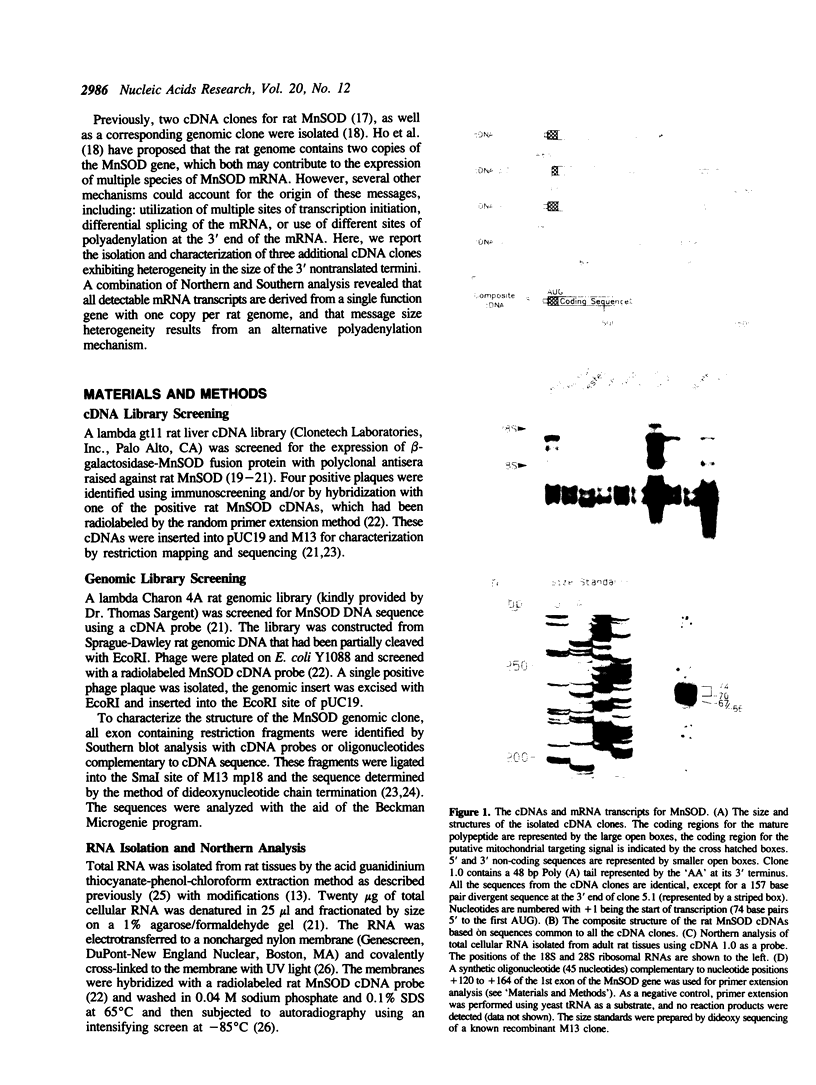
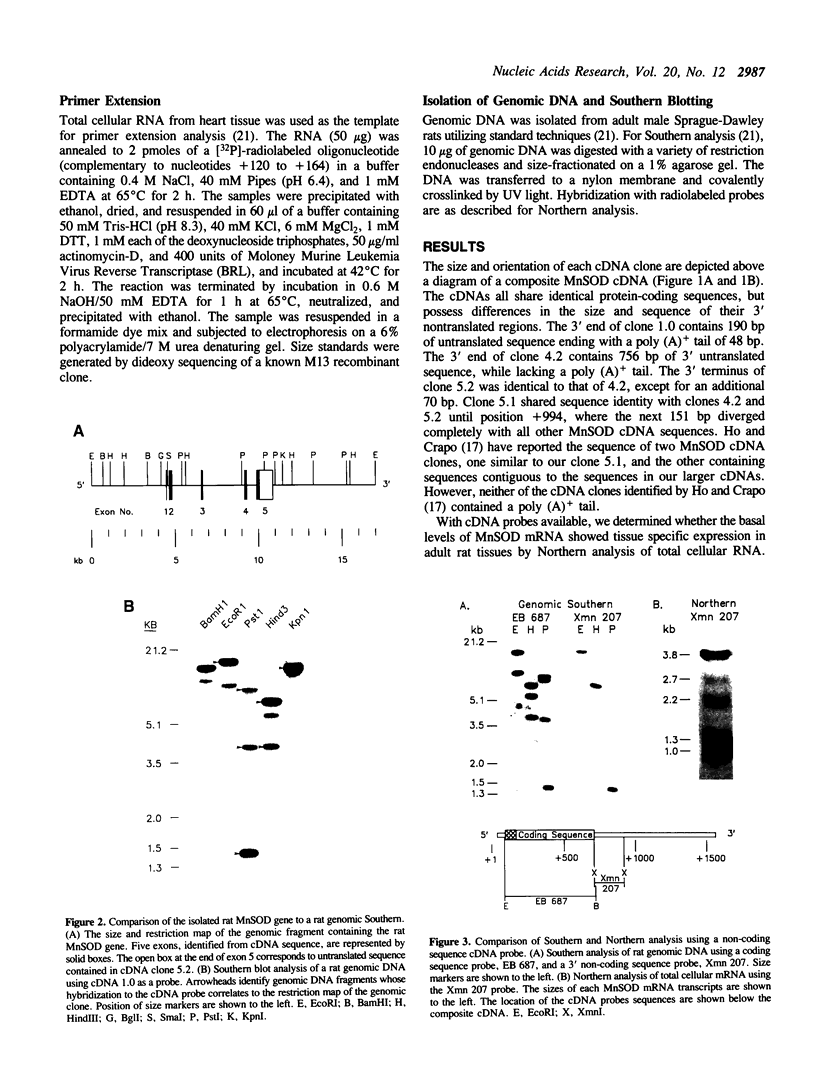
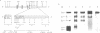Abstract
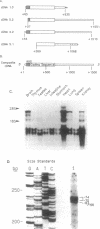
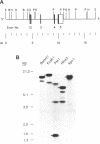
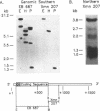
The mitochondrial enzyme, manganese superoxide dismutase (MnSOD) is an integral component of the cell's defense against superoxide-mediated cellular damage. We have isolated and characterized four cDNA clones and the structural gene for rat MnSOD. Northern analyses using MnSOD cDNA probes detected at least five mRNAs in all tissues and cell types examined. Southern and Northern analysis using a 3' non-coding sequence probe, common to all the cDNAs, showed hybridization only to genomic restriction fragments that correspond to our genomic clone and the five MnSOD mRNAs. These data demonstrate that all of the rat MnSOD transcripts are derived from a single functional gene. Primer extension data indicate that transcription initiation is clustered within a few bases. Northern analysis using intron probes demonstrates that all five transcripts are fully processed. Northern analysis using cDNA and genomic probes from sequences progressively 3' to the end of the coding sequence indicates that size heterogeneity in the MnSOD transcripts results from variations in the length of the 3' non-coding sequence. From this data and the location of potential polyadenylation signals near the expected sites of transcript termination, we conclude that the existence of multiple MnSOD mRNA species originate as the result of alternate polyadenylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asayama K., Burr I. M. Rat superoxide dismutases. Purification, labeling, immunoassay, and tissue concentration. J Biol Chem. 1985 Feb 25;260(4):2212–2217. [PubMed] [Google Scholar]
- Asayama K., Janco R. L., Burr I. M. Selective induction of manganous superoxide dismutase in human monocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C393–C397. doi: 10.1152/ajpcell.1985.249.5.C393. [DOI] [PubMed] [Google Scholar]
