2.2 ± 3.3 | 2.4 ± 3.9 | 0.0 ± 0.0 |
| Medical-grade | 0.4 ± 1.0 | 0.4 ± 0.5 | 0.2 ± 0.4 |
| General-duty | 1.0 ± 1.2 | 2.6 ± 3.5 | 0.7 ± 1.3 |
| Low-modulus | 0.8 ± 1.6 | 1.3 ± 1.6 | 2.0 ± 3.0 |
|
| |||
| Percentage of total leaks | 30.3% | 51.4% | 18.3% |
Logistic Regression
Results of the logistic regression analyses of glove leaks by the experimental variables in this study are presented in Table VIII and are consistent with the previously reported results. Movement was not found to have a significant effect on glove leaks (p = 0.25). In contrast, glove type significantly affected glove leaks (p ≤ 0.01). On average, the medical grade gloves were about six times (OR = 0.17) less likely to have leaks than the cleanroom gloves. The low-modulus gloves were about 3.5 times (OR = 0.27) less likely to have leaks than the cleanroom gloves. The general duty gloves were about 2 times (OR = 0.55) less likely to have leaks than the cleanroom gloves.
TABLE VIII.
Logistic Regression of Glove Failure by Experimental Variables
| Variable | Odds Ratio | 95% Confidence Interval | p value |
|---|---|---|---|
| Exposure | |||
| No MovementA | 1.00 | ||
| Movement | 1.26 | 0.84–1.89 | 0.25 |
| Glove Type | |||
| CleanroomA | 1.00 | ||
| Medical grade | 0.17 | 0.08–0.35 | 0.00 |
| General duty | 0.55 | 0.34–0.89 | 0.01 |
| Low-modulus | 0.27 | 0.12–0.57 | 0.00 |
| Glove Product (ID number) | |||
| Glove 1A | 1.00 | ||
| Glove 3 | 4.99 | 1.15–21.68 | 0.03 |
| Glove 23 | 4.43 | 1.01–19.43 | 0.05 |
| Additional water required | |||
| NoneA | 1.00 | ||
| Additional 0.5 L | 0.48 | 0.26–0.89 | 0.02 |
| Additional 1.0 L | 1.82 | 0.97–3.42 | 0.06 |
Note: Bolded text = significantly less or more leaks than the reference category (p ≤ 0.05).
Reference category.
Logistical regression analysis of the individual glove products revealed only two gloves with significantly higher leak failures than the other gloves. For Glove 3, a cleanroom product, the odds of experiencing a leak was about five times the odds of another glove experiencing a leak (p = 0.03). For Glove 23, a general duty product, the odds of experiencing a leak was about four times the odds of another glove experiencing a leak (p=0.05). The overall variability in failure percentages was low between glove products, with the exception of two gloves.
Logistical regression analysis of the additional water required in the modified water-leak test provided further indication that significant differences in polymer formulation existed between the glove products. On average, the glove products that required an additional 0.5 L of water were about two times (OR = 0.48) less likely to have leaks than those products requiring no additional water (p ≤ 0.05). Although not significantly different (p = 0.06), the glove products that required an additional 1.0 L of water were more likely to have leaks than those products requiring no additional water. Only two glove products, number 16 (medical grade) and 28 (general duty), required an additional 1.0 L of water. Differences in polymer formulation are expected to account for this observed variation.
DISCUSSION
Simulated Movement
Results summarized in Tables IV, V, and VIII indicated that simulated movement did not have a significant effect on glove integrity. In addition, it must be noted that the glove inflation pressure used provided a worst-case scenario, equivalent to an improperly fitted glove. This served to strengthen the conclusion that normal conditions and hand movement would not have a significant effect on glove integrity. Overall, the statistical results were not significant (p>0.05) for the comparisons made with individual glove products and all glove products combined.
