Abstract
Resting and exercise fuel metabolism was assessed in three different phases of the menstrual cycle, characterized by different levels of estrogen relative to progesterone: early follicular (EF, low estrogen and progesterone), midfollicular (MF, elevated estrogen, low progesterone), and midluteal (ML, elevated estrogen and progesterone). It was hypothesized that exercise glucose utilization and whole body carbohydrate oxidation would decrease sequentially from the EF to the MF to the ML phase. Normal-weight healthy females, experiencing a regular menstrual cycle, were recruited. Subjects were moderately active but not highly trained. Testing occurred after 3 days of diet control and after an overnight fast (12-13 h). Resting (2 h) and exercise (50% maximal O2 uptake, 90 min) measurements of whole body substrate oxidation, tracer-determined glucose flux, and substrate and hormone concentrations were made. No significant difference was observed in whole body fuel oxidation during exercise in the three phases (nonprotein respiratory exchange ratio: EF 0.84 ± 0.01, MF 0.85 ± 0.01, ML 0.85 ± 0.01) or in rates of glucose appearance or disappearance. There were, however, significantly higher glucose (P < 0.05) and insulin (P < 0.001) concentrations during the first 45 min of exercise in the ML phase vs. EF and MF phases. In conclusion, whole body substrate oxidation and glucose utilization did not vary significantly across the menstrual cycle in moderately active women, either at rest or during 90 min of moderate-intensity exercise. During the ML phase, however, this similar pattern of substrate utilization was associated with greater glucose and insulin concentrations. Both estrogen and progesterone are elevated during the ML phase of the menstrual cycle, suggesting that one or both of these sex steroids may play a role in this response.
Keywords: substrate oxidation, female sex steroids, glucose metabolism
IN PREMENOPAUSAL WOMEN, the menstrual cycle represents a continuous state of change in terms of the female sex steroid environment. Determining how the menstrual cycle phase affects various aspects of metabolism is necessary to provide a comprehensive understanding of normal physiology in women. It is also important to establish the necessity of controlling for sex steroid hormone status when women are included in metabolic studies. This is relevant both to the resting condition and during perturbations common to everyday life, such as exercise.
Data would suggest that the normal cyclical variation in estrogen and/or progesterone could affect a number of aspects of lipid and carbohydrate metabolism. For example, studies in animals have shown that an isolated change in estrogen favors lipid mobilization, whereas an isolated change in progesterone promotes lipid storage (19, 29, 30, 41, 43). Either estrogen or progesterone can increase glycogen deposition in muscle and liver (2, 37), whereas estrogen decreases hepatic gluconeogenesis (2, 37). Furthermore, the combination of estrogen and progesterone can decrease hepatic glycogenolysis in response to epinephrine or glucagon (1). These data suggest that, in humans, glucose conservation and lipid mobilization would be favored during the luteal vs. follicular phase of the menstrual cycle (43), as is observed during the rat estrous cycle (48). Furthermore, there are small but potentially influential increases in growth hormone (4, 56), cortisol (17), sympathetic nerve activity (39, 58), and decreased insulin action (15, 51) in the luteal phase of the menstrual cycle that together would suggest that a greater lipid utilization would occur in this phase.
Although no differences have been observed across the menstrual cycle in resting whole body respiratory exchange ratio (RER) (42), basal free fatty acid (FFA) (22), and glucose (12) turnover, the effect of the menstrual cycle on fuel metabolism may be more apparent under conditions of metabolic stress, such as during exercise. Indeed, animal studies show that greater lipid, and lower carbohydrate, utilization occurs during exercise when estrogen, and possibly progesterone, are elevated (14, 21). Exercise nutrient utilization in women throughout the menstrual cycle has generally been characterized by the measurement of RER. Certain data suggest that there may be greater lipid oxidation and lower carbohydrate oxidation with mild- to moderately high-intensity exercise [<75% of maximal O2 uptake ] performed in the luteal vs. follicular phase of the menstrual cycle (10, 18, 40, 57), although this has not always been observed (3, 7, 11, 27). In terms of exercise substrate changes and the associated neuroendocrine response, results are inconsistent regarding menstrual cycle effects (6, 16, 27, 49). Recent work has provided some interesting, but conflicting, data with respect to menstrual cycle changes in exercise glucose kinetics. Braun et al. (7) reported no difference in glucose rate of appearance (Ra)or disappearance (Rd) between the mid- to late follicular (MF) and midluteal (ML) phases of the menstrual cycle during moderate exercise (~50% of , ≤45 min), whereas Zderic et al. (57) found glucose Ra and Rd to be significantly lower in the ML vs. early follicular (EF) phase. Subjects in both studies were habitually active but not highly trained. Braun et al. made their observations twice, once at sea level and again at high altitude. Recently, it has been reported that, in women athletes, significantly lower glucose Ra and Rd are observed after only 75 min of exercise at 70% of during the ML vs. MF phase of the menstrual cycle (10). Thus clarification of the effect of the menstrual cycle on substrate utilization and the neuroendocrine response to exercise is needed, in relation to both exercise intensity and duration and the training status of women.
The lack of clarity in results of menstrual cycle effects on exercise metabolism may be due partly to variations in prestudy control and designation of cycle phase. Careful control of prestudy diet and activity is very important, as variations in the state of energy balance before testing can significantly impact glycogen stores, substrate utilization, and the neuroendocrine response, as can prior activity. Also, testing in the follicular phase has not always distinguished between the times when estrogen is low and when it is elevated. With the start of menstruation, indicated as day 1 of the cycle, there is approximately a 4- to 6-day interval when both estrogen and progesterone remain low (EF) (32). In the MF phase (excluding the preovulatory peak), estrogen is increased but progesterone remains low. After ovulation and during the ML phase, both progesterone and estrogen are high. Thus dividing the menstrual cycle into the EF, MF, and ML phases covers the majority of days in which the female physiology resides (the preovulatory surge in estrogen lasts only 1-2 days). Because each of these three phases is characterized by a different estrogen-to-progesterone ratio, this may be a more appropriate way to compare effects across the complete menstrual cycle. Also, the EF phase has estrogen and progesterone levels closer to those observed in males, who have a different pattern of whole body substrate oxidation compared with women studied randomly throughout the follicular phase (24).
The current study, therefore, aimed to determine substrate oxidation, glucose kinetics, and the substrate and hormone response to moderate-intensity exercise of 90-min duration across three phases of the menstrual cycle (EF, MF, and ML) in moderately active women. This has never previously been assessed and was performed within subjects under controlled conditions of diet and exercise. It was hypothesized that “carbohydrate conservation,” that is, a lower whole body carbohydrate oxidation and glucose utilization, would be greatest when the female sex steroids were lowest (EF phase) and significantly reduced when both sex steroid hormones were elevated (ML phase).
METHODS
Subjects
Eumenorrheic, lean, healthy females (18-39 yr) were recruited for the study. Subjects were required to have a regular menstrual cycle (>11 cycles over the past year) and were habitually active (>90 min of aerobic exercise/wk) but not highly trained, competitive athletes. Medical exclusions included past or present history of cardiovascular disease, high blood pressure, diabetes, any hormonal imbalance or metabolic abnormality, and use of oral contraceptives or other hormones. A total of 13 subjects took part in the study. One subject was excluded on the basis of her study day hormone concentrations, which suggested she was anovulatory. Subject characteristics are shown in Table 1. The study protocol was approved by the University of Colorado Committee Institutional Review Board for the Protection of Human Subjects. All subjects read and signed an informed consent form before admission into the study.
Table 1.