- Asayama K., Sharp R. A., Burr I. M. Purification and radioimmunoassays for superoxide dismutases in the mouse: tissue concentrations in different strains. Int J Biochem. 1985;17(11):1171–1178. doi: 10.1016/0020-711x(85)90006-0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church S. L. Manganese superoxide dismutase: nucleotide and deduced amino acid sequence of a cDNA encoding a new human transcript. Biochim Biophys Acta. 1990 Oct 23;1087(2):250–252. doi: 10.1016/0167-4781(90)90213-l. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler J. E., Van Wyk J. J., Lund P. K. Different half-lives of insulin-like growth factor I mRNAs that differ in length of 3' untranslated sequence. Endocrinology. 1990 Sep;127(3):1550–1552. doi: 10.1210/endo-127-3-1550. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. Nucleotide sequences of cDNAs coding for rat manganese-containing superoxide dismutase. Nucleic Acids Res. 1987 Dec 10;15(23):10070–10070. doi: 10.1093/nar/15.23.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S., Howard A. J., Crapo J. D. Molecular structure of a functional rat gene for manganese-containing superoxide dismutase. Am J Respir Cell Mol Biol. 1991 Mar;4(3):278–286. doi: 10.1165/ajrcmb/4.3.278. [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Manley J. L. Polyadenylation of mRNA precursors. Biochim Biophys Acta. 1988 May 6;950(1):1–12. doi: 10.1016/0167-4781(88)90067-x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiki Y., Meyrick B. O., Brigham K. L., Burr I. M. Endotoxin increases superoxide dismutase in cultured bovine pulmonary endothelial cells. Am J Physiol. 1987 Apr;252(4 Pt 1):C436–C440. doi: 10.1152/ajpcell.1987.252.4.C436. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Freeman B. A., Crapo J. D. Intracellular localization of the copper-zinc and manganese superoxide dismutases in rat liver parenchymal cells. Lab Invest. 1986 Sep;55(3):363–371. [PubMed] [Google Scholar]
- Visner G. A., Block E. R., Burr I. M., Nick H. S. Regulation of manganese superoxide dismutase in porcine pulmonary artery endothelial cells. Am J Physiol. 1991 Jun;260(6 Pt 1):L444–L449. doi: 10.1152/ajplung.1991.260.6.L444. [DOI] [PubMed] [Google Scholar]
- Visner G. A., Dougall W. C., Wilson J. M., Burr I. A., Nick H. S. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. J Biol Chem. 1990 Feb 15;265(5):2856–2864. [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de los Santos R., Seidenfeld J. J., Anzueto A., Collins J. F., Coalson J. J., Johanson W. G., Jr, Peters J. I. One hundred percent oxygen lung injury in adult baboons. Am Rev Respir Dis. 1987 Sep;136(3):657–661. doi: 10.1164/ajrccm/136.3.657. [DOI] [PubMed] [Google Scholar]