There were indications that individual glove products responded differently to movement. First, only cleanroom and general duty gloves had failure percentages above 4.0% after exposure to movement. This was a clear indication that the medical grade and low-modulus glove products were formulated and manufactured differently from some of the cleanroom and general duty products. Second, glove failure results were inconsistent between the glove products. Two glove products failed the 4.0% adulteration level after exposure to simulated movement. For Glove 5, the out-of-box failure percentage was 1.25% and the simulated movement failure percentage was 4.6% (p=0.27). For Glove 23, the out-of-box failure percentage was 2.5%, and the simulated movement failure percentage was 6.25% (p=0.19). However, an equal number of gloves failed prior to movement and then passed after exposure to movement. For Glove 21, the out-of-box failure percentage was 6.25%, and the simulated movement failure percentage was 2.2% (p=0.06). For Glove 28 the out-of-box failure percentage was 6.25% and the simulated movement failure percentage was 3.5% (p=0.27). The remaining Glove 3 had failure percentages above 4.0% for both treatments. These inconsistencies indicated that movement may have either a positive (e.g. relaxation) or negative (e.g., tearing or enlargement of holes) effect on the polymer. Polymer formulation and tensile properties are most likely associated with these observed differences. Ongoing research will evaluate the influence of polymer formulation (e.g., acrylonitrile and plasticizer content) and tensile properties on glove integrity.
The overall effect of movement on the glove integrity of nitrile exam gloves was low. For the combined results of 5790 water-leak tests, the overall change was small and less than 1% between movement (2.1%) and no movement (1.5%) exposures. Results were not significantly different (p=0.068). For select glove products, increases greater than 1% were observed but not significant (p>0.05). For Glove 5, there was a 3.3% increase in glove holes detected with simulated movement (p=0.27). For Glove 23 there was a 4% increase in glove holes detected with simulated movement (p=0.19). These failure percentages are still lower than observed in other glove studies. Reported failure percentages associated with clinical use are often higher. Most data exist for latex exam and surgical gloves; whereas studies including nitrile exam gloves involve simulated in-use conditions. Pitten et al.(17) reported glove failure percentages ranging from 15–50% in latex exam gloves after glove use by dentistry personnel. Hansen et al.(18) found a 6.9% increase over the control (1.0%) in latex exam glove failures after use in an emergency department. Korniewicz et al.(19) reported failure percentages ranging from 0–50% (average 9.3%) for thicker surgical nitrile gloves used in medical and dental surgeries. In addition, simulated clinical and abrasion exposures with nitrile exam gloves have resulted in failure percentages ranging from about 0.8 to 12%.(2,19,20) In most cases the effect of simulated use was not significant (p>0.05), with the exception of one glove product. Kerr et al.(20) found a failure percentage of 12% (7% higher than the control) associated with a simulated clinical method. In general, the overall effect of simulated movement on nitrile exam glove integrity was small in comparison with the in-use clinical effects caused largely by abrasion, cuts, and punctures.
The results of this study did not agree with the robotic hand study by Phalen et al.,(8) which resulted in 100% glove failures associated with movement in an accelerator-free nitrile exam glove. All failures were in the knuckle region, which was prominent in the robotic hand model. It is possible that excessive stretching in the knuckle region, abrasion against the knuckle, and constant water submersion played a role in the glove failures. On the other hand, no failures were observed in the other glove products, which would indicate that glove formulation played a central role in the observed differences. The same accelerator-free glove product (Glove 7) was included in this study; however, the lot was different. No glove failures were observed with Glove 7, both with and without simulated movement. Thus, it is likely that glove formulation played a significant role in the observed differences. The influence of polymer formulation, surface wetting, and abrasion on glove integrity under conditions of simulated movement requires further investigation.
Glove Type
Glove type had a significant effect on glove integrity. A significant difference in the number of leaks was observed between the different glove types (p<0.001). Between the two glove types expected to be most different, the cleanroom gloves had the highest proportion of leaks at 3.1%, on average, and the low-modulus gloves had the second lowest proportion of leaks at 1.0%. In addition, only the cleanroom gloves had failure percentages greater than 4.0%. Fifty percent of the cleanroom glove products failed the glove integrity tests. These results indicated prominent differences in glove integrity and formulation. Differences in formulation were anticipated. Cleanroom gloves were expected to have lower plasticizer and filler content, as reported on the label claims of two products. In contrast, the low-modulus gloves were expected to have increased plasticizer content, used to “soften” the polymer and reduce tensile modulus. As stated earlier, excess plasticizer (often an oil or nonpolar organic) is likely to repel water and result in a favorable water resistance.
Results of this study were consistent with previously observed differences in glove integrity between different manufacturers and brands.(5,6,8) It was apparent that polymer formulation played a role in glove integrity. Polymer formulation and variability should be considered when establishing exam glove standards and certifications. Ongoing research will investigate the influence of acrylonitrile, plasticizer, and filler content on glove integrity. Potential trade-offs with chemical resistance will also be evaluated. The primary goal is to provide supporting evidence and guidance for improved nitrile exam glove standards and certifications, which would address the observed disparities between glove brands and types.
