Abstract
Linked recognition of antigen by B and T lymphocytes is ensured in part by a state of tolerance acquired by CD4 T cells to germline-encoded sequences within the B cell antigen receptor (BCR). We sought to determine how such tolerance is attained when a peptide from the BCR variable (V) region is expressed by small numbers of B cells as it is in the physiological state. Mixed bone marrow (BM) chimeras were generated using donor BM from mice with B cells that expressed a transgene (Tg)-encoded kappa light chain and BM from T cell receptor (TCR) Tg mice in which the CD4 T cells (CA30) were specific for a Vκ peptide encoded by the kappa transgene (κTg). In chimeras where few B cells express the κTg, many CA30 cells were deleted in the thymus. However, a substantial fraction survived to the CD4 single-positive stage. Among single-positive CA30 thymocytes, few reached maturity and migrated to the periphery. Maturation was strongly associated with, and likely promoted by, expression of an endogenous TCR alpha chain. CD4+ CA30 cells that reached peripheral lymphoid tissues were antigen-experienced and anergic, and some developed into regulatory cells. These findings reveal several checkpoints and mechanisms that enforce a state of self-tolerance in developing T cells specific for BCR V region sequences, thus ensuring that T cell help to B cells occurs through linked recognition of foreign antigen.
Introduction
The generation of high-avidity antibody responses requires linked recognition of antigen by specific B cells and CD4 T follicular helper (TFH) cells in the context of a germinal center (GC) reaction. Within the GC, B cells mutate genes encoding the BCR V region in a process that ultimately results in the maturation of antibody affinity and fine specificity (1–4). A requirement for antigen-specific T cell help to B cells during the GC reaction is thought to be an important regulatory checkpoint, ensuring that only B cells with high-avidity BCR for foreign antigens receive appropriate signals from TFH cells that promote B cell growth and differentiation.
A potential caveat in this scenario is that, along with foreign antigen, peptides from the BCR are also processed and presented on the B cell surface in MHC II (5–12). CD4 T cells with specificity for V region peptides derived from the BCR could potentially provide an avenue of help to the B cell, in violation of the principle of linked antigen recognition (13). Use of this pathway is plausible due to the enormous sequence diversity within the repertoire of V regions expressed by B cells. Some of this diversity is germline-encoded, and some is generated by somatic recombination during lymphopoiesis in the bone marrow (BM) and by somatic hypermutation in the periphery. Antigen-unlinked help to the B cell, directed by BCR peptides, is potentially dangerous, as underscored in transgene models where such help results in autoantibody development and manifestations of systemic autoimmune disease (14, 15).
Prior studies have demonstrated that CD4 T cells attain a state of tolerance to germline-encoded antibody diversity. This was shown by immunizing mice with unmutated monoclonal antibodies (mAb) and sampling T cell hybridomas for responses to the mAb V region peptides in the context of MHC II (16, 17). Additional studies using transgene models revealed that this special case of self-tolerance among CD4 T cells takes place by central deletion within the thymus. However, these studies were performed in mice with nearly monoclonal populations of B and T cells and with high concentrations of serum mAb bearing antigenic V region peptides (14, 18, 19). In these “monoclonal” models, even maternally transmitted mAb resulted in thymic deletion of CD4 T cells specific for peptides from the mAb (14, 20). Complementary experiments demonstrated that large quantities of injected IgG could similarly induce thymic deletion in CD4 T cells reactive to a V region peptide (18).
While it is clear that CD4 T cells in wildtype, nontransgenic mice are rendered tolerant to germline-encoded peptides derived from immunoglobulin (Ig) V regions, and that T cells specific for such peptides are deleted in the thymus of Ig transgenic mice, the mechanism(s) of tolerance to BCR and Ig V regions present at physiological levels are unknown. To gain insight into this problem, we generated mixed BM chimeras in which Vκ peptide-specific T cells developed in the presence of physiological numbers of B cells expressing the cognate kappa V region. Our experiments reveal multiple checkpoints in tolerance culminating in the development of rare Vκ-specific regulatory T cells (Treg) in the periphery.
