Abstract
Purpose of review
The concept of the developmental origins of health and disease susceptibility is rapidly attracting interest and gaining prominence as a complementary approach to understanding the causation of many complex common disorders that confer a major burden of disease; however several important issues and questions remain to be addressed, particularly in the context of humans.
Recent findings
In this review we enunciate some of these questions and issues, review empirical evidence primarily from our own recent studies on prenatal stress and stress biology, and discuss putative maternal–placental–fetal endocrine and immune/inflammatory candidate mechanisms that may underlie and mediate short-term and long-term effects of prenatal stress on the developing human embryo and fetus, with a specific focus on body composition, metabolic function, and obesity risk.
Summary
The implications for research and clinical practice are discussed with a summary of recent advances in noninvasive methods to characterize fetal, newborn, infant, and child developmental and health-related processes that, when coupled with available state-of-the-art statistical modeling approaches for longitudinal, repeated measures time series analysis, now afford unprecedented opportunities to explore and uncover the developmental origins of human health and disease.
Keywords: childhood obesity, developmental programming, maternal–placental–fetal endocrine and immune processes, metabolic function, prenatal stress
Introduction
The origins of health and disease susceptibility for many complex, common disorders that confer a major burden of disease in developed societies and other societies in rapid transition, including but not limited to body composition, metabolic function, and obesity risk, can be traced back to the intrauterine period of life. Development is a plastic process, wherein a range of different phenotypes can be expressed from a given genotype. The unfolding of all developmental processes across the multicontoured landscape from genotype to phenotype is context-dependent, wherein the developing embryo/fetus responds to, or is acted upon by, conditions in the internal or external environment during sensitive periods of cellular proliferation, differentiation, and maturation, resulting in structural and functional changes in cells, tissues, and organ systems. These changes may, in turn, either independently or through interactions with subsequent developmental processes and environments, have short-term and/or long-term consequences for health and disease susceptibility. These concepts have variously been referred to as the fetal or developmental origins of health and disease [1].
With respect to developmental programming specifically during fetal life some important issues currently being addressed include identification of salient intrauterine conditions; exposition of structural and functional changes produced in response to or by these intrauterine exposures; detection of critical time periods during gestation when intrauterine conditions exert their greatest impact; determination of the nature of the exposure-outcome relationship (whether a threshold, or a linear, dose-dependent, or a J-shaped or U-shaped function); elucidation of the underlying physiological mechanisms by which intrauterine exposures produce their programming effects; clarification of whether the long-term health effects are necessarily mediated by unfavorable birth outcomes; and amplification of the role of subsequent postnatal conditions in either potentiating or mitigating effects of earlier intrauterine exposures.
Human studies on prenatal stress exposure and subsequent health outcomes
Over the past several years the UC Irvine Development, Health, and Disease Research Program has examined the interface between biological, behavioral, and social processes in human pregnancy, with a focus on the impact of maternal psychosocial stress and stress biology on fetal development, birth outcomes, and subsequent newborn, infant, and child developmental and health outcomes. Findings from our studies converge to suggest that after accounting for the effects of other established sociodemographic and obstetric risk factors, maternal psychosocial stress exposure is significantly and independently associated with increased risk of adverse pregnancy and birth outcomes related to the length of gestation (preterm birth) and fetal growth [low birth weight/small-for-gestational age (SGA) birth] [2–5]. Our studies also suggest that the effects of maternal psychosocial stress are mediated, in part, by stress-related alterations in maternal–placental–fetal (MPF) endocrine and immune processes [6–9]. Ongoing work in this area addresses the role of maternal–fetal gene–environment interactions, with a focus on candidate genes implicated in the regulation of key enzyme systems, steroid hormones and other peptides that regulate fetal development and birth outcomes. Other studies address the role of mitochondrial genetic variation, and the biobehavioral basis for the well documented racial/ethnic disparities and the Hispanic acculturation paradox in reproductive health outcomes. The significance of this area of research derives from the well established fact that adverse birth outcomes represent the major problem in maternal-child health in the USA and other developed and developing nations [10].
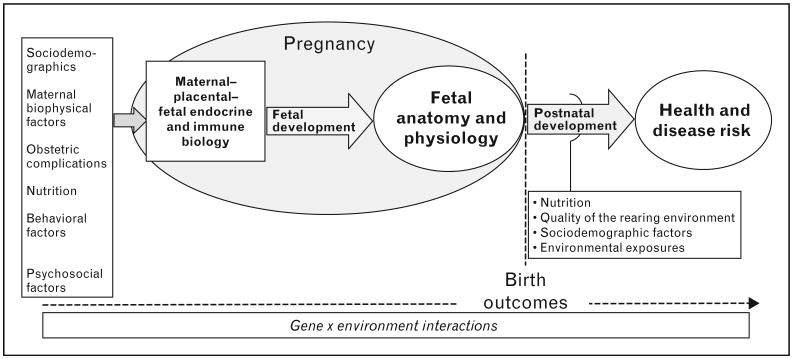
A more recent set of studies, described below, has extended our research agenda to the long-term effects of prenatal psychosocial stress exposure on adult physiology and health. A large number of studies of fetal programming have focused on the critical role of prenatal and perinatal nutrition and have produced important findings and insights (reviewed in [11•]). Based on the consideration that key environmental conditions that have shaped evolutionary selection include not only a variation in the amount and constituents of energy substrate availability and utilization (nutrition) but also conditions and challenges that may impact the physical integrity and survival of living organisms (stress), we and others have proposed that prenatal stress exposure likely represents yet another important adverse intrauterine environment that may impact the anatomy and physiology of the developing organism, with important implications for the developmental programming of health outcomes and disease susceptibility [12]. Moreover, emerging evidence supports a bi-directional interaction between nutrition and stress, such that the consequences on target tissues of either one are moderated by the other [13–17]. Last, we submit the application of a prenatal stress perspective which offers an excellent model system for the study of early development because it is increasingly apparent that the developing fetus acquires and incorporates information about the nature of its environment in part via the same systems that in an already-developed individual mediate adaptation and central and peripheral responses to endogenous and exogenous challenges [12] (see conceptual framework in Fig. 1).
Figure 1. Conceptual framework of a biobehavioral model in humans of prenatal stress-related maternal–placental–fetal endocrine and immune processes and programming of health and disease risk.