Modified Water-Leak Test
The modified water-leak test used in this study was designed to address inconsistencies with the existing ASTM and FDA water-leak tests.(5,6,15) A primary concern was the ability of the 1 L water-leak test to detect holes in the fingers and thumb. Preliminary investigation indicated this was an issue with holes made by a 21-gauge needle. The testing was modified to detect a 30-gauge needle puncture in an attempt to develop a sensitive method that would improve statistical power. While the 1 L water-leak test was not designed to reliably detect a 30-gauge hole; it has been shown that a virus can still readily penetrate the opening.(15) The need for a modified water-leak test was evident before the study was initiated.
The need for a modified water-leak test was also apparent in the results of this study. In about 27% of the glove products tested, the traditional 1 L water-leak test would not have sufficiently detected holes in the finger and thumb regions. This was also true for 2 of the 9 medical grade products, which are evaluated against the 1 L water-leak test. In addition, and quite unexpectedly, more than 80% of the glove leaks detected in this study were in the finger and thumb regions. This could be due in part to optimization of current manufacturing processes to meet the 1 L water-leak test criteria, which does not properly address these glove regions or defect morphology.
The inability of the 1 L approach to detect leaks also appears to be linked to variations in glove formulation and tensile properties. It is recommended that the existing water-leak tests be re-evaluated and standardized to account for variability in polymer formulation. Standardizing the test to a known hole size and common shape (e.g., crescent-shaped needle puncture) has distinct advantages over standardizing the water volume. Currently, it appears that a number of available nitrile exam glove products may pass the 1 L water-leak test but still contain small holes equivalent to a 0.15 ± 0.05 mm crescent-shaped hole from a 30-gauge needle. Kotilainen et al.(15) indicated that virus penetration was 100% for 30-gauge needle punctures. The need for further investigation and improvement is evident.
CONCLUSIONS
Simulated whole-glove movement did not have a significant on effect glove integrity (p>0.05). However, significant variability in glove integrity was observed between different glove types (p ≤ 0.05). Cleanroom gloves, on average, had the highest proportion of leaks, and 50% of the products tested failed the water-leak test. Low-modulus and medical grade gloves had the lowest proportions of leaks, and none of the products tested failed the water-leak test. Variability in polymer formulation and plasticizer content are suspected to account for the observed discrepancies. In addition, polymer variability is also suspected to affect the ability of the 1 L water-leak test in detecting small holes in the finger and thumb regions of gloves. It is recommended that the existing 1 L water-leak tests be re-evaluated and standardized to account for product variability. Future studies should investigate the influence of NBR formulation, plasticizer, and filler contents on glove integrity. This information will provide necessary guidance for improved nitrile exam glove standards and certifications, which would address the observed disparities in performance between glove brands and types.
Acknowledgments
The research activities were supported by Grant 1R21OH009327-01A2 from CDC-NIOSH. Funding for preliminary results and equipment was also provided by the National Institutes of Health (NIH Grant HD052368). Special thanks go to student research assistants Paul Lawrence, Brett Griffin, Carrie Cushman, Thi Le, and Hou Ung for there assistance in the development and testing of the methods described in this project.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the National Institute for Occupational Safety and Health.