Material and Methods
Mice
A complementary pair of mice expressing either a complete Igκ Tg containing a Vκ36–71 exon (κTg mouse), or a Tg encoding an αβTCR (Vα1/Vβ8) specific for a peptide from Vκ36–71 in the context of I-Ak (CA30 mouse) has been described (14). These transgenes are carried by mice with an A/J genetic background through more than 25 backcross generations. Large populations of lymphocytes expressing the respective transgenes are present in a resting state, as assessed in the CA30 mouse by low frequencies of T cells expressing activation markers. In the κTg mouse, this resting state is evidenced by large numbers of high-density B cells (ρ > 1.079) (5). B6.PL-Thy1<a>/CyJ were purchased from The Jackson Laboratory (Bar Harbor, ME). BM from (A/J. κTg × C57BL/6)F1 and (A/J.CA30 × B6.PL-Thy1<a>/CyJ)F1 mice were used to create mixed BM chimeras in this study. In some experiments, transgenic mice expressing the F508αβTCR on a B6.PL-Thy1<a>/CyJ background were crossed with the A/J.CA30 mice to produce an F1 mouse expressing two T cell receptors (2TCR). F508 T cells (Vα2/Vβ14) are specific for the 3K peptide presented by I-Ab (21). All experiments were conducted in accordance with an IACUC-approved protocol.
Mixed Bone Marrow Chimeras
C57BL6 × A/J F1 (B6AF1) recipient mice (Jackson Laboratories) were lethally irradiated [1300 Rads in 2 doses (800R + 500R), 3 hours apart] and reconstituted i.v. with a total of 2 × 106 BM cells, previously depleted of mature leukocytes with the EasySep BM progenitor enrichment kit (STEMCELL Technologies, Vancouver, BC). Various ratios of BM cells from CA30 F1 and κTg F1 or control B6AF1 mice were used as described in figures and legends. Chimeras were maintained under specific pathogen-free conditions in microisolator cages on a food source fortified with antibiotics (SEPTRA, Harlan Laboratories, Denver, CO) until sacrificed at 12–16 weeks for various analyses of tolerance.
Flow Cytometry
Lymph nodes and spleen cells were pooled, and cells were incubated for 30 minutes on ice with a cocktail of labeled antibodies in staining buffer (PBS + 2% FCS + 0.01% NaN3). The following labeled mAb were purchased from Biolegend (San Diego, CA): anti-B220 (clone RA3-B2), anti-PD-1(clone RMP1–30), anti-CD44 (clone IM7), anti-CD8 (clone 53–6.7), anti-Thy1.1 (OX-7) and anti-CD4 (RM4–5). Anti-TCR Vβ8 (clone KJ16) and anti-Foxp3 (clone FJK-16) were purchased from eBiosciences (San Diego, CA). The anti-TCR Vα2 (clone B20.1), anti-CD5 (clone 53–7.3) and anti-CD24 (M1/69) mAb were purchased from BD Biosciences (San Jose, CA). Intracellular staining for Foxp3 was performed according to the manufacturer’s (eBiosciences) recommendation. The Vκ36–71 FR1-I-Ak tetramer used to identify the CA30 TCR has been described (14). A mAb specific for the Vκ of mAb36–71 (mAb17–63) was produced in-house and was used to identify κTg B cells and corresponding serum Igκ. Flow cytometry was performed on a BD™ LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar, Ashland, OR).
Quantifying serum Ig
To quantify serum IgG and IgM, 96-well trays were coated overnight at 4° C with 1 μg/ml of heavy chain-specific goat anti-mouse IgG or IgM (Sigma, St Louis, MO). Trays were incubated with blocking buffer for 1–2 hours at 37°C. Serial dilutions of mouse sera (starting at 1:1000) were incubated for 1 h at 37°C. Plate-bound Ig was detected with biotinylated goat anti-mouse Igκ (Southern Biotechnology, Birmingham, AL) followed by europium-labeled streptavidin (Wallac, Turku, Finland). Europium fluorescence at 615 nm was measured on a Wallac Victor2 1420 multilabel counter using an excitation wavelength of 340 nm as described (22). Standard curves for quantification were generated with known quantities of IgG and IgM mAb.