Subject characteristics
| Menstrual Cycle Length, days |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, yr | Height, m | Body Weight, kg | BMI kg/m2 | Body Fat, % | , ml·kg-1 ·min-1 | Total | Follicular phase | Luteal phase | |
| Mean ± SD | 29 ± 5 | 1.67 ± 0.06 | 59.6 ± 7.1 | 21.4 ± 1.8 | 25.4 ± 1.5 | 39.9 ± 5.8 | 28.0 ± 3.2 | 14.0 ± 2.5 | 14.0 ± 1.7 |
BMI, body mass index; , maximal O2 intake.
Preliminary assessments
Measurement of , body composition, and resting metabolic rate (RMR) was performed in the follicular phase of the menstrual cycle. does not appear to be affected by menstrual cycle phase (11, 26), and body composition, assessed by densitometry, would not be detectably affected by cycle phase. The RMR was used to estimate energy requirements during the period of prestudy diet control. Any increase in energy requirements during the luteal phase was accommodated by provision of optional food modules (described in the prestudy diet control section), because food intake and energy expenditure can increase slightly in the luteal phase of the menstrual cycle (35, 42).
was determined using a graded exercise test on a cycle ergometer (Monark, Varberg, Sweden). Subjects cycled at a rate of 70 rpm while the resistance was gradually increased (commencing at 50 W and increasing by 25 W every 2 min) until volitional exhaustion. Heart rate was continuously monitored via a 12-lead electrocardiogram, with blood pressure and perceived rate of exertion measured in the final minute of each work level. To ensure maximum effort was achieved, two of the following criteria had to be fulfilled: whole body RER at or >1.1; achieved maximum heart rate within 5% of the age-predicted maximum; or an increase in O2 consumption in response to the final workload of <2.0 ml·kg body wt-1·min-1.
Body composition
Body composition was determined by measuring body density with underwater weighing, as previously described (23). Percent body fat was estimated from body density by use of the revised equation of Brozek et al. (8).
RMR
RMR was measured using indirect calorimetry via a metabolic cart system (Sensormedics 2900, Sensormedics, Yorba Linda, CA). Subjects were tested in the morning after a 12-h fast. After 30 min of rest, a 15- to 20-min measurement of metabolic rate was made using a ventilated canopy. Gas concentrations were measured in the air exiting the hood. and carbon dioxide production () were used to calculate metabolic rate (53). The RMR value was used to determine energy intake of subjects during the period of prestudy diet control.
Health and physical examination
A health and physical examination was performed on subjects to confirm that there was no medical reason for their exclusion from the study.
Menstrual cycle monitoring
Subjects provided details of prior menstrual history as far back as possible. Subjects then monitored basal body temperature for ≥3-4 mo before their first study day. Ovulation was indicated by a sustained increase in basal body temperature averaging ≥0.3°C. An ovulation prediction kit (First Response Ovulation Predictors Test, Tambrands, Lake Success, NY) was also used to more precisely identify the time of ovulation. However, these kits produced false negative results in some subjects and were not always reliable. Menstrual cycle length was estimated from the first day of menses (day 1) to the day preceding the next menses. Using a typical cycle length of 28 days, and ovulation at day 14, the EF studies were performed between days 1 and 4. This time interval avoided any increase in estrogen. The MF studies were performed between days 8 and 11 of the cycle. This time interval was characterized by moderately elevated estrogen but low progesterone, and it avoided the preovulatory surge in estrogen. The ML phase studies were between days 19 and 23 to avoid the high pulsatility in progesterone in the early luteal phase and any decline in the sex steroids toward the end of the luteal phase. Adjustments in the study days were made if cycle length and/or day of ovulation differed from the typical 28-day pattern. Measurements were made in the same or in consecutive cycles, with ≥2 wk separating study days.
Prestudy diet and exercise control
Subjects were fed a controlled diet for 3 days before each study day. All food was prepared by the General Clinical Research Center (GCRC) diet kitchen at the University of Colorado, and subjects were required to consume breakfast in the GCRC, with other food prepared to take away. No other food was permitted, and subjects were required to consume all the food given. The only optional part of the diet was two food modules (840 kJs each), one or both of which the subjects could eat if they were hungry. The diet composition was 25% fat, 15% protein, and 60% carbohydrate, and initial energy intake was calculated at 1.6-1.8 × RMR based on subjects' habitual activity level. Body weight was measured daily before breakfast was eaten to ascertain weight stability. Table 2 shows the dietary intake information for the three menstrual cycle phases. Subjects were allowed to follow their usual activity routine for the first 2 days of the diet, and on the last day they refrained from any planned exercise.
Table 2.
Dietary intake and body weight change before each study day
| Menstrual Phase | No. in Group | Energy Intake, kJ/day | Fat Intake, g/day | Carbohydrate Intake, g/day | Protein Intake, g/day | Weight Change, kg |
|---|---|---|---|---|---|---|
| Early follicular | 10 | 9,811 ± 294 | 66 ± 2 | 356 ± 11 | 89 ± 3 | -0.25 ± 0.3 |
| Midfollicular | 11 | 9,761 ± 302 | 66 ± 2 | 354 ± 11 | 88 ± 3 | 0.0 ± 0.1 |
| Midluteal | 10 | 9,845 ± 286 | 66 ± 2 | 356 ± 10 | 89 ± 3 | -0.1 ± 0.2 |
Values are means ± SE. Each individual's average of 3-day dietary intake was used to calculate group means. Weight change was estimated from fasting body weight measured on the morning of the 1st day of diet control - fasting body weight measured on the morning of the study day.
Study Days
Subjects spent the evening before each study in the GCRC. The evening meal was consumed between 1900 and 2000. The study began at 0800 the next day, with samples being drawn for resting measurements at ∼0930-1000 (∼13 h of fast). The aim was for all subjects to complete three test days (EF, MF, and ML). The order of testing was initially randomized, but this proved difficult to adhere to because of problems in correctly anticipating the desired menstrual cycle phase in some subjects. This was despite these individuals previously having had a very regular cycle (based on menstrual cycle records and basal temperature monitoring). Consequently, study days had to be canceled and rescheduled on certain occasions, but testing order remained unbiased with respect to cycle phase. In three different subjects, the data from one day had to be excluded because they had been obtained in an incorrect cycle phase. Two subjects were able to complete only two study days. Confirmation of cycle phase was always based on serum estrogen and progesterone levels measured on the day of the study.
Study protocol
On the morning of the study, subjects were awakened at ∼0545. An intravenous catheter was placed in an antecubital vein for infusion of stable isotopes. In the contralateral arm, a sampling catheter was placed retrograde fashion into a dorsal hand vein or, if necessary, in a wrist vein. The heated hand technique (38) was used to obtain arterialized blood samples. Initial blood samples were drawn for determination of background enrichment followed by a primed (17.6 μmol/kg), constant (0.2 μmol·kg-1·min-1) infusion of [6,6-2H2]glucose. Glycerol and palmitate isotopes were infused at the same time, but the data on the glycerol and palmitate kinetics will be presented elsewhere. Resting substrate kinetics were determined on blood samples taken at 90, 100, 110, and 120 min. Subjects remained resting in bed during this time. They then moved to a stationary bike and began the exercise. The infusion rate of the glucose isotope was increased twofold to minimize changes in isotope enrichment (44). Blood samples were drawn after a 5-min warm-up (time 0 for steady-state exercise) and at 10, 20, 30, 45, 60, 75, and 90 min of steady-state exercise. Plasma samples were analyzed for glucose enrichment. Serum or plasma samples were analyzed for glucose, FFA, glycerol, insulin, cortisol, estradiol, progesterone, epinephrine, norepinephrine, and glucagon. Lactate was measured on perchloric acid extracts of whole blood.