References
- 1.Klingner TD, Boeniger MF. A critique of assumptions about selecting chemical-resistant gloves: A case for workplace evaluation of glove efficacy. Appl Occup Environ Hyg. 2002;17:360–367. doi: 10.1080/10473220252864969. [DOI] [PubMed] [Google Scholar]
- 2.Rego A, Roley L. In-use barrier integrity of gloves: Latex and nitrile superior to vinyl. Am J Infect Control. 1999;27(5):405–410. doi: 10.1016/s0196-6553(99)70006-4. [DOI] [PubMed] [Google Scholar]
- 3.Edlich RF, Suber F, Neal JG, Jackson EM, Williams FM. Integrity of powder-free examination gloves to bacteriophage penetration. J Biomed Mater Res. 1999;48(5):755–758. doi: 10.1002/(sici)1097-4636(1999)48:5<755::aid-jbm23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Jackson EM, Williams FM, Neal JG, Suber F, Thacker JG, Edlich RF. Biomechanical performance of examination gloves. J Biomed Mater Res. 1999;48(4):572–577. doi: 10.1002/(sici)1097-4636(1999)48:4<572::aid-jbm25>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Zinner NL. How safe are your gloves? A study of protective barrier properties of gloves. AORN J. 1994;59(4):876, 879–882. doi: 10.1016/s0001-2092(07)65347-2. [DOI] [PubMed] [Google Scholar]
- 6.Muto CA, Sistrom MG, Strain BA, Farr BM. Glove leakage rates as a function of latex content and brand - Caveat emptor. Arch Surg. 2000;135(8):982–985. doi: 10.1001/archsurg.135.8.982. [DOI] [PubMed] [Google Scholar]
- 7.Phalen RN, Que Hee SS, Xu W, Wong WK. Acrylonitrile content as a predictor of the captan permeation resistance for disposablenitrile rubber gloves. J Appl Polymer Sci. 2007;103(3):2057–2063. [Google Scholar]
- 8.Phalen RN, Que Hee SS. A moving robotic hand system for whole-glove permeation and penetration: Captan and nitrile gloves. J Occup Environ Hyg. 2008;5:258–270. doi: 10.1080/15459620801934786. [DOI] [PubMed] [Google Scholar]
- 9.Edlich RF, Taylor CC, Winters K, et al. Scientific basis for selection of emergency medical examination gloves for emergency medical technicians, paramedics, firefighters, and emergency department personnel. J Long Term Eff Med Implants. 2004;14(1):51–66. doi: 10.1615/jlongtermeffmedimplants.v14.i1.50. [DOI] [PubMed] [Google Scholar]
- 10.Edlich RF, Winters KL, Martin ML, Long WB, III, Werner CL, Gubler KD. Scientific basis for the selection of emergency medical examination gloves for emergency medical technicians, paramedics, firefighters, and emergency department personnel. An update. J Long Term Eff Med Implants. 2005;15(2):161–83. doi: 10.1615/jlongtermeffmedimplants.v15.i2.50. [DOI] [PubMed] [Google Scholar]
- 11.Korniewicz DM, Garzon L, Seltzer J, Feinleib M. Failure rates in nonlatex surgical gloves. Am J Infect Control. 2004;32(5):268–273. doi: 10.1016/j.ajic.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Barker R, editor. National Personal Protection Technology Laboratory (NPPTL), National Institute for Occupational Safety and Health (NIOSH) A Review of Gaps and Limitations in Test Methods for First Responder Protective Clothing and Equipment. Pittsburgh, Pa: NPPTL, NIOSH; 2005. [Google Scholar]
- 13.American Society for Testing and Materials (ASTM) Standard Test Method for Rubber Detection of Holes in Medical Gloves (D 5151–99). [Standard] Philidelphia, Pa: ASTM; 2003. [Google Scholar]
- 14.Food and Drug Administration (FDA) Medical Glove Guidance Manual, Document shelf number 852. Rockville, Md: Department of Health and Human Services, FDA; 1999. [Google Scholar]
- 15.Kotilainen HR, Cy WH, Truscott W, Gantz NM, Routson LB, Lytle CD. Ability of 1000 ml water leak test for medical gloves to detect gloves with potential for virus penetration. In: McBriarty JP, Henry NW, editors. Performance of Protective Clothing. Vol. 4. Philadelphia, Pa: American Society for Testing and Materials; 1992. pp. 38–49. [Google Scholar]
- 16.Patient examination gloves and surgeons’ gloves; sample plans and test method for leakage defects; adulteration. Code of Federal Regulations Title 21. 2000;800
- 17.Pitten FA, Herdemann G, Kramer A. The integrity of latex gloves in clinical dental practice. Infection. 2000;28(6):388–392. doi: 10.1007/s150100070011. [DOI] [PubMed] [Google Scholar]
- 18.Hansen KN, Korniewicz DM, Hexter DA, Kornilow JR, Kelen GD. Loss of glove integrity during emergency department procedures. Ann Emerg Med. 1998;31(1):65–72. doi: 10.1016/s0196-0644(98)70283-5. [DOI] [PubMed] [Google Scholar]
- 19.Korniewicz DM, El-Masri M, Broyles JM, Martin CD, O’Connell KP. Performance of latex and nonlatex medical examination gloves during simulated use. Am J Infect Control. 2002;30(2):133–138. doi: 10.1067/mic.2002.119512. [DOI] [PubMed] [Google Scholar]
- 20.Kerr LN, Chaput MP, Cash LD, et al. Assessment of the durability of medical examination gloves. J of Occup Environ Hyg. 2004;1:607–612. doi: 10.1080/15459620490491803. [DOI] [PubMed] [Google Scholar]