A competition immunoassay was used to quantify serum Ig containing the transgenic Vκ36–71 light chain. 96-well trays were coated overnight at 4° C with a mixture of mAb17–63 (1.66 μg/ml) and normal bovine gamma globulin (3.33 μg/ml, Sigma). Trays were blocked as above and incubated with serial dilutions of sera (starting at 1:25) mixed with biotinylated mAb36–71 mAb (25 ng/well). Biotinylated antibodies were detected by europium-labeled streptavidin. A standard curve for quantification was generated with sera of κTg mice containing known quantities of IgM and IgG. Statistical analyses were performed with GraphPad Prism (GraphPad Software, San Diego, CA).
T cell Proliferation Assays
CD4+ T cells were purified from splenocytes, lymph node cells or thymocytes by negative selection using the CD4+ T cell enrichment kit EasySep (STEMCELL Technologies). 2 × 105 CFSE-labeled [5-(and-6)-carboxyfluorescein diacetate succinimidyl ester, Invitrogen, Carlsbad, CA] T cells were incubated in 0.250 ml with 105 irradiated (1100 Rads) B6AF1 splenocytes with or without the Vκ36–71 FR1 peptide (DIQMTQIPSSLSA, 1μM) (23). Triplicate cell cultures were harvested at day 6–7 and analyzed by flow cytometry. In some experiments, exogenous recombinant (r) IL-2 (Biolegend) was provided to the cell cultures at 50 U/ml. The suppressive function of peripheral CA30 T cells from CA30/κTg chimeras was also assessed in proliferation assays. Briefly purified CA30 CD4+ naïve T cells were labeled with CFSE. 5×103 of these cells were incubated with CD4+ CD25+ Thy1.1+ cells at various ratios in 96 well culture trays. CD4+ cells from B6AF1 were used as filler cells to achieve a total of 105 CD4+ T cells per 250 μl culture. Proliferation assays were performed in the presence of 105 irradiated nonTg splenocytes in the presence or not of Vκ36–71 peptide (1 μM). Cultures were harvested at days 5–7 and analyzed by flow cytometry.
Results
Analyzing CD4 T cell tolerance to germline BCR V regions in mixed BM chimeras
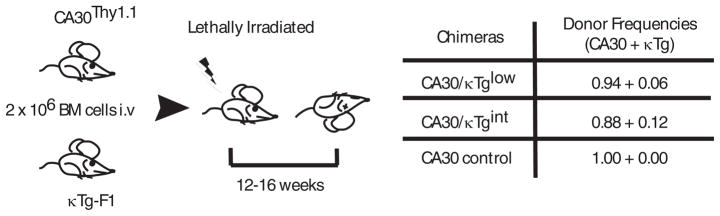
To analyze mechanisms of CD4 T cell tolerance to germline-encoded BCR V regions, we developed a mixed BM chimera model using a complementary pair of donor Tg mice. The κTg animal expresses a complete Igκ transgene with a Vκ-Jκ exon derived from the 36–71 hybridoma (24). The first 13 amino acids of framework 1 in this Vκ comprise an immunogenic MHC II epitope. B cells of this line exhibit good isotypic exclusion, reside in the context of normal lymphoid architecture, and contain a substantial fraction that are in a resting state, as assessed by a variety of criteria (5). CA30 Tg mice carry αβTCR transgenes that encode a TCR reactive to the Vκ epitope in the context of I-Ak. CD4 T cells in this mouse are present in normal numbers and largely antigen inexperienced (14). Both lines were on an A/J genetic background. To produce F1 donors for the chimera studies, A/J κTg mice were crossed with B6 mice, and A/J CA30 mice were crossed with B6 Thy1.1 mice to permit tracking of CA30 T cells. For tolerance studies, B6AF1 recipients were reconstituted with donor BM at low CA30/κTg ratios to generate chimeras in which frequencies of κTg B cells were approximately those expected for B cells expressing any given kappa variable region in a wildtype mouse (Figure 1).
Figure 1. Generation of mixed bone marrow chimeras (BMC).
Lethally irradiated B6AF1 recipient mice were reconstituted i.v. with 2 × 106 BM cells depleted of mature leukocytes. CA30 and κTg BM cells were mixed at the indicated frequencies. Recipient mice were sacrificed for analysis at 12–16 weeks.