During the last 30 min of the resting period and during 20 min of each 30-min exercise period, measurements of respiratory gas exchange were made using the metabolic cart system. Carbohydrate and fat oxidation was calculated from the volume of O2 consumed and volume of CO2 expired, after correction for protein oxidation (25, 47). Protein oxidation during exercise was estimated from urinary nitrogen excretion. Urinary nitrogen excretion during exercise was calculated from the minute rate of nitrogen excretion calculated from 24-h urine collection. This time frame was used rather than the study period itself, as there is a time delay in the excretion of nitrogen resulting from exercise protein oxidation, and urine volume can be minimal after exercise. Previously, we calculated a difference of less than ±0.5% in the estimation of carbohydrate or fat grams oxidized when we used the study period urine collection vs. the rate of nitrogen excretion estimated from the 24-h urine collection (24).
Determination of glucose isotope enrichment
This was measured via gas chromatography-mass spectrometry (GC-MS; GC models 5992 and 5985B, Hewlett-Packard, Palo Alto, CA) by use of the pentaacetate derivative of glucose. Before GC-MS analysis, plasma samples were deproteinized with iced ethanol, and the supernatant was lyophilized. Samples were then derivatized by using 200 μl of acetic anhydridepyridine solution (1:1) and heating for 10 min at 60°C. Samples were transferred to GC-MS vials for analysis. Injector temperature of the GC-MS was set at 250°C, and initial oven temperature was set at 195°C. Oven temperature was increased 10°C/min until a final temperature of 265°C was achieved. Helium was used as the carrier gas with a 35-to-1 ml/min splitless injection ratio; transfer line temperature was set at 250°C, source temperature at 200°C, and quadruple temperature at 116°C; electron impact ionization was used to monitor selctive ions with a mass-to-charge ratio of 242/244 atomic mass units.
Calculations
Glucose enrichment and concentration data were spline fitted (52) to remove noise introduced by analytic and sampling errors (55). Glucose Ra and Rd were then calculated with the Steele equation, as modified for use with stable isotopes (44, 55)
where Ra equals the rate of appearance of tracee (μmol/min), F is the infusion rate of tracer (μmol·ml-1·min-1), Ep is plasma enrichment, Vd is the effective volume of tracee distribution [100 ml/kg body wt (44)], t1 is time 1 of sampling, t2 is time 2 of sampling, C1 is [tracee] at t1, C2 is [tracee] at t2, E1 is plasma enrichment at t1, and E2 is plasma enrichment at t2.
Determination of circulating hormone and substrate levels
Two to three milliliters of blood were added to EDTA tubes for the analysis of stable isotopes and plasma substrate concentrations. The sample was immediately placed on ice and spun, and the plasma was separated. Approximately 0.5 ml of whole blood was added to a preweighed tube containing 1.5 ml of iced perchloric acid (8%). After vortexing, the tube was postweighed and spun to separate the supernatant. This was used to measure blood lactate. Two and a half milliliters of whole blood were added to 40 μl of preservative [EGTA (3.6 mg) plus glutathione (2.4 mg) in distilled water] for plasma catecholamine determinations. Blood for glucagon measurement (2 ml) was added to tubes containing EDTA plus 500 killikrein-inhibitor units of aprotinin. Samples were immediately placed on ice and spun. Approximately 7 ml of whole blood were allowed to clot, and the serum was separated off after spinning. This was used for determination of hormone and substrate concentrations. All plasma, serum, and supernatant samples were stored at -70°C until analysis. Catecholamines were determined in duplicate by high-performance liquid chromotography with electrochemical detection [intra-assay coefficients of variation (CV) 6.2% epinephrine, 4.9% norepinephrine] (50). Radioimmunoassays were used to determine serum insulin (Kabi Pharmacia, Piscataway, NJ), cortisol, progesterone, estradiol (Diagnostic Products, Los Angeles, CA), and glucagon (Linco Research, St. Louis, MO). Samples were run in duplicate with intra-assay CV of 10, 6.7, 8.8, 7.5, and 9.4%, respectively. Serum glucose was measured enzymatically by use of the hexokinase method on an automated Roche COBAS Mira Plus analyzer (intra-assay CV 0.7%). Enzymatic assays were used to determine serum glycerol (Boehringer Mannheim Diagnostics, Indianapolis, IN) and FFA (Wako Chemical USA, Richmond, VA) (intra-assay CV of 7.8 and 1.2%, respectively) on the COBAS Mira Plus analyzer. Blood lactate (Sigma Diagnostics, St. Louis, MO) was run in duplicate with an intra-assay CV of 4.2%. For each subject, samples from all study days were run simultaneously for all assays.
Data Analysis
For each dependent variable, the effects of menstrual cycle phase, time, and their interaction were examined. The mixed model (31) was preferable to the more commonly used general linear multivariate model, because of missing values at certain time points and because the covariance structure was of interest. Missing values occurred randomly across subjects and cycle phases because of occasional problems with blood sampling. Random effects for subject and cycle phase were tested for each outcome variable by use of likelihood ratio tests to compare nested models. Evaluating these random effects established whether measurements for any given parameter were more highly correlated within subject and cycle phase. Phase, time, and a phase-by-time interaction were included as fixed effects in a preliminary modeling strategy and then were removed from the model if not significant at the P < 0.05 level.
RESULTS
Energy Expenditure
Resting metabolic rate tended to be greater (to a nonsignificant degree) in the ML than in the EF and MF phases of the menstrual cycle (Table 3, 4.43 ± 0.12, 4.29 ± 0.12, and 4.39 ± 0.11 kJ/min, respectively). Energy expenditure, , and heart rate during the 90-min cycle exercise were not different among cycle phases (Table 3). On average, subjects exercised at 51.1, 50.1, and 50.6% of during the EF, MF, and ML phases, respectively.
Table 3.
Resting metabolic rate and exercise energy expenditure
| Exercise EE |
Exercise |
||||||
|---|---|---|---|---|---|---|---|
| Menstrual Phase | No. in Group | RMR, kJ/min | Rate, kJ/min | Total, kJ/90 min | Exercise HR, beats/min | ml·kg-1 ·min-1 | % of |
| Early follicular | 10 | 4.29 ± 0.12 | 25.6 ± 1.6 | 2,308 ± 146 | 130 ± 4 | 20.4 ± 0.9 | 51.1 |
| Midfollicular | 11 | 4.39 ± 0.11 | 25.2 ± 1.6 | 2,271 ± 146 | 126 ± 5 | 20.0 ± 0.9 | 50.1 |
| Midluteal | 10 | 4.43 ± 0.12 | 25.7 ± 1.6 | 2,313 ± 142 | 131 ± 4 | 20.2 ± 0.9 | 50.6 |
Values are means ± SE. RMR, resting metabolic rate; EE, energy expenditure; HR, heart rate; , O2 uptake.
Whole Body Substrate Oxidation
Resting RER did not differ among the three phases of the menstrual cycle (Table 4). Likewise, RER during the entire 90-min exercise period was not significantly different among phases (0.84 ± 0.01, 0.85 ± 0.01, and 0.85 ± 0.01, EF, MF, and ML phases, respectively; Table 4), nor were there any differences in RER over time during exercise (Table 4). Consequently, whole body exercise nutrient oxidation (protein, fat, and carbohydrate) were not different among phases of the menstrual cycle (Table 5).
Table 4.