Our studies are designed to address the primary question of elucidating the long-term health effects in adult human offspring of exposure to maternal psychosocial stress during pregnancy. Other corollaries addressed include whether these long-term effects are independent from those of other established obstetric, newborn, and childhood risk factors; whether the effects are outcome specific, or whether they influence a range of outcomes; and whether the effects are necessarily mediated by unfavorable birth outcomes. Experimental studies in animals suggest maternal exposure to psychosocial stress during gestation can independently exert long-term effects simultaneously on several central and peripheral systems in the offspring, and that titration of the prenatal stress exposure dose can produce significant long-term effects without altering the birth phenotype [18–22]. However, only a very small number of studies have addressed these questions in humans.
As a first step to addressing these questions, we employed a retrospective case–control design in a sample of healthy young adults born to mothers with healthy pregnancies. One half of the study population of young adults was born to mothers who had experienced a major stressful life event during the index pregnancy (prenatal stress group), whereas the other half was a sociodemographically matched population with no history of maternal exposure to prenatal stress (comparison group). We selected a study population of younger as opposed to older adults in order to focus on predisease markers of physiological dysregulation of metabolic, endocrine and immune systems as early predictors of disease susceptibility. The potential effects of other established obstetric, newborn, and childhood risk factors on adult health were controlled using a stringent set of exclusionary criteria. Maternal and child medical records were obtained and screened to exclude presence of any maternal acute or chronic diseases, obstetric complications (e.g., gestational diabetes, hypertension/preeclampsia, infection), unhealthy behaviors (smoking), adverse birth outcomes (preterm birth, low birth weight), newborn complications, and history of any major childhood or current diseases (obesity, diabetes, asthma, and adverse neuro-developmental or psychiatric conditions). Study assessments were performed to quantify health and physiological markers of disease risk, including body composition and glucose-insulin metabolism (BMI and percentage fat mass; basal and postoral glucose tolerance test levels of glucose, insulin, leptin, adiponectin; fasting lipid profile), endocrine function (basal and post-behavioral/pharmacological stress levels of pituitary– adrenal stress hormones, chronobiological regulation of adrenal function, and assessment of HPA-axis feedback sensitivity), immune function [immune cell trafficking and lipopolysaccharide (LPS)-stimulated production of proinflammatory and anti-inflammatory and TH1/TH2 cytokines], and cognitive function (working memory under basal and hydrocortisone conditions). Because subtle physiological differences in disease susceptibility are often not detected in basal state we employed appropriate challenge tests to quantify the function of these systems under stimulated conditions (e.g., oral glucose challenge, ACTH stimulation test, LPS-stimulated immune responses).
Our results indicated that the young adults exposed during intrauterine life to maternal psychosocial stress consistently exhibited significant dysregulation of all these key physiological parameters, thereby placing them at increased risk for developing clinical disorders. Specifically, individuals in the prenatal stress group exhibited higher BMI and percentage body fat, primary insulin resistance, and a lipid profile consistent with the metabolic syndrome [23], see Fig. 2; altered immune function with a TH2 shift in the TH1/TH2 balance (consistent with increased risk of asthma and autoimmune disorders [24]); altered endocrine function, with an increased adrenocorticotrophic hormone (ACTH) and reduced Cortisol levels during pharmacological and psychological stimulation paradigms (consistent with the high-risk endocrine profile exhibited by individuals exposed to early life abuse [25]); and impaired prefrontal cortex (PFC)-related cognitive performance (impairments in working memory performance after hydrocortisone administration) [26]. Consistent with the finding on cognitive function are results from one of our other recent prospective, longitudinal studies on the long-term effects of prenatal stress (anxiety) on child brain morphology. After excluding cases with low birth weight and adjusting for total gray matter volume, age, gestational age at birth, handedness and postpartum stress, maternal pregnancy-specific anxiety in mid-gestation was associated with gray matter volume reductions in several child brain regions, including the prefrontal cortex [27].
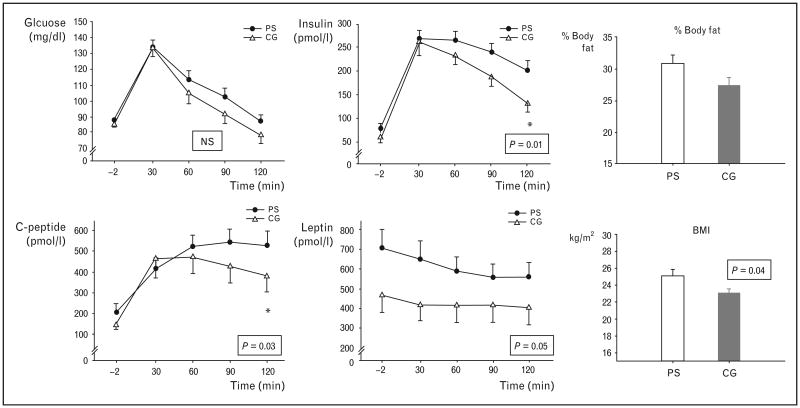
Figure 2. Mean glucose, insulin, C-peptide, and leptin responses (±SEM) to an oral glucose tolerance test in prenatally stressed (PS, ‘black circles’) and comparison group (CG, ‘white triangles’) individuals (left panel).
Glucose levels were not significantly different across the groups, however, PS individuals showed significantly elevated 2 h insulin (P=0.01) and C-peptide levels (P=0.03), as well as higher leptin levels at all time points during the oral glucose tolerance test (OGTT) (P=0.05). In addition, PS individuals had a higher % body fat and a higher BMI (P=0.04, right panel). Adapted with permission from [23].
Taken together, our findings suggest that in-utero exposure to prenatal psychosocial stress may confer increased long-term risk of a range of negative physiological and cognitive health outcomes in humans; these effects are independent from those of other established obstetric and childhood risk factors; and these long-term effects are not necessarily mediated by unfavorable birth outcomes. It is noteworthy that our above-described finding on body composition is consistent with a more recent report in a large, national cohort sample linking prepregnancy and prenatal stress exposure related to maternal bereavement to risk of childhood overweight [28], and our finding on immune function is consistent with another recent report linking prenatal maternal anxiety with infant illnesses and antibiotic use [29].