Chimeras with low frequencies of tolerogenic κTg B cells
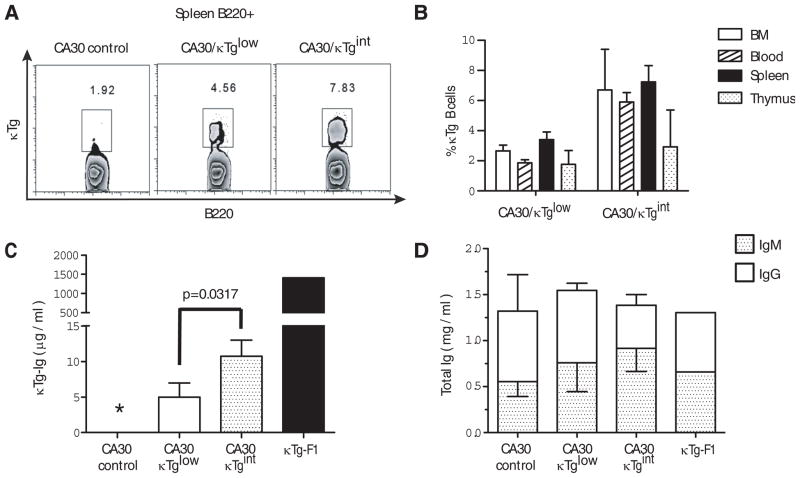
In initial experiments, we reconstituted mice with donor BM enriched for hematopoietic precursors at two CA30/κTg cell ratios. At 12 weeks, the degree of chimerism was assessed in blood, spleen, lymph nodes and thymus using a monoclonal antibody (mAb 17–63) directed against the κTg variable region. Flow cytometric analyses indicated that chimeras containing low frequencies of κTg B cells had been generated in both cases (Figure 2A–B). Moreover, the frequencies of κTg B cells and the amount of κTg-derived serum antibody were roughly proportional to the ratios of reconstituting κTg and CA30 BM (Figure 2C). In mice that received the lowest proportion (6%) of κTg BM (κTglow), κTg B cells comprised from ~2–3% of all B220+ cells, and κTg antibody was present in sera at ~5 μg/ml. These approximate the physiological frequency of B cells and quantity of antibody expected for any given Vκ gene in a wildtype mouse. In mice reconstituted with twice as many κTg BM cells (κTgint), these numbers were approximately twice as high (Figures 2B–C). Moreover, as shown in Figure 2D, the low levels of κTg serum Ig in mixed chimeras were not a consequence of a reduced quantity of total Ig, as mg/ml quantities of serum Ig were present in chimeric mice.
Figure 2. The frequency of κTg B cells and serum κTg Ig in chimeras is proportional to the frequency of donor κTg BM cells.
A, Percent ofκTg B cells among B220+ splenocytes from representative mixed BMC. B, Mean percent of κTg B cells in primary and secondary lymphoid organs. Mean percentages were obtained after subtracting values observed in the CA30-only chimeras. C, Mean total κTg Ig concentrations in sera of mixed chimeras. κTg-F1 denotes pooled sera of 3 κTg mice. D, Mean total Igκ concentration (IgMκ + IgGκ) in mixed chimeras. Error bars indicate mean plus SEM for n=5 mice per group. * Indicates undetectable.
Incomplete negative selection of BCR peptide-specific thymocytes in chimeric mice
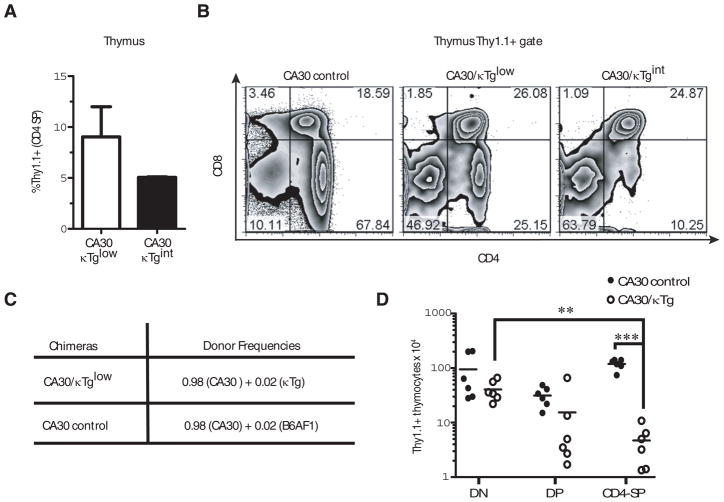
Prior studies using double Tg mice carrying 36–71 kappa and CA30 TCR transgenes demonstrated that CA30 T cells were deleted during thymocyte development. To determine whether this was also true in chimeras with small numbers of κTg B cells, we stained thymocytes for CA30 T cells using an anti-Thy1.1 antibody. In CA30/κTglow mice, <10% of CD4 single positive (SP) thymocytes were Thy1.1+, and in CA30/κTgint mice they were <5% (Figure 3A), suggesting that CA30 thymocytes were deleted during development in the thymus. Thymic deletion was also suggested by the reduced frequencies of CD4 SP cells among total Thy1.1+ thymocytes in mixed BM chimeras (Figure 3B). In thymi of control mice that were reconstituted only with CA30 BM, most of the cells (~68%) were CD4 SP, as expected for an MHC II-restricted αβTCR. In contrast, this number dropped to ~25% and ~10% in CA30/κTglow and CA30/κTgint chimeras respectively (Figure 3B). Although negative selection in the thymus was evident, it was nevertheless incomplete. This distinguishes tolerance in the chimeric model from that observed in prior studies of double transgenic CA30 × κTg mice and in a related double Tg model where CD4 SP cells were undetectable (14, 18). Incomplete tolerance indicated that either some CA30 T cells were able to escape tolerance in the chimeras or that there was an additional tolerance checkpoint.