Respiratory exchange ratio at rest and during exercise
| Exercise |
||||||
|---|---|---|---|---|---|---|
| Menstrual Phase | No. in Group | Rest | 0-30 min | 31-60 min | 61-90 min | Total |
| Early follicular | 10 | 0.715 ± 0.011 | 0.868 ± 0.007 | 0.836 ± 0.007 | 0.821 ± 0.007 | 0.842 ± 0.007 |
| Midfollicular | 11 | 0.721 ± 0.006 | 0.872 ± 0.005 | 0.844 ± 0.005 | 0.833 ± 0.005 | 0.849 ± 0.005 |
| Midluteal | 10 | 0.722 ± 0.010 | 0.880 ± 0.006 | 0.848 ± 0.006 | 0.828 ± 0.005 | 0.852 ± 0.005 |
Values are means ± SE.
Table 5.
Whole body exercise nutrient oxidation
| Absolute Nutrient Oxidation, g/90 min |
||||
|---|---|---|---|---|
| Menstrual Cycle Phase | No. in Group | CHO | Fat | Protein |
| Early follicular | 10 | 60.1 ± 4.5 | 29.3 ± 3.1 | 4.2 ± 0.2 |
| Midfollicular | 11 | 64.5 ± 4.9 | 27.0 ± 2.1 | 3.7 ± 0.3 |
| Midluteal | 10 | 65.8 ± 4.1 | 27.1 ± 2.4 | 4.5 ± 0.3 |
Values are means ± SE. CHO, carbohydrate.
Glucose kinetics
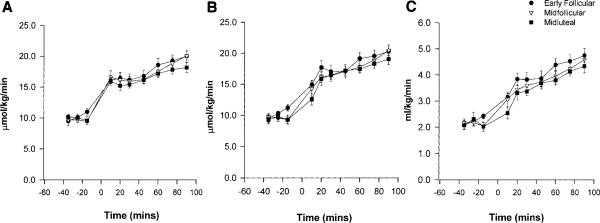
Figure 1 shows the mole percent excess (MPE, %) for glucose enrichment at rest and during the 90-min exercise. Values were relatively stable, both at rest and during exercise, showing that large changes in isotope enrichment were avoided by doubling the infusion rate of the glucose isotope at the onset of exercise. At rest, glucose turnover did not differ among the EF, MF, or ML phases (10.17 ± 0.53, 9.59 ± 0.33, or 9.90 ± 0.44 μmol·kg-1·min-1, respectively). During exercise, glucose Ra and Rd increased over time and were approximately twofold higher at the end of exercise than resting values (Fig. 2). Glucose Ra was not significantly different among cycle phases. There was a tendency for a lower glucose Rd during the ML phase, in the first half of the exercise, but this did not result in a significant phase-by-time interaction. Metabolic clearance rate (MCR) was not significantly different among phases. For both glucose Ra and Rd, there were significant random effects of subject and phase (P < 0.0001 vs. model with no random effects). This means that values were more highly correlated within subject and within phase than within subject only.
Fig. 1.
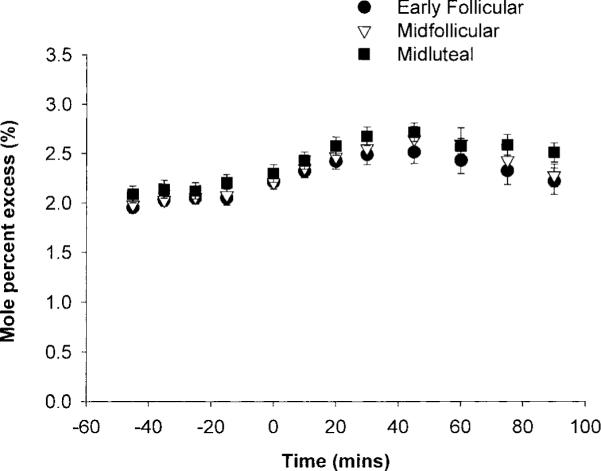
Glucose tracer enrichment expressed as mole percent excess (MPE).
Fig. 2.
A: glucose rate of appearance (Ra), at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.02). B: glucose rate of disappearance (Rd), at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.0001). C: glucose metabolic clearance rate, at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05).
Circulating substrate and hormone concentrations
Resting concentrations of hormones and substrates are shown in Table 6. As expected, progesterone was significantly greater in the ML phase (P < 0.0001) than in the EF and ML phases. Estradiol concentration was significantly lower in the EF phase than in either the MF (P < 0.0008) or the ML (P < 0.0001) phase. There was no significant difference in the resting estradiol concentration between the MF and ML phases.
Table 6.
Resting substrate and hormone concentrations
| Early Follicular Phase | Midfollicular Phase | Midluteal Phase | |
|---|---|---|---|
| Estradiol, pg/ml | 24 ± 3 | 72 ± 9† | 107 ± 9* |
| Progesterone, ng/dl | 0.41 ± 0.04 | 0.38 ± 0.04 | 11.5 ± 1.6‡ |
| E/P ratio | 59 | 190 | 9.3 |
| Glucose, mg/dl | 83 ± 1 | 81 ± 1 | 81 ± 2 |
| Insulin, μU/ml | 4.7 ± 0.5 | 4.2 ± 0.3 | 5.1 ± 0.5 |
| FFA, μeq/1 | 641 ± 29 | 608 ± 28 | 657 ± 53 |
| Glycerol, μmol/l | 63 ± 5 | 58 ± 4 | 62 ± 5 |
| Lactate, mmol/l | 1.3 ± 0.10 | 1.2 ± 0.08 | 1.3 ± 0.10 |
| Epinephrine, pg/ml | 35 ± 11 | 23 ± 1.0 | 30 ± 3 |
| Norpinephrine, pg/ml | 141 ± 26 | 158 ± 28 | 163 ± 26 |
| Glucagon, pg/ml | 38 ± 5 | 41 ± 4 | 48 ± 5 |
| Cortisol, μg/dl | 5.6 ± 0.6 | 6.8 ± 1.0 | 5.4 ± 0.8 |
Values are means ± SE of all subjects. E/P ratio, ratio of estradiol concentration to progesterone concentration. FFA, free fatty acid.
P < 0.0001 vs. early follicular (EF) phase
P < 0.0008 vs. EF phase.
P < 0.0001 vs. midfollicular phase.