Stress-related maternal–placental–fetal endocrine and immune processes as potential mediators of fetal programming of health and disease
The fetal programming hypothesis has led to the search for underlying mechanisms by which disparate intrauterine insults exert a multitude of effects on different physiological systems in the developing offspring. A question of further interest relates to whether these biological mechanisms are outcome specific, or whether there may be some common mechanisms that influence a range of disparate outcomes. We suggest that stress-related maternal–placental–fetal endocrine and immune processes in gestation constitute an attractive underlying common candidate mechanism because they are responsive to many classes of intrauterine perturbations and they act on multiple targets of fetal programming [12]. Unlike exposure to toxins and teratogens, it is important to appreciate that maternal–placental–fetal hormones and cytokines play an essential and obligatory role in orchestrating key events underlying cellular growth, replication and differentiation in the brain and peripheral tissues [30–35]. Thus, perturbations in the level and/or time of exposure of these biologic effectors are likely to produce alterations of normal structure and function. It also is important to appreciate that the state of pregnancy itself produces major and progressive alterations in the function of these systems, and that these changes may have important implications for altering the responsivity of these systems to exogenous or endogenous perturbations.
The maternal–placental–fetal endocrine and immune systems in mammalian pregnancy
Pregnancy produces major alterations in neuroendocrine and immune function, including changes in hormone and cytokine levels and control mechanisms (feedback loops), that are crucial in providing a favorable environment within the uterus and fetal compartment for growth, differentiation, and maturation and conveying signals when the fetus is ready for extrauterine life. Starting very early in gestation the placenta, the first fetal organ to develop and function, produces hormones, neuropeptides, growth factors, and cytokines, and appears to function in a manner resembling that of compressed hypothalamic–pituitary–target systems [36,37]. Glucocorticoid physiology (Cortisol in humans) has received extensive and well placed consideration as a critical endocrine mediator of fetal programming, with an emphasis on not only hormone production but also hormone action mediated by tissue-specific glucocorticoid receptor expression, sensitivity and affinity, and by maternal–fetal transfer mediated by the activity of the placental 11β-hydroxysteriod dehydrogenase enzyme system (see [38••] for a recent review). Less well recognized is the potential and perhaps equally important role of the peptide corticotrophin-releasing hormone (CRH). In primates, but not other mammals, the placenta synthesizes and releases CRH in large amounts into the fetal and maternal circulations. In contrast to the inhibitory influence on hypothalamic CRH production, Cortisol stimulates placental CRH production [39], and this positive feedback loop results in a progressive amplification of CRH and Cortisol production over the course of gestation [40]. With respect to the immune axis, a major endeavor of pregnancy-related alterations in immune function is to achieve and maintain the optimal balance between tolerating the fetal semi-allograft while not suppressing maternal immune responses to an extent that increases maternal or fetal susceptibility to infection. Thus, a generalized reduction of maternal immune responsiveness occurs during pregnancy, mediated by hormonal changes (e.g., increased levels of progesterone), trophoblast expression of key immunomodulatory molecules, and a progressive switch from a TH1/TH2 balance to a predominantly T-helper 2-type pattern of cytokines [41•].
Prenatal stress and maternal–placental–fetal endocrine and immune function
Substantial evidence in nonpregnant humans and animals demonstrates that stress exposure produces activation of the neuroendocrine system (e.g., HPA axis) and exaggerated inflammatory responses [42,43]; however, these associations cannot be assumed to also be present in the pregnant state because the above-described changes in endocrine and immune physiology have consequences for attenuating the responsivity of these systems to stress. With respect to prenatal psychosocial stress-related biological pathways, some of our earlier studies were among the first to demonstrate that despite the large pregnancy-associated changes in maternal endocrine physiology, the system is responsive to maternal psychosocial states (such as high stress and low social support) [6]. Our more recent studies on maternal stress responses in human pregnancy are among the first to demonstrate that maternal psychophysiological stress responses are progressively attenuated with advancing gestation [44•], and that after accounting for the effects of other established risk factors, the degree of attenuation is a significant predictor of shortened length of gestation and earlier delivery [45].
Studies by other groups have reported that elevated psychosocial stress in pregnant women is associated with higher circulating levels of inflammatory markers like C-reactive protein (CRP) and the proinflammatory cytokines IL-1b, IL-6, and TNF-α, with lower circulating levels of the anti-inflammatory cytokine IL-10 and ex-vivo endotoxin (LPS)-stimulated levels of IL-1b and IL-6 [46,47]. Another recent study of proinflammatory responses to an in-vivo antigen challenge (influenza virus vaccination) in pregnant women reported an association between depressive symptoms and sensitization of the inflammatory cytokine responses [48].
In addition to psychosocial stress, substantial in-vitro and in-vivo evidence indicates that maternal–placental–fetal endocrine and immune processes during pregnancy respond to a variety of other maternal and intrauterine perturbations, including biological effectors of stress [7,49–53], obstetric risk conditions such as preeclampsia, pregnancy-induced hypertension [54–67], gestational diabetes [68,69], infection [54–56,70], reduced uteroplacental blood flow [57,58], and behavioral factors such as the constituents of maternal diet, over nutrition and under nutrition, and smoking [59–67].
Based on these findings, it is apparent that measures of maternal–fetal endocrine and immune/inflammatory stress markers capture physiological responses to a wide range of intrauterine perturbations including, but not limited to prenatal stress. In accordance with our suggestion that stress-related maternal–placental–fetal endocrine and immune processes in gestation constitute an attractive candidate mechanism for fetal programming, a recent JAMA editorial [71] on an article reporting an increase in the prevalence of several categories of chronic illness in childhood, including obesity, asthma, and ADHD [72], speculates there may be common early risks underlying these conditions that are triggering development of aberrant physiologic pathways. The editorial suggests that adverse early experiences that affect stress-sensitive physiologic systems (endocrine/metabolic, immune) may contribute to not only the onset of childhood illness but also predispose the same individuals to develop age-related diseases as adults.
Fetal programming of body composition, metabolic function, and obesity risk
Continuing with the theme of a common underlying biological mechanism, in this section we address the issue of the potential impact of prenatal stress biology on multiple targets of fetal programming. We consider here, as an example, phenotypic outcomes related to body composition, metabolic function, and obesity risk.