Figure 3. Partial deletion of CA30 thymocytes in chimeras with κTg B cells.
A, Mean percent of total CD4+ SP thymocytes that were Thy1.1+ in CA30/κTg mixed BM chimeras. B, Distribution of CA30 (Thy1.1+) thymocyte subpopulations in representative CA30/κTg chimeras. Thymocytes were segregated on the basis of the Thy1.1 marker (CA30 cells), and analyzed for the expression of CD4 and CD8. Error bars indicate SEM (n=5). C, Donor BM ratios used to reconstitute lethally irradiated B6AF1 mice. D, Numbers of thymocytes in mixed chimeras generated as indicated in C (red blood cell lysed). Statistics were calculated using a one-tailed paired t test. Data represent the composite of two independent experiments. DN (CD4− CD8− double negative), DP (CD4+ CD8+ double positive) and CD4 SP (single positive).** p=0.0023 *** p< 0.0001.
Deletion of BCR peptide-specific thymocytes in mixed BM chimeras
Less than 10% of the all CD4 SP thymocytes were CA30 (Thy1.1+) in CA30/κTg chimeras. We interpret this to indicate that thymic deletion by cognate self-antigen rather than competition between CA30 and wildtype thymocytes (from the κTg donor) was responsible for this low frequency because in both sets of chimeras a large proportion (94% and 88%) of reconstituting BM was from CA30 donors. Also, the 2-fold drop in the proportion of CD4 SP CA30 thymocytes between the CA30/κTglow and CA30/κTgint chimeras did not reflect the small change in the proportion of reconstituting CA30 BM (Figure 3A). To determine if competition by nonTg thymocytes could account for the low numbers of CA30 Tg thymocytes in mixed BM chimeras, we reconstituted B6AF1 mice with equal numbers of BM cells from B6AF1 and CA30 mice. In these chimeras, wildtype CD4 SP thymocytes demonstrated an advantage over CA30 CD4 SP thymocytes (~29% of the CD4 SP thymocytes were Thy1.1+). However, this appeared insufficient to account for the low frequencies of CD4 SP CA30 thymocytes observed in the mixed BM chimeras (Figure S1).
To provide a definitive test of clonal deletion mediated by κTg B cells, we generated mixed BM chimeras in which the proportion of κTg BM in the experimental group (2%) was matched in the control group by wildtype B6AF1 BM. As shown in Figure 3C and D, CA30 Tg thymocytes decreased in number as they progressed to the CD4 SP stage in CA30/κTg chimeras, but not in the control CA30/B6AF1 chimeras. The absolute numbers of Thy1.1+ CD4 SP thymocytes were also severely reduced in the experimental group relative to those in the control group (p<0.0001). Collectively, these results indicate that CA30 T cells were subject to thymic deletion in chimeric mice harboring low frequencies of κTg B cells, but that deletion was incomplete.