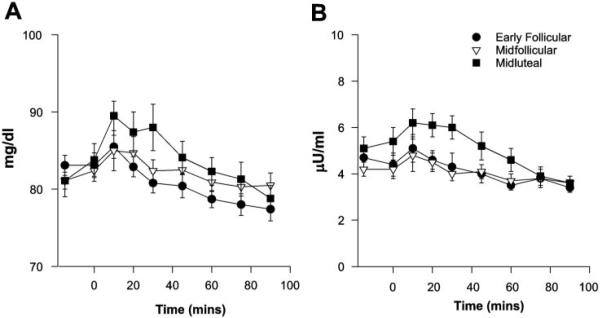
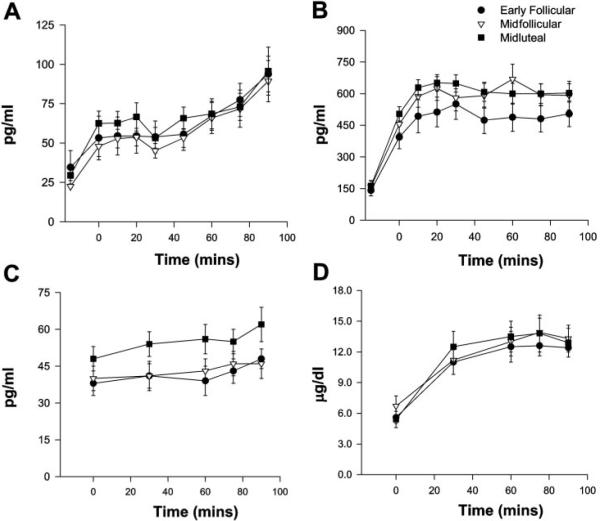
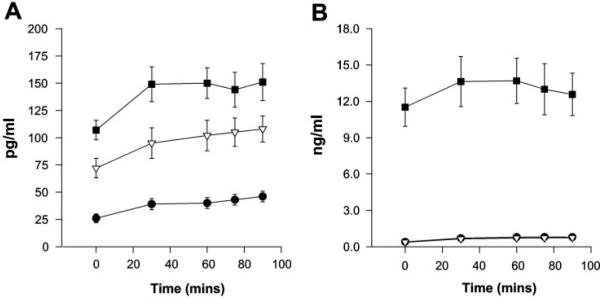
Exercise-induced changes in circulating glucose and insulin concentrations, although small, differed by cycle phase (time-by-phase interaction: P < 0.05 glucose, P < 0.001 insulin). This was mainly due to a consistent increase in both glucose and insulin during the first 45 min of exercise in the ML phase compared with the EF and MF phases (Fig. 3). There was no main effect of cycle phase on FFA or glycerol concentrations (data not shown). Circulating lactate concentrations changed over time but were not different across the menstrual cycle (Fig. 4). Exercise concentrations of the counterregulatory hormones epinephrine, norepinephrine, cortsiol, and glucagon are shown in Fig. 5. All of these counterregulatory hormones increased significantly over time during exercise. The relationship between norepinephrine and time was significantly different in the MF phase vs. the EF and ML phases (P < 0.02). The sex steroid hormones increased in all three phases, and there was a significant phase-by-time interaction for both estradiol (P < 0.02) and progesterone (P < 0.001; Fig. 6).
Fig. 3.
A: glucose concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05) and phase × time interaction (P < 0.05). B: insulin concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05) and phase × time interaction (P < 0.001).
Fig. 4.
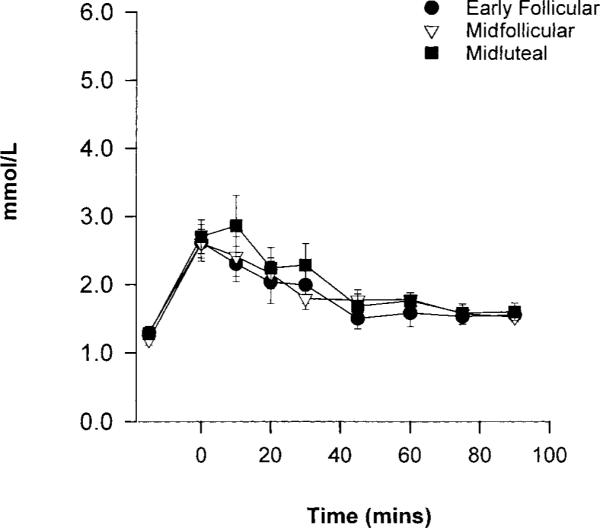
Lactate concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05).
Fig. 5.
A: epinephrine concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.0001). B: norepinephrine concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.0001) and phase × time interaction (P < 0.01). C: glucagon concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05). D: cortsiol concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05).
Fig. 6.
A: estradiol concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05) and phase × time interaction (P < 0.02). B: progesterone concentrations at rest and during 90 min of cycle exercise. Significant effect of time (P < 0.05) and phase × time interaction (P < 0.0005).
For concentrations of glucose, FFA, glycerol, lactate, glucagon, estradiol, progesterone, and cortisol, values were more highly correlated with measurements made in the same phase of the menstrual cycle than with those in the other two phases (P < 0.0001). Cycle phase was not a significant random effect for insulin, indicating that variation within phase was no greater than the variation within subjects.
DISCUSSION
This study determined the effect of the normal menstrual cycle on resting and exercise substrate metabolism. It was unique in that it compared the response to moderate-intensity, long-duration exercise across three phases of the menstrual cycle characterized by very different estrogen-to-progesterone concentrations: EF, low estrogen and low progesterone; MF, elevated estrogen and low progesterone; and ML, elevated estrogen and progesterone. Contrary to what was hypothesized, we observed no difference in whole body carbohydrate or lipid oxidation during moderate, prolonged exercise in the EF, MF, or ML phases. In addition, there were no differences in plasma glucose kinetics across cycle phases, despite a greater exercise increase in glucose and insulin concentrations in the luteal compared with both follicular phases.
The present data agree with previous studies that show no effect of the menstrual cycle on resting whole body substrate oxidation (3, 7, 33) and resting concentrations of glucose, FFA, glycerol, and lactate (3, 7, 16, 27, 34). We also observed no significant mean differences in resting concentrations of insulin, epinephrine, norepinephrine, and cortisol, as others have reported (3, 7, 16, 28, 34, 57). Changes in estrogen and progesterone across the normal menstrual cycle do not appear to be of sufficient magnitude to significantly affect resting substrate oxidation or glucose flux, as observed in the current study and by others (7, 57).
Contrary to what was originally hypothesized, and under carefully controlled conditions of prestudy diet and activity, we observed no significant mean differences in whole body lipid and carbohydrate oxidation across the menstrual cycle during moderate-intensity long-duration exercise. This is in agreement with other studies (3, 7, 11, 26), which have measured exercise RER over a range of exercise intensities (from 35 to 80% ). Others have reported, however, that lipid oxidation is greater, and carbohydrate oxidation lower, in the luteal vs. early or midfollicular phases of the menstrual cycle (10, 18, 57). These latter studies do not show consistent findings with respect to the intensity of exercise at which such an effect may be observed. For example, in the study of Hackney et al. (18), greater luteal phase lipid oxidation was observed only when exercise was performed at low to moderate intensities (35 and 60, but not 75% of ), whereas other studies have reported cycle phase differences in substrate oxidation only with exercise >50% peak () (10, 57). Interestingly, estrogen supplementation studies have found no effect of this hormone on exercise RER (9, 46). The reason for these inconsistencies with respect to menstrual cycle effects on exercise fuel oxidation could be factors such as prestudy diet and exercise control. For example, inadequate energy and/or carbohydrate intake before testing would affect glycogen stores (liver and muscle), as would variations in exercise training before different study days. A further consideration is the timing and duration of the respiratory gas exchange measurements. For example, in the study of Zderic et al. (57), respiratory gas collections were made for 5 min at the end of a 25-min exercise period. In contrast, the current study measured RER for 20 min, on three occasions, during steady-state exercise. Whether short-duration measurements at the end of a longer exercise bout are sufficient and/or representative of the whole exercise period needs to be considered. However, significant differences in whole body substrate oxidation may be observed only when there are differences in glucose kinetics, as is discussed below.