Obesity (or, to be more precise, adiposity) is recognized as one of the most serious health problems in the USA and other societies. At the individual level, obesity results when energy intake exceeds energy expenditure. However, the relationship between excess energy intake and adiposity is not linear and monotonic; there is a wide variation among children or adults at identical levels of excess energy intake in their propensity to gain weight and accrue fat mass. This variation across individuals defines susceptibility for developing obesity/adiposity. Once an individual becomes obese, it is difficult to lose weight, and even more difficult to sustain weight loss, because of the remarkable efficiency of energy balance homeostasis mechanisms [73,74••]. For these reasons, it is important to gain a better understanding of the origins of individual differences in the propensity for weight and fat mass gain, in order to predict obesity risk and develop strategies for primary prevention [74••].
Targets of programming of obesity: potential role of the maternal–placental–fetal endocrine and immune/inflammatory pathway
It is well established that the primary targets of programming of body composition, metabolic function, and obesity risk are the neural networks that regulate energy balance (appetite, feeding, and basal energy expenditure) and peripheral organs and tissues involved in fat synthesis/breakdown, storage, and metabolic function (adipocyte, liver, and pancreas). In this section, we consider and review findings that pertain to the potential role of prenatal stress biology in programming these major targets of interest.
Stress-related endocrine and immune processes in human pregnancy are associated with not only fetal development and birth outcomes but also with later disease risk. For example, we have reported that placental CRH concentrations in human pregnancy significantly predict the rate of fetal growth and size at birth [8], which, in turn, is a significant predictor of childhood and adult adiposity [75–77]. Other researchers have found a positive association between CRH levels in pregnancy and an increase in central adiposity [78] and alterations in adiponectin levels in 3-year-old children [79••]. Yet others have reported a positive association between maternal levels of interleukin-6 (IL-6) in pregnancy and neonatal adiposity [80].
Neural circuits
The central role of ventromedial hypothalamic (VMH) circuits in regulating feeding and energy balance is well established. VMH neurons contain receptors for and receive afferent signals related to fat stores (leptin), nutrient metabolism (insulin), hunger (ghrelin), and satiety (peptide YY), and they integrate peripheral signals of effectors of food intake and energy expenditure so as to prevent substantial variations in the level of energy balance [81]. Also involved in the regulation of appetite and food intake are brain regions that make food intake rewarding (limbic structures), and higher cortical structures (e.g., prefrontal cortex) that are important for learned patterns of eating behavior and executive control [82]. A growing body of literature suggests that intrauterine perturbations can produce reorganization of these neural pathways that regulate energy intake and expenditure in ways that enhance the development of obesity. Several studies have convincingly demonstrated that biological (endocrine, immune) stress during gestation, triggered by a variety of nutritional, inflammatory, vascular, behavioral, or psychosocial perturbations, can promote obesity in the offspring by reorganizing central neural pathways through programming of energy balance ‘set points’ (see [83••] for recent review). One key system involved in the regulation of energy balance is the hypothalamic (CRH)–pituitary (ACTH)–adrenal (Cortisol) neuroendocrine stress axis, which forms a network of neuronal pathways capable of interacting with brain circuits controlling energy balance [84]. For instance, the adipogenic hormone leptin which is the afferent loop informing the hypothalamus about the states of fat stores, participates in the expression of hypothalamic CRH, interacts at the adrenal with ACTH, and is regulated by Cortisol. Cortisol increases leptin secretion and limits CNS leptin-induced efferents [85].
Adipocytes
Obesity is impacted by increases in fat cell number, size, or both. Fetal adipose tissue development is regulated by the complex interaction of maternal, endocrine, and paracrine influences that initiate specific changes in angiogenesis, adipogenesis, and metabolism [86]. Adipogenesis, the process of adipocyte development from mesenchymal stem cell precursors, occurs primarily during late fetal and early postnatal life in humans, and the number of adipocytes is relatively fixed after young adulthood [86–88], supporting the notion that fetal and early postnatal periods are crucial windows in the development of adipose depots. Adipogenesis is highly sensitive to the intrauterine biological environment, in particular to concentrations of insulin-like growth factors, glucose, insulin, and glucocorticoids [86,87]. In addition, CRH seems to be an important regulator of adipocyte function, and CRH receptors are expressed in both white and brown adipocytes [89]. The role of cytokines as regulators of adipose tissue metabolism is well established. Proinflammatory cytokines are elevated in obese individuals, and they seem to modulate leptin secretion from adipocytes [90]. Furthermore, animal studies have shown that fat cells exposed to an excess substrate supply during crucial windows in their development have an increased capacity for storing lipid in postnatal life [91,92]. This enhanced lipogenic capacity renders these individuals more likely to store excess energy in the form of fat and increases their susceptibility to weight gain and obesity and its metabolic sequelae. In individuals exposed to low nutrition levels before birth, adipocyte development is initially sacrificed in favor of ‘essential’ organs [93,94]. If an in-utero ‘restricted’ individual is born into a postnatal environment in which nutrient supply is no longer constrained, a period of ‘catch-up’ fat deposition ensues, mainly in the visceral adipose depot [95]. These individuals are at increased risk of visceral obesity [77] and, consequently, to the development of insulin resistance and type 2 diabetes [96].
Liver and pancreas
The liver controls the production and fate of metabolic fuels through the action of hepatic enzymes. Phosphoenolpyruvate carboxykinase (PEPCK), a key enzyme in hepatic gluconeogenesis, is under potent glucocorticoid regulation. In animals, prenatal exposure to dexamethasone produces an increased expression of hepatic glucocorticoid receptors as well as increased levels and activity of PEPCK [97], thereby predisposing these animals to glucose intolerance later in life. Furthermore, manipulation of diet during pregnancy is associated with epigenetic changes in the promotor regions of the genes encoding PPARα and the glucocortiocid receptors in the liver in offspring after birth, thereby altering their metabolic phenotype [98,99]. Insulin is produced by the beta cells in the pancreas in response to elevated blood glucose levels. Increased glucocorticoid exposure and malnutrition during fetal development have the potential to permanently reduce the pancreatic beta cell mass and lower pancreatic insulin content, thereby increasing the risk for metabolic disease later in life (reviewed in [100•]).