Escape of BCR peptide-specific T cells to the periphery
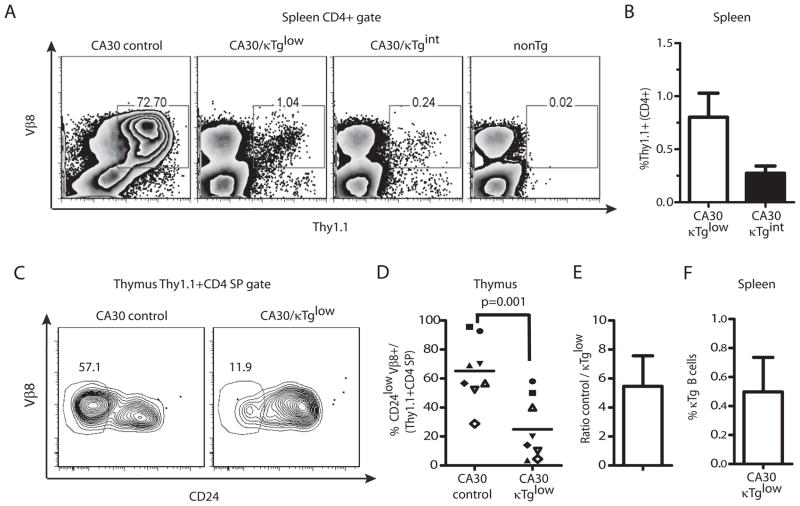
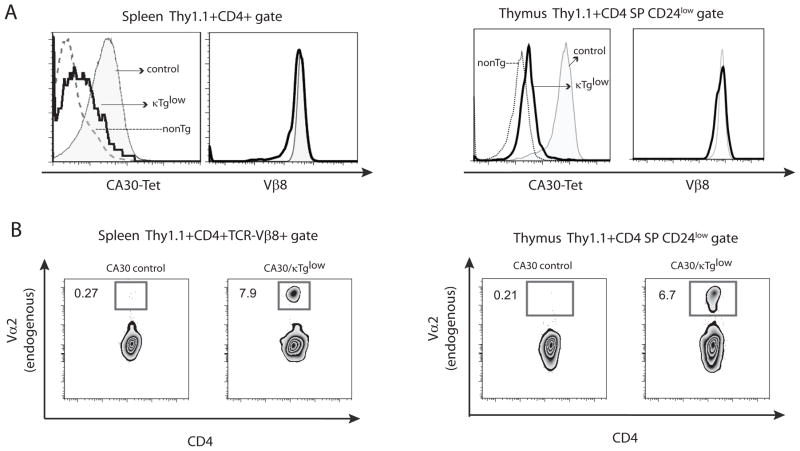
To determine if any CA30 T cells from mixed BM chimeras attained maturity and seeded the periphery, we stained lymphocytes from spleen and lymph node with anti-Thy1.1. Figures 4A and 4B show that Thy1.1+ CD4 T cells were present in low numbers in peripheral lymphoid organs. In CA30/κTglow chimeras, approximately 1% of spleen and lymph node CD4+ T cells stained with anti-Thy1.1, while in the CA30/κTgint group, this number was ~0.25%. These low frequencies indicated that there were 10- to 20-fold reductions in the proportion of peripheral CD4 cells that were Thy1.1+ (CA30) relative to the proportion of Thy1.1+ cells among CD4+ thymocytes.
Figure 4. A majority of CD4 SP CA30 thymocytes in mixed BMC have an immature phenotype.
A, Percent of CD4+ splenocytes that were Thy1.1+ Vβ8+. NonTg denotes splenocytes of negative control Thy1.2+/+ mouse. B, Summary of A. Error bars indicate SEM (n=5). C, Representative frequencies of mature CA30 CD4 SP thymocytes defined on the basis of CD24 expression levels. D, Percent of mature CA30 thymocytes among total CD4 SP CA30 thymocytes. Paired experimental and control mice represented by matching symbols. Horizontal bars depict mean value for all animals in each group. E, Fold-difference in percent of mature CA30 thymocytes between control and experimental chimeras. Graph depicts mean fold-difference calculated using individual fold-differences for paired experimental and control chimeras. F, Low frequencies of κTg B cells among B220+ CD19+ splenocytes in mixed bone marrow chimeras generated with a CA30/κTg or CA30/B6AF1 (control) donor BM ratio of 98:2 for experiments in this figure. Error bars indicate SEM (n=8, combined results of 3 experiments).