In the current study, we observed no significant difference in glucose Ra or Rd during moderate-intensity exercise in three phases of the menstrual cycle characterized by very different estrogen-to-progesterone ratios. This is in agreement with the study of Braun et al. (7), who studied 15 women during moderate exercise (50% ) in the MF and ML phases. In contrast, Zderic et al. (57) reported a significantly lower glucose Ra and Rd during the ML vs. EF phase of the menstrual cycle in six women (recreational athletes) during exercise performed at 90% lactate threshold (LT: ∼52% ) but not at 70% LT (∼43% ). In the current study and that of Zderic et al., subjects were studied after an overnight fast, whereas Braun et al. studied subjects 4-5 h after a breakfast meal. Because hepatic glycogen stores would have been higher in the study of Braun et al., this may have minimized any potential effect the sex steriods may have had on glucose production (liver glycogen not being limited). One possible reason for the difference in the results between the current study and that of Zderic et al. is the method of determining exercise intensity. Unlike Zderic et al., we calculated exercise intensity relative to subject , rather than LT. Although in both studies subjects exercised at a similar intensity relative to (50-52%), subjects in the current study could have been exercising at slightly different levels of LT. It is possible, therefore, that this may have obscured our ability to observe a consistent difference among cycle phases in glucose kinetics. Nevertheless, in the data of Zderic et al., there is no explanation as to why luteal phase glucose kinetics were reduced only with exercise at 90% LT but not at 70% LT (which represents a very small change in ), for example, in terms of differences in the endocrine and/or metabolic response. Differences in exercise duration between the current study (90 min) and that of Zderic et al. (25 min @ 70% LT, immediately followed by 25 min @ 90% LT, resepectively) are unlikely to be the cause of different results, because the current study found no difference in glucose kinetics at any time point during exercise in the EF, MF, or ML phases.
A further possible explanation for the differences between the current study and that of Zderic et al. (57) is the training status of subjects. In the current study, subjects appeared to be less trained than the “recreational athletes” in the study of Zderic et al. ( of 42 vs. 48.2 ml·kg-1·min-1, respectively, with current study values adjusted to sea level equivalent). Because subjects were better trained in the study of Zderic et al., a higher rate of total fuel oxidation, and thus glucose utilization, could be achieved when they exercised at ∼50% . At this intensity only, and not at the lower exercise intensity (43% , when total fuel oxidation and glucose kinetics were lower), did Zderic et al. observe a significant effect of menstrual cycle phase. This emphasizes what may be an important factor in determining whether or not menstrual cycle differences are observed in glucose kinetics, and that is the demands placed on endogenous glucose production. For example, in the study of Zderic et al., glucose Ra and Rd were much greater during exercise at 52% (90% LT) (33.4 μmol·kg-1·min-1 in the follicular phase vs. 28.4 μmol·kg-1·min-1 in the luteal phase) compared with 43% (70% LT) (18-19 μmol·kg-1·min-1, in both phases) and compared with the current study (∼19-21 μmol·kg-1· min-/-1, in all phases). Furthermore, in highly trained women exercising at 70% , glucose Ra between 75 and 120 min was ~32-35 vs. 25 μmol·kg--1min-1, in the MF vs. ML phases, respectively, a difference that was highly significant (10). In the latter study, cycle phase differences were obliterated when the subjects consumed a glucose drink that increased exogenous glucose Ra and diminished the need for endogenous glucose Ra (10). Hence, it appears that, under overnight fasted conditions, the primary factor determining whether or not menstrual cycle phase differences are observed in glucose kinetics is the demand placed on endogenous glucose production. In turn, this is related to a lower glucose Rd and total carbohydrate oxidation (10, 57). It could be hypothesized, therefore, that a significant effect of luteal phase increases in estrogen and/or progesterone on exercising glucose kinetics is observed only when the demands on glucose utilization exceed a “critical” level. The plausibility of such a hypothesis remains to be determined.
At the rates of glucose utilization observed during the current study, the effects of the sex steroids on glucose kinetics may be subtle and difficult to detect as significant mean differences. Indeed, both glucose Ra and Rd were nonsignificantly reduced in the ML vs. EF and MF phases. There also appeared to be a time lag in the early increase in glucose Rd, relative to Ra, in the luteal phase. Although not statistically significant, this was sufficient to cause a small, but significant, increase in blood glucose during the first 45 min of exercise in the ML phase. Others have also reported a greater luteal phase increase in exercise blood glucose concentration in overnight-fasted subjects (16, 57). The subtle difference in glucose Rd and/or Ra with this rate of glucose utilization may have been too small to detect by tracer measurements but appeared to be sufficient to produce a transient increase in blood glucose.
The level of circulating estrogen may be another factor that is important in determining whether menstrual cycle differences in glucose kinetics are observed during perturbations such as exercise. In the studies by Zderic et al. (57) and Campbell et al. (10), a slightly higher circulating estradiol was reported during the luteal phase of the menstrual cycle compared with the current study. Estrogen supplementation studies, in relatively estrogen-“naïve” subjects (men or amenorrheic women), have also found a significant reduction in exercise glucose Ra, Rd, and MCR (9, 46). Carter et al. (9) reported that circulating estrogen levels reached ~650 pg/ml in the male subjects, which is sixfold higher than those observed in the current study during the ML phase. Whether there is a threshold above which estrogen needs to be before effects on glucose Ra and/or Rd are observed is a possibility. Furthermore, the effects of elevated estrogen may be more pronounced when glucose Ra and Rd approach a “critical” level, as we have discussed.
In our current study, we observed a significant rise in exercise glucose concentration only in the ML phase (high estrogen and progesterone) and not in the MF phase (high estrogen, low progesterone). Campbell et al. (10) observed a significantly lower carbohydrate oxidation and glucose Ra and Rd in the ML vs. the MF phase. This suggests that estrogen and progesterone may interact to affect exercise glucose metabolism during exercise in the normal menstrual cycle. Although the data from the estrogen supplementation studies do support a greater role for estrogen (9, 46), the menstrual cycle data suggest that progesterone cannot be dismissed. Elevated progesterone and estradiol during the luteal phase of the menstrual cycle have been shown to impair non-insulin-mediated glucose uptake (13, 54). Effects on contraction-mediated glucose transport are not known. Estrogen alone, on the other hand, is required for normal contraction-mediated glucose transport and does not appear to be inhibitory (20). With respect to glucose production, animal studies have shown that estrogen or progesterone can each decrease hepatic glycogenolysis (1), whereas estrogen appears to be more effective at decreasing gluconeo-genesis (2, 36, 37). Although a lower glucose Ra in and of itself can decrease glucose utilization, progesterone could complement a reduction in glucose Ra by impairing glucose uptake directly. In concert, the two hormones may exert the greatest effect on diminishing glucose utilization, particularly when glucose requirements are greatly increased.
We observed a significantly greater insulin concentration during the first part of exercise in the ML phase compared with the EF and MF phases, and this has previously been reported (5, 6). In the current study, this increase in insulin was small in absolute terms (1.1 μU/ml) but coincided with the increase in glucose concentration, suggesting that the rise in blood glucose may have stimulated insulin secretion. A decrease in insulin action is also suggested in the luteal phase of the menstrual cycle, because exercise glucose utilization was not significantly different from that in both follicular phases, but insulin concentration was significantly greater (at least initially). Because we observed no cyclic difference in glycerol concentrations, it appeared that the slight increase in circulating insulin had no effect on whole body lipolysis. Tracer measurements of glycerol kinetics are needed to confirm this. In agreement with previous studies, we observed no difference in the epinephrine, norepinephrine (3, 7), or cortisol (3, 6, 7, 16, 28) response to exercise during the menstrual cycle. There was a significant time-by-phase interaction for norepinephrine because of a different pattern of response during exercise in the MF phase vs. the EF and ML phases. This does not appear, however, to have had any significant metabolic consequences. We did observe a tendency for a greater glucagon response in the luteal phase relative to both follicular phases, which has previously been reported (34). Nevertheless, the lack of a main effect of menstrual cycle phase on the counterregulatory hormones measured is in agreement with a lack of menstrual cycle effects on glucose kinetics and whole body substrate oxidation.