Genetics, gene–environment interactions, and epigenetics
Although weight and body composition are highly heritable, known genes account for only a modest proportion of their variance [101–103]. Genetic makeup alone cannot explain the rapid increase in obesity prevalence in the population because the genetic characteristics of the human population have not changed in the last three decades, but the prevalence of obesity has tripled during that time [104]. Estimates of maternal transmission of heritability are stronger than those for paternal transmission, which argues in favor of intrauterine effects and/or mitochondrial DNA effects. Moreover, the strongest genetic associations seem to vary as a function of the environment (e.g., effects are seen at specific times but not other times in the life cycle). These observations suggest that gene–environment interactions are particularly relevant in the context of the obesity phenotype. Interestingly, a variant in the gene encoding the glucocorticoid receptor has been associated with increased body fatness in children [105], and we and others have described the association of the same variant with altered physiological stress responses [106]. Potential epigenetic mechanisms are areas of great interest in this context. A detailed review of epigenetics is beyond the scope of the current paper, and we have elaborated on this issue elsewhere [107•,108].
Future directions: implications for research and clinical practice
By incorporating the developmental programming approach into the traditional paradigm of causation of complex common health disorders, the focus shifts to placing a far greater emphasis on the health and well being of young women of reproductive age prior to conception and across gestation, in order to more effectively address health and disease risk-related issues in their offspring from infancy and childhood through adolescence and into adult life. A multilevel approach is required, including molecular and cellular studies, the use of appropriate animal models, and well designed human studies. In the context of human research, opportunities are limited for experimental manipulations of prenatal stress and the intrauterine environment, and for access to many of the target tissues of interest, particularly in fetal life. The value of prospective, longitudinal, follow-up studies, ideally starting before conception, and extending through pregnancy and birth into childhood and beyond, is emphasized. For these studies, deployment of state-of-the art methods, including 3D/4D fetal ultrasonography for quantification of fetal growth (biometry), regional blood flow (uterine, umbilical, cerebral, hepatic and renal [109,110]), volume and growth trajectory of organs (placenta, brain, liver, kidneys, adrenals) [111,112], and body composition (arm, thigh and visceral fat/lean mass [113•, 114]), coupled with reliable assessments in newborns, infants, and children of body composition [with magnetic resonance imaging (MRI) or dual energy X-ray absorptiometry; DXA] and energy expenditure [total energy expenditure using the doubly labeled water method (DLW), and resting metabolic state using the indirect calorimetry approach], will move the field forward in an informed manner. Furthermore, recent advances in imaging techniques will likely enable the developments of protocols in infants and children for subcutaneous and visceral fat quantification (especially intrahepatic fat) [115•], and characterization of and differentiation between white and brown adipose tissue [116••]. These observational studies, in conjunction with parallel molecular studies including studies of human placental, multipotent (stromal) stem cells, and adipose tissue culture systems [117••], and coupled with state-of-the-art statistical modeling techniques for parametric and nonparametric repeated measures, time-series data [118–121], will contribute to further defining technical capabilities in this field.
Regarding clinical implications, it is apparent that current approaches to the prevention and management of obesity and associated metabolic disorders have yielded only very limited success. Once an individual becomes obese, it is difficult to lose weight, and even more difficult to sustain weight loss [73,74••]; systematic studies of the efficacy of current weight loss programs has provide the sobering statistic that approximately 80–90% of obese people who have lost weight regain it within 1 year [122–124]. Clearly, it is critical to adopt a developmental framework in order to arrive at a better understanding of the origins of individual differences in the propensity for weight and fat mass gain, and to develop and test hypotheses that set the stage for translational research to inform the subsequent development of primary intervention strategies before an individual becomes overweight or obese, or secondary interventions to increase the likelihood of a favorable and more sustained response to weight-loss strategies.
Conclusion
Based on the conceptual framework and empirical findings presented here, we suggest that prenatal stress exposure may represent an important consideration in arriving at a better understanding of developmental programming of health and disease susceptibility. Moreover, we submit that stress-related maternal–placental–fetal endocrine and immune processes in human gestation represent a potentially attractive underlying candidate mechanism for elucidating the common biological basis (pathway) for mediating not only the long-term effects of prenatal stress but also those of a host of other intrauterine perturbations that have been implicated in this area.
Acknowledgments
Preparation of this manuscript was supported, in part, by US PHS (NIH) grants RO1 HD-06028 and PO1 HD-047609 to PDW and RO1 HD-065825 to SE.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 569).
- 1.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 2.Glynn LM, Wadhwa PD, Dunkel-Schetter C, et al. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 3.Feldman PJ, Dunkel-Schetter C, Sandman CA, Wadhwa PD. Maternal social support predicts birth weight and fetal growth in human pregnancy. Psychosom Med. 2000;62:715–725. doi: 10.1097/00006842-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18:333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 5.Wadhwa PD, Sandman CA, Porto M, et al. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 6.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, et al. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996;58:432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Wadhwa PD, Sandman CA, Chicz-DeMet A, Porto M. Placental CRH modulates maternal pituitary adrenal function in human pregnancy. Ann N Y Acad Sci. 1997;814:276–281. doi: 10.1111/j.1749-6632.1997.tb46163.x. [DOI] [PubMed] [Google Scholar]
- 8.Wadhwa PD, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 9.Wadhwa PD, Porto M, Garite TJ, et al. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079–1085. doi: 10.1016/s0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 10.Behrman RE, Stith ButlerA, editors. Committee on understanding premature birth and assuring healthy outcomes. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 11•.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98. doi: 10.1159/000273066. A review of mechanisms underlying nutritional programming of obesity and metabolic function. [DOI] [PubMed] [Google Scholar]
- 12.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Tataranni PA, Larson DE, Snitker S, et al. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271(2 Pt 1):E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 14.George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated Cortisol release and food intake in healthy, nonobese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Bono E, Rohleder N, Hellhammer DH, et al. Glucose but not protein or fat load amplifies the Cortisol response to psychosocial stress. Horm Behav. 2002;41:328–333. doi: 10.1006/hbeh.2002.1766. [DOI] [PubMed] [Google Scholar]
- 16.Hitze B, Hubold C, van Dyken R, et al. How the selfish brain organizes its supply and demand. Front Neuroenergetics. 2010;2:7. doi: 10.3389/fnene.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced Cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 18.Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61(5 Pt 1):520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- 19.Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Dev Psychobiol. 2002;41:178–185. doi: 10.1002/dev.10063. [DOI] [PubMed] [Google Scholar]
- 21.Coe CL, Lubach GR, Karaszewski JW. Prenatal stress and immune recognition of self and nonself in the primate neonate. Biol Neonate. 1999;76:301–310. doi: 10.1159/000014172. [DOI] [PubMed] [Google Scholar]
- 22.Bowman RE, MacLusky NJ, Sarmiento Y, et al. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 23.Entringer S, Wust S, Kumsta R et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;199:498.e1–498.e7. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Entringer S, Kumsta R, Nelson EL, et al. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev Psychobiol. 2008;50:579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 26.Entringer S, Buss C, Kumsta R, et al. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behav Neurosci. 2009;123:886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buss C, Davis EP, Muftuler LT, et al. High pregnancy anxiety during midgestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35:141–153. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Olsen J, Vestergaard M, et al. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS One. 2010;5:e11896. doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126:e401–e409. doi: 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- 30.Merrill JE. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Dev Neurosci. 1992;14:1–10. doi: 10.1159/000111642. [DOI] [PubMed] [Google Scholar]
- 31.Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47:291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Zhao B, Schwartz JP. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J Neurosci Res. 1998;52:7–16. doi: 10.1002/(SICI)1097-4547(19980401)52:1<7::AID-JNR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Trejo JL, Cuchillo I, Machin C, Rua C. Maternal adrenalectomy at the early onset of gestation impairs the postnatal development of the rat hippocampal formation: effects on cell numbers and differentiation, connectivity and calbindin-D28k immunoreactivity. J Neurosci Res. 2000;62:644–667. doi: 10.1002/1097-4547(20001201)62:5<644::AID-JNR4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Garbrecht MR, Klein JM, Schmidt TJ, Snyder JM. Glucocorticoid metabolism in the human fetal lung: implications for lung development and the pulmonary surfactant system. Biol Neonate. 2006;89:109–119. doi: 10.1159/000088653. [DOI] [PubMed] [Google Scholar]
- 35.Cole TJ, Blendy JA, Monaghan AP, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 36.Yen SC. Endocrinology of pregnancy. In: Creasy RK, Resnick R, editors. Maternal–fetal medicine: principles and practice. Philadelphia, PA: WB Saunders; 1994. [Google Scholar]
- 37.Mastorakos G, Ilias I. Maternal and fetal hypothalamic–pituitary–adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 38••.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.06.007. [Epub ahead of print] An important, comprehensive review of animal and human studies on glucocorticoid programming, with an emphasis on molecular mechanisms and effects on the brain. [DOI] [PubMed] [Google Scholar]
- 39.Cheng YH, Nicholson RC, King B, et al. Corticotropin-releasing hormone gene expression in primary placental cells is modulated by cyclic adenosine 3′,5′-monophosphate. J Clin Endocrinol Metab. 2000;85:1239–1244. doi: 10.1210/jcem.85.3.6420. [DOI] [PubMed] [Google Scholar]
- 40.Lowry PJ. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found Symp. 1993;172:108–115. discussion 15-28. [PubMed] [Google Scholar]
- 41•.Weetman AP. Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol. 2010;6:311–318. doi: 10.1038/nrendo.2010.46. A review of major pregnancy-induced changes in immune function. [DOI] [PubMed] [Google Scholar]
- 42.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 43.Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 44•.Entringer S, Buss C, Shirtcliff EA, et al. Attenuation of maternal psychophysiological stress responses and the maternal Cortisol awakening response over the course of human pregnancy. Stress. 2010;13:258–268. doi: 10.3109/10253890903349501. The first report to show a progressive attenuation of maternal psychophysiological stress responses over the course of human gestation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buss C, Entringer S, Reyes JF, et al. The maternal Cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009;201:398.e1–398.e8. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 46.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 47.Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christian LM, Franco A, Iams J, et al. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain Behav Immun. 2010;24:49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petraglia F, Calza L, Garuti GC, et al. New aspects of placental endocrinology. J Endocrinol Invest. 1990;13:353–371. doi: 10.1007/BF03349579. [DOI] [PubMed] [Google Scholar]
- 50.Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987;328:717–719. doi: 10.1038/328717a0. [DOI] [PubMed] [Google Scholar]
- 51.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160:247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 52.Goland RS, Conwell IM, Warren WB, Wardlaw SL. Placental corticotropin-releasing hormone and pituitary–adrenal function during pregnancy. Neuroendocrinology. 1992;56:742–749. doi: 10.1159/000126302. [DOI] [PubMed] [Google Scholar]
- 53.Chan EC, Smith R, Lewin T, et al. Plasma corticotropin-releasing hormone, beta-endorphin and Cortisol inter-relationships during human pregnancy. Acta Endocrinol (Copenhagen) 1993;128:339–344. doi: 10.1530/acta.0.1280339. [DOI] [PubMed] [Google Scholar]
- 54.Florio P, Zatelli MC, Reis FM, et al. Corticotropin releasing hormone: a diagnostic marker for behavioral and reproductive disorders? Front Biosci. 2007;12:551–560. doi: 10.2741/2081. [DOI] [PubMed] [Google Scholar]