Finally, it is important to emphasize the results from the statistical analysis when random effects for the different dependent variables measured in this study are analyzed. Previous exercise studies have not examined potential correlations between repeated measurements from the same woman, or from the same woman in the same phase of the menstrual cycle. We found that, for all the dependent time-varying parameters we measured, with the exception of insulin and glucose MCR, an individual's data were better correlated with values measured in the same cycle phase than with values measured in the other two cycle phases. This suggests that, if studies are performed in women randomly throughout the menstrual cycle, there will be much greater variability in the hormone, substrate, and/or substrate kinetic data than if the women were studied in a similar cycle phase. If control is not made for menstrual cycle phase or other sex-steroid hormone variability, e.g., oral contraceptive use or menopause, then the statistical power may be decreased when assessing the impact of an intervention and/or sex-based differences in metabolism is attempted. Consequently, it is strongly recommended that sex-steroid hormone status be determined and controlled for in metabolic studies including women.
In summary, menstrual cycle variations in the levels of estrogen to progesterone, ranging from low to high, had no significant effect on mean values of whole body substrate oxidation during prolonged moderate-intensity exercise in moderately active women. There was a suggestion, however, of a subtle effect of elevated estrogen and progesterone on glucose and insulin concentrations in the midluteal phase, but this was not reflected in measures of glucose kinetics. Despite no significant differences in the mean responses of most substrates and hormones, and glucose kinetics, menstrual cycle phase was a significant predictor of the variance observed in most of these measures. Thus variations in the sex steroids should be controlled for in metabolic studies using female subjects.
Acknowledgments
We thank all the subjects who volunteered for the study for their time and cooperation. We thank the GCRC nursing, dietary, and laboratory staff for their valuable assistance, as well as the Mass Spectrometry and Energy Balance Core Laboratories of the Colorado Nutrition Research Unit for their important provision of testing and analytical services. Finally, many thanks to Dr. Michael Pagliassotti for extremely useful advice on many aspects of this study, and valuable guidance on tracer techniques.
This investigation was supported by National Institutes of Health Grants HL-59331, HL-04226, DK-48520, and Public Health Services Research Grant M01-RR-00051 from the Division of Research Resources.
REFERENCES
- 1.Ahmed-Sorour H, Bailey CJ. Role of ovarian hormones in the long-term control of glucose homeostasis. Interaction with insulin, glucagon and epinephrine. Hormone Res. 1980;13:396–403. doi: 10.1159/000179307. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed-Sorour H, Bailey CJ. Role of ovarian hormones in the long-term control of glucose homeostasis. Glycogen formation and gluconeogenesis. Ann Nutr Metab. 1981;25:212. doi: 10.1159/000176496. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SP, Zacher CM, Mittleman KD. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J Appl Physiol. 2000;88:690–697. doi: 10.1152/jappl.2000.88.2.690. [DOI] [PubMed] [Google Scholar]
- 4.Bhathena SJ, Berlin E, Judd J, Nair PP, Kennedy BW, Jones J, Smith PM, Jones Y, Taylor PR, Campbell WS. Hormones regulating lipid and carbohydrate metabolism in premenopausal women: modulation by dietary lipids. Am J Clin Nutr. 1989;49:752–757. doi: 10.1093/ajcn/49.5.752. [DOI] [PubMed] [Google Scholar]
- 5.Bonen A, Haynes FJ, Graham TE. Substrate and hormonal responses to exercise in women using oral contraceptives. J Appl Physiol. 1991;70:1917–1927. doi: 10.1152/jappl.1991.70.5.1917. [DOI] [PubMed] [Google Scholar]
- 6.Bonen A, Haynes FJ, Watson-Wright W, Sopper MM, Pierce GN, Low MP, Graham TE. Effects of menstrual cycle on metabolic responses to exercise. J Appl Physiol. 1983;55:1506–1513. doi: 10.1152/jappl.1983.55.5.1506. [DOI] [PubMed] [Google Scholar]
- 7.Braun BJ, Mawson T, Muza SR, Dominick SB, Brooks GA, Horning MA, Rock PB, Moore LG, Mazzeo RS, Ezeji-Okoye SC, Butterfield GE. Women at altitude: carbohydrate utilization during exercise at 4,300 m. J Appl Physiol. 2000;88:246–256. doi: 10.1152/jappl.2000.88.1.246. [DOI] [PubMed] [Google Scholar]
- 8.Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 9.Carter S, McKenzie S, Mourtzakis M, Mahoney DJ, Tarnopolsky MA. Short-term 17β-estradiol decreases glucose Ra but not whole body metabolism during endurance exercise. J Appl Physiol. 2001;90:139–146. doi: 10.1152/jappl.2001.90.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SE, Angus DJ, Febbraio MA. Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion. Am J Physiol Endocrinol Metab. 2001;281:E817–E825. doi: 10.1152/ajpendo.2001.281.4.E817. [DOI] [PubMed] [Google Scholar]
- 11.De Souza MJ, Maguire MS, Rubin KR, Maresh CM. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med Sci Sports Exerc. 1990;22:575–580. doi: 10.1249/00005768-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Diamond MP, Grainger DA, Rossi G, Connolly-Diamond M, Sherwin RS. Counter-regulatory response to hypoglycemia in the follicular and luteal phases of the menstrual cycle. Fertil Steril. 1993;60:988–993. [PubMed] [Google Scholar]
- 13.Diamond MP, Simonson DC, DeFronzo RA. Menstrual cyclicity has a profound effect on glucose homeostasis. Fertil Steril. 1989;52:204–208. [PubMed] [Google Scholar]
- 14.Ellis GS, Lanza-Jacoby S, Gow A, Kendrick ZV. Effects of estradiol on lipoprotein lipase activity and lipid availability in exercised male rats. J Appl Physiol. 1994;77:209–215. doi: 10.1152/jappl.1994.77.1.209. [DOI] [PubMed] [Google Scholar]
- 15.Escalante Pulido J, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30:19–22. doi: 10.1016/s0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 16.Galliven EA, Singh A, Michelson D, Bina S, Gold PW, Deuster PA. Hormonal and metabolic responses to exercise across time of day and menstrual cycle phase. J Appl Physiol. 1997;83:1822–1831. doi: 10.1152/jappl.1997.83.6.1822. [DOI] [PubMed] [Google Scholar]
- 17.Genazzani AR, Lemarchand-Beraud TH, Aubert ML, Felber JP. Pattern of plasma ACTH, hGH, and cortisol during menstrual cycle. J Clin Endocrinol Metab. 1975;41:431–437. doi: 10.1210/jcem-41-3-431. [DOI] [PubMed] [Google Scholar]
- 18.Hackney AC, McCracken-Compton MA, Ainsworth B. Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle. Int J Sport Nutr. 1994;4:299–308. doi: 10.1123/ijsn.4.3.299. [DOI] [PubMed] [Google Scholar]
- 19.Hansen FM, Fahmy N, Nielson JH. The influence of sexual hormones on lipogenesis and lipolysis in rat fat cells. Acta Endocrinol (Copenh) 1980;95:566–570. doi: 10.1530/acta.0.0950566. [DOI] [PubMed] [Google Scholar]
- 20.Hansen PA, McCrathy TJ, Pasia EN, Spina RJ, Gulve EA. Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats. J Appl Physiol. 1996;80:1605–1611. doi: 10.1152/jappl.1996.80.5.1605. [DOI] [PubMed] [Google Scholar]