- 55.Petraglia F, Aguzzoli L, Florio P, et al. Maternal plasma and placental immunoreactive corticotrophin-releasing factor concentrations in infection-associated term and preterm delivery. Placenta. 1995;16:157–164. doi: 10.1016/0143-4004(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 56.Stallmach T, Hebisch G, Joller H, et al. Expression pattern of cytokines in the different compartments of the feto-maternal unit under various conditions. Reprod Fertil Dev. 1995;7:1573–1580. doi: 10.1071/rd9951573. [DOI] [PubMed] [Google Scholar]
- 57.Harville EW, Savitz DA, Dole N, et al. Stress and placental resistance measured by Doppler ultrasound in early and mid-pregnancy. Ultrasound Obstet Gynecol. 2008;32:23–30. doi: 10.1002/uog.5344. [DOI] [PubMed] [Google Scholar]
- 58.LaMarca BD, Ryan MJ, Gilbert JS, et al. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. 2007;9:480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 59.Varvarigou AA, Petsali M, Vassilakos P, Beratis NG. Increased Cortisol concentrations in the cord blood of newborns whose mothers smoked during pregnancy. J Perinat Med. 2006;34:466–470. doi: 10.1515/JPM.2006.091. [DOI] [PubMed] [Google Scholar]
- 60.Shen Q, Li ZQ, Sun Y, et al. The role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophrenia. Schizophr Res. 2008;99:48–55. doi: 10.1016/j.schres.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Paul K, Boutain D, Agnew K, et al. The relationship between racial identity, income, stress and C-reactive protein among parous women: implications for preterm birth disparity research. J Natl Med Assoc. 2008;100:540–546. doi: 10.1016/s0027-9684(15)31300-6. [DOI] [PubMed] [Google Scholar]
- 62.Ford SP, Zhang L, Zhu M, et al. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009;297:R835–R843. doi: 10.1152/ajpregu.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bloomfield FH, Oliver MH, Hawkins P, et al. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic–pituitary–adrenal axis in late gestation. Endocrinology. 2004;145:4278–4285. doi: 10.1210/en.2004-0424. [DOI] [PubMed] [Google Scholar]
- 64.Chadio SE, Kotsampasi B, Papadomichelakis G, et al. Impact of maternal undernutrition on the hypothalamic–pituitary–adrenal axis responsiveness in sheep at different ages postnatal. J Endocrinol. 2007;192:495–503. doi: 10.1677/JOE-06-0172. [DOI] [PubMed] [Google Scholar]
- 65.Dwyer CM, Stickland NC. The effects of maternal undernutrition on maternal and fetal serum insulin-like growth factors, thyroid hormones and Cortisol in the guinea pig. J Dev Physiol. 1992;18:303–313. [PubMed] [Google Scholar]
- 66.Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999;846:236–242. doi: 10.1016/s0006-8993(99)02058-2. [DOI] [PubMed] [Google Scholar]
- 67.Bispham J, Gopalakrishnan GS, Dandrea J, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and Cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- 68.Florio P, Imperatore A, Sanseverino F, et al. The measurement of maternal plasma corticotropin-releasing factor (CRF) and CRF-binding protein improves the early prediction of preeclampsia. J Clin Endocrinol Metab. 2004;89:4673–4677. doi: 10.1210/jc.2004-0186. [DOI] [PubMed] [Google Scholar]
- 69.Ng EK, Leung TN, Tsui NB, et al. The concentration of circulating corticotropin-releasing hormone mRNA in maternal plasma is increased in preeclampsia. Clin Chem. 2003;49:727–731. doi: 10.1373/49.5.727. [DOI] [PubMed] [Google Scholar]
- 70.Florio P, Romero R, Chaiworapongsa T, et al. Amniotic fluid and umbilical cord plasma corticotropin-releasing factor (CRF), CRF-binding protein, adrenocorticotropin, and Cortisol concentrations in intraamniotic infection and inflammation at term. J Clin Endocrinol Metab. 2008;93:3604–3609. doi: 10.1210/jc.2007-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halfon N, Newacheck PW. Evolving notions of childhood chronic illness. JAMA. 2010;303:665–666. doi: 10.1001/jama.2010.130. [DOI] [PubMed] [Google Scholar]
- 72.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303:623–630. doi: 10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- 73.Butte NF, Christiansen E, Sorensen TIA. Energy imbalance underlying the development of childhood obesity. Obesity. 2007;15:3056–3066. doi: 10.1038/oby.2007.364. [DOI] [PubMed] [Google Scholar]
- 74••.Muhlhausler B, Smith SR. Early-life origins of metabolic dysfunction: role of the adipocyte. Trends Endocrinol Metab. 2009;20:51–57. doi: 10.1016/j.tem.2008.10.006. A comprehensive review of early life factors influencing adipocyte development. [DOI] [PubMed] [Google Scholar]
- 75.Kensara OA, Wootton SA, Phillips DI, et al. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82:980–987. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 76.Ibanez L, Suarez L, Lopez-Bermejo A, et al. Early development of visceral fat excess after spontaneous catch-up growth in children with low birth weight. J Clin Endocrinol Metab. 2008;93:925–928. doi: 10.1210/jc.2007-1618. [DOI] [PubMed] [Google Scholar]
- 77.Ibanez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91:2153–2158. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- 78.Gillman MW, Rich-Edwards JW, Huh S, et al. Maternal corticotropin-releasing hormone levels during pregnancy and offspring adiposity. Obesity (Silver Spring) 2006;14:1647–1653. doi: 10.1038/oby.2006.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79••.Fasting MH, Oken E, Mantzoros CS, et al. Maternal levels of corticotropin-releasing hormone during pregnancy in relation to adiponectin and leptin in early childhood. J Clin Endocrinol Metab. 2009;94:1409–1415. doi: 10.1210/jc.2008-1424. The first paper reporting an association between CRH levels in pregnancy and alterations in adiponectin and leptin levels in 3-year-old children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Radaelli T, Uvena-Celebrezze J, Minium J, et al. Maternal interleukin-6: marker of fetal growth and adiposity. J Soc Gynecol Investig. 2006;13:53–57. doi: 10.1016/j.jsgi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Devaskar SU. Neurohumoral regulation of body weight gain. Pediatr Diabetes. 2001;2:131–144. doi: 10.1034/j.1399-5448.2001.002003131.x. [DOI] [PubMed] [Google Scholar]
- 82.Mietus-Snyder ML, Lustig RH. Childhood obesity: adrift in the ‘limbic triangle’. Annu Rev Med. 2008;59:147–162. doi: 10.1146/annurev.med.59.103106.105628. [DOI] [PubMed] [Google Scholar]
- 83••.Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 1):S31–S38. doi: 10.1097/MPG.0b013e3181977375. An important, comprehensive review of the actions of perinatal hormones and nutrition in programming the development and organization of hypothalamic circuits that regulate body weight and energy balance. [DOI] [PubMed] [Google Scholar]
- 84.Richard D, Huang Q, Timofeeva E. The corticotropin-releasing hormone system in the regulation of energy balance in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S36–S39. doi: 10.1038/sj.ijo.0801275. [DOI] [PubMed] [Google Scholar]
- 85.Leal-Cerro A, Soto A, Martinez MA, et al. Influence of Cortisol status on leptin secretion. Pituitary. 2001;4:111–116. doi: 10.1023/a:1012903330944. [DOI] [PubMed] [Google Scholar]
- 86.Martin RJ, Hausman GJ, Hausman DB. Regulation of adipose cell development in utero. Proc Soc Exp Biol Med. 1998;219:200–210. doi: 10.3181/00379727-219-44333. [DOI] [PubMed] [Google Scholar]
- 87.Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- 88.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 89.Grammatopoulos D. The family of corticotropin-releasing hormone (CRH) peptides: important regulators of adipocyte function. Endocr Abstracts. 2008;16:S19.1. [Google Scholar]
- 90.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 91.Muhlhausler BS, Adam CL, Findlay PA, et al. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 2006;20:1257–1259. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- 92.Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-gamma, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology. 2007;148:878–885. doi: 10.1210/en.2006-1115. [DOI] [PubMed] [Google Scholar]
- 93.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 94.Padoan A, Rigano S, Ferrazzi E, et al. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am J Obstet Gynecol. 2004;191:1459–1464. doi: 10.1016/j.ajog.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 95.Crescenzo R, Samec S, Antic V, et al. A role for suppressed thermogenesis favoring catch-up fat in the pathophysiology of catch-up growth. Diabetes. 2003;52:1090–1097. doi: 10.2337/diabetes.52.5.1090. [DOI] [PubMed] [Google Scholar]
- 96.Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 2000;85:1401–1406. doi: 10.1210/jcem.85.4.6544. [DOI] [PubMed] [Google Scholar]
- 97.Nyirenda MJ, Lindsay RS, Kenyon CJ, et al. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lillycrop KA, Phillips ES, Torrens C, et al. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr. 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lillycrop KA, Slater-Jefferies JL, Hanson MA, et al. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100•.Schwitzgebel VM, Somm E, Klee P. Modeling intrauterine growth retardation in rodents: impact on pancreas development and glucose homeostasis. Mol Cell Endocrinol. 2009;304:78–83. doi: 10.1016/j.mce.2009.02.019. A review of animal models invetigating the effects of an adverse intrauterine environment on fetal and postnatal pancreatic islet development. [DOI] [PubMed] [Google Scholar]
- 101.Speiser PW, Rudolf MC, Anhalt H, et al. Childhood obesity. J Clin Endocrinol Metab. 2005;90:1871–1887. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- 102.O'Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905–2910. doi: 10.2337/db08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clement K, Ferre P. Genetics and the pathophysiology of obesity. Pediatr Res. 2003;53:721–725. doi: 10.1203/01.PDR.0000059753.61905.58. [DOI] [PubMed] [Google Scholar]
- 104.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 105.Voorheuve PG, van den Akker EL, van Rossum EF, et al. Glucocorticoid receptor gene variant is associated with increased body fatness in youngsters. Clin Endocrinol. 2009;71:518–523. doi: 10.1111/j.1365-2265.2009.03538.x. [DOI] [PubMed] [Google Scholar]
- 106.Kumsta R, Entringer S, Koper JW, et al. Sex specific associations between common glucocorticoid receptor gene variants and hypothalamus–pituitary–adrenal axis responses to psychosocial stress. Biol Psychiatry. 2007;62:863–869. doi: 10.1016/j.biopsych.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 107•.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. A review of effects of overnutrition and stress during pregnancy on outcomes in childhood and adulthood, and potential epigenetic mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009;27:391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flo K, Wilsgaard T, Acharya G. Relation between utero-placental and fetoplacental circulations: a longitudinal study. Acta Obstet Gynecol Scand. 2010;89:1270–1275. doi: 10.3109/00016349.2010.512069. [DOI] [PubMed] [Google Scholar]
- 110.Ebbing C, Rasmussen S, Godfrey KM, et al. Fetal superior mesenteric artery: longitudinal reference ranges and evidence of regulatory link to portal liver circulation. Early Hum Dev. 2009;85:207–213. doi: 10.1016/j.earlhumdev.2008.09.412. [DOI] [PubMed] [Google Scholar]
- 111.Chang CH, Yu CH, Chang FM, et al. Assessment of fetal adrenal gland volume using three-dimensional ultrasound. Ultrasound Med Biol. 2002;28:1383–1387. doi: 10.1016/s0301-5629(02)00650-6. [DOI] [PubMed] [Google Scholar]
- 112.Chang CH, Yu CH, Ko HC, et al. Predicting fetal growth restriction with liver volume by three-dimensional ultrasound: efficacy evaluation. Ultrasound Med Biol. 2006;32:13–17. doi: 10.1016/j.ultrasmedbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 113•.Lee W, Balasubramaniam M, Deter RL, et al. Fetal growth parameters and birth weight: their relationship to neonatal body composition. Ultrasound Obstet Gynecol. 2009;33:441–446. doi: 10.1002/uog.6317. An important empircal paper investigating the relationship between prenatal sonographic parameters and birth weight in predicting neonatal body composition in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee W, Deter RL, McNie B, et al. Individualized growth assessment of fetal soft tissue using fractional thigh volume. Ultrasound Obstet Gynecol. 2004;24:766–774. doi: 10.1002/uog.1779. [DOI] [PubMed] [Google Scholar]
- 115•.Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring) 2010;18:841–847. doi: 10.1038/oby.2009.352. An empirical paper validating an MRI protocol to image fatty infiltration in organs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116••.Hu HH, Smith DL, Jr, Nayak KS, et al. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging. 2010;31:1195–1202. doi: 10.1002/jmri.22162. An important method development paper to noninvasively characterize brown adipose tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117••.Kirchner S, Kieu T, Chow C, et al. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24:526–539. doi: 10.1210/me.2009-0261. A very important empirical paper showing an effect of prental exposure to environmental toxins on sensitizing multipotent stromal stem cells to differentiate into adipocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 2004;99:673–686. [Google Scholar]
- 119.Hedeker D, Gibbons R. In: Longitudinal data analysis. Balding DJ, Cressie NAC, Fisher NI, et al., editors. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 120.Raudenbush SW, Liu X. Effect of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychol Methods. 2001;6:387–401. [PubMed] [Google Scholar]
- 121.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurence. New York: Oxford University Press; 2003. [Google Scholar]
- 122.Astrup A, Buemann B, Toubro S, et al. Low resting metabolic rate in subjects predisposed to obesity: a role for thyroid status. Am J Clin Nutr. 1996;63:879–883. doi: 10.1093/ajcn/63.6.879. [DOI] [PubMed] [Google Scholar]
- 123.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 124.Kaplan RM. Should medicare reimburse providers for weight loss interventions? Am Psychol. 2007;62:217–219. doi: 10.1037/0003-066X.62.3.217. [DOI] [PubMed] [Google Scholar]