- 21.Hatta H, Atomi Y, Shinohara S, Yamamoto Y, Yamada S. The effects of ovarian hormones on glucose and fatty acid oxidation during exercise in female ovariectomized rats. Horm Metab Res. 1988;20:609–611. doi: 10.1055/s-2007-1010897. [DOI] [PubMed] [Google Scholar]
- 22.Heiling VJ, Jensen MD. Free fatty acid metabolism in the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 1992;74:806–810. doi: 10.1210/jcem.74.4.1548345. [DOI] [PubMed] [Google Scholar]
- 23.Horton TJ, Drougas HJ, Sharp TA, Martinez LA, Reed GW, Hill JO. Energy balance in endurance-trained female cyclists and untrained controls. J Appl Physiol. 1994;76:1937–1945. doi: 10.1152/jappl.1994.76.5.1937. [DOI] [PubMed] [Google Scholar]
- 24.Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol. 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- 25.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Ann Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 26.Jurkowski JE, Jones NL, Walker WC, Younglai EV, Sutton JR. Ovarian hormonal responses to exercise. J Appl Physiol. 1978;44:109–114. doi: 10.1152/jappl.1978.44.1.109. [DOI] [PubMed] [Google Scholar]
- 27.Kanaley JA, Boileau RA, Bahr JM, Misner JE. Substrate oxidation and GH responses to exercise are independent of menstrual phase and status. Med Sci Sports Exerc. 1992;24:873–880. [PubMed] [Google Scholar]
- 28.Kanaley JA, Boileau RA, Bahr JM, Misner JE, Nelson RA. Cortisol levels during prolonged exercise: the influence of menstrual phase and menstrual status. Int J Sports Med. 1992;13:332–336. doi: 10.1055/s-2007-1021276. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Kalkhoff RK. Sex steroid influence on triglyceride metabolism. J Clin Invest. 1975;56:888–896. doi: 10.1172/JCI108168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krotkiewski M, Bjorntorp P. The effect of progesterone and of insulin administration on regional adipose tissue cellularity in the rat. Acta Physiol Scand. 1976;96:122–127. doi: 10.1111/j.1748-1716.1976.tb10177.x. [DOI] [PubMed] [Google Scholar]
- 31.Laird NM, Wart JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 32.Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94:89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- 33.Larivière F, Moussalli R, Garrel DR. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am J Physiol Endocrinol Metab. 1994;267:E422–E428. doi: 10.1152/ajpendo.1994.267.3.E422. [DOI] [PubMed] [Google Scholar]
- 34.Lavoie JM, Dionne N, Helie R, Brisson GR. Menstrual cycle phase dissociation of blood glucose homeostasis during exercise. J Appl Physiol. 1987;62:1084–1089. doi: 10.1152/jappl.1987.62.3.1084. [DOI] [PubMed] [Google Scholar]
- 35.Lissner L, Stevens J, Levtisky DA, Rasmussen KM, Strupp B. Variation in energy intake during the menstrual cycle: implications for food-intake research. Am J Clin Nutr. 1988;48:956–962. doi: 10.1093/ajcn/48.4.956. [DOI] [PubMed] [Google Scholar]
- 36.Mandour T, Kissebah AH, Wynn V. Mechanism of oestrogen and progesterone effects on lipid and carbohydrate metabolism: alteration in the insulin:glucagon molar ratio and hepatic enzyme activity. Eur J Clin Invest. 1976;7:181–187. doi: 10.1111/j.1365-2362.1977.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 37.Matute ML, Kalkhoff RK. Sex steroid influence on hepatic gluconeogenesis and glycogen formation. Endocrinology. 1972;92:762–768. doi: 10.1210/endo-92-3-762. [DOI] [PubMed] [Google Scholar]
- 38.McGuire EAH, Helderman Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976;41:565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- 39.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 40.Nicklas BJ, Hackney AC, Sharp RL. The menstrual cycle and exercise: performance, muscle glycogen, and substrate responses. Int J Sports Med. 1989;10:264–269. doi: 10.1055/s-2007-1024913. [DOI] [PubMed] [Google Scholar]
- 41.Pasquier YN, Pecquery R, Giudicelli Y. Increased adenylate cyclase catalytic activity explains how estrogens “in vivo” promote lipolytic activity in rat white fat cells. Biochem Biophys Res Commun. 1988;154:1151–1159. doi: 10.1016/0006-291x(88)90261-6. [DOI] [PubMed] [Google Scholar]
- 42.Piers LS, Doggavi SN, Rijskamp J, van Raaij JMA, Shetty PS, Hautvast J. Resting metabolic rate and thermic effect of a meal in the follicular and luteal phases of the menstrual cycle in well-nourished Indian women. Am J Clin Nutr. 1995;61:296–302. doi: 10.1093/ajcn/61.2.296. [DOI] [PubMed] [Google Scholar]
- 43.Reinke U, Ansah B, Voigt KD. Effect of the menstrual cycle on carbohydrate and lipid metabolism in normal females. Acta Endocrinol. 1972;69:762–768. doi: 10.1530/acta.0.0690762. [DOI] [PubMed] [Google Scholar]
- 44.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 45.Rondinone CM, Baker ME, Rodbard D. Progestins stimulate the differentiation of 3T3-L1 preadipocytes. J Steroid Biochem Molec Biol. 1992;42:795–802. doi: 10.1016/0960-0760(92)90087-y. [DOI] [PubMed] [Google Scholar]
- 46.Ruby BC, Robergs RA, Waters DL, Burge M, Mermier C, Stolarczyk L. Effects of estradiol on substrate turnover during exercise in amenorrheic females. Med Sci Sports Exerc. 1997;29:1160–1169. doi: 10.1097/00005768-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretive problems. Am J Physiol Endocrinol Metab. 1990;258:E399–E412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- 48.Sladek CD. Gluconeogenesis and hepatic glycogen formation in relation to the rat estrous cycle. Horm Metab Res. 1974;6:217–221. doi: 10.1055/s-0028-1093856. [DOI] [PubMed] [Google Scholar]
- 49.Sutton JR, Jurkowski JE, Keane P. The effect of the menstrual cycle on the plasma catecholamine response to exercise in normal females (Abstract) Clin Res. 1978;26:867A. [Google Scholar]
- 50.Terjung RL, Kaciuba-Uscilko H. Lipid metabolism during exercise: influence of training. Diabetes Metab Rev. 1986;2:35–51. doi: 10.1002/dmr.5610020103. [DOI] [PubMed] [Google Scholar]
- 51.Valdes CT, Elkind-Hirsch E. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72:642–646. doi: 10.1210/jcem-72-3-642. [DOI] [PubMed] [Google Scholar]
- 52.Wahba G. Spline Models for Observational Data. Society for Industrial and Applied Mathematics; Philadelphia, PA: 1990. [Google Scholar]
- 53.Weir JB, de V. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Widom B, Diamond MP, Simonson DC. Alterations in glucose metabolism during the menstrual cycle in women with IDDM. Diabetes Care. 1992;15:213–220. doi: 10.2337/diacare.15.2.213. [DOI] [PubMed] [Google Scholar]
- 55.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Wiley-Liss; New York: 1992. [Google Scholar]
- 56.Yen SSC, Vela P, Rankin J, Littell AS. Hormonal relationships during the menstrual cycle. JAMA. 1970;211:1513–1517. [PubMed] [Google Scholar]
- 57.Zderic TW, Coggan AR, Ruby BC. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol. 2001;90:447–453. doi: 10.1152/jappl.2001.90.2.447. [DOI] [PubMed] [Google Scholar]
- 58.Zuspan FP, Zuspan KJ. Ovulatory plasma amine (epinephrine and norepinephrine) surge in the woman. Am J Obstet Gynecol. 1973;117:654–661. doi: 10.1016/0002-9378(73)90207-x. [DOI] [PubMed] [Google Scholar]