CD4 SP thymocytes specific for a BCR peptide are mostly immature
The large difference in frequency of Thy1.1+ CD4+ cells between the thymus and the periphery could be due to deletion of immature thymocytes at the CD4 SP stage. To test for this, we assessed thymocytes for surface expression of CD24, which distinguishes immature cells poised for apoptosis upon TCR signaling (CD24high) from mature, apoptosis-resistant cells (CD24low) (25). In experimental BM chimeras, an average of only 25% of Thy1.1+ CD4 SP thymocytes were mature. In contrast ~67% were mature in the CA30-only controls (Figure 4C and D). This difference was even larger when experimental and control chimeras analyzed on the same day were individually compared and fold-differences averaged (Figure 4E). This indicated that in addition to deletion at or before the CD4+CD8+ double-positive stage, late-stage deletion of CD4 SP cells also occurred. Moreover, in these chimeras, the proportion of all CD4 SP thymocytes that were mature was close to the proportion of peripheral CD4+ cells that were Thy1.1+, indicating that the reduction of CD4+ CA30 cells occurring between the thymus and the periphery could be largely accounted for by late-stage deletion of immature CD4 SP thymocytes. Notably, in this cohort of chimeras generated using donor BM at a CA30/κTg reconstitution ratio of 98:2, only 0.5% of peripheral B cells were κTg+ (Figure 4F).
Poor allelic exclusion in BCR peptide-specific T cells in secondary lymphoid tissue
To analyze TCR expression by peripheral CD4+ Thy1.1+ T cells of CA30/κTg chimeric mice, we stained splenocytes with a Vκ36–71 FR1-I-Ak tetrameric reagent. As shown in Figure 5A, Thy1.1+CD4+ T cells expressed the CA30 TCR, albeit at reduced levels compared to cells in the control mice reconstituted with CA30 and B6AF1 BM. This suggested that peripheral CA30 T cells in the mixed chimeras had down-modulated their TCR. When stained with an antibody directed to the Vβ8.2 component of the CA30 TCR, however, Thy1.1+ CD4+ T cells in the chimeras and controls were nearly indistinguishable, indicating that the low level of staining with the MHC-peptide tetramer was due instead to expression of a second TCR (Figure 5A). To confirm this interpretation, we stained lymphocytes from the chimeras with an antibody to Vα2, which is distinct from the Vα component (Vα1) of the CA30 TCR. As shown in Figure 5B, peripheral CA30 T cells (Thy1.1+CD4+Vβ8+) in chimeras stained at a frequency that was ~30 times greater than that of CA30 T cells from control animals, indicating frequent dual TCRα chain expression in CA30 T cells of chimeric mice (Figure 5B, top panels).
Figure 5. Poor TCRα allelic exclusion in CA30 T cells of mixed bone marrow chimeras.
A, Tetramer and Vβ8 stains of CD4+ Thy1.1+ splenocytes and mature thymocytes from CA30/κTg chimera (thick line) or control CA30/B6AF1 chimeras (solid histogram). NonTg denotes CD4+ Thy1.1− cells from CA30/κTg chimera (dotted line). Representative data for one of 3 experiments (8 mice total). B, Poor TCRα allelic exclusion in CA30 splenocytes (left panel) and thymocytes (right panel) in CA30/κTg chimeras. Representative data for one of 3 experiments (8 mice total).
To determine whether this elevated frequency of peripheral CA30 T cells expressing an endogenous TCRα chain was due to high frequency expression in mature thymocytes, we analyzed mature Thy1.1+ thymocytes (CD4 SP Vβ8 +CD24low) from CA30/κTg chimeras by flow cytometry and found that they indeed expressed Vα2 at high frequency relative to controls and at a similar frequency to that seen in the periphery (Figure 5B lower panels). Mature Th1.1+ thymocytes from CA30/κTg mice also stained more weakly with the Vκ36–71 FR1-I-Ak tetramer than did controls from CA30 control mice (Figure 5A). Collectively, these results suggested that expression of an endogenous Vα due to receptor editing or failed allelic exclusion promoted survival and maturation of CA30 thymocytes in BM chimeras containing κTg B cells.
BCR peptide-specific thymocytes protected from late thymic deletion by a second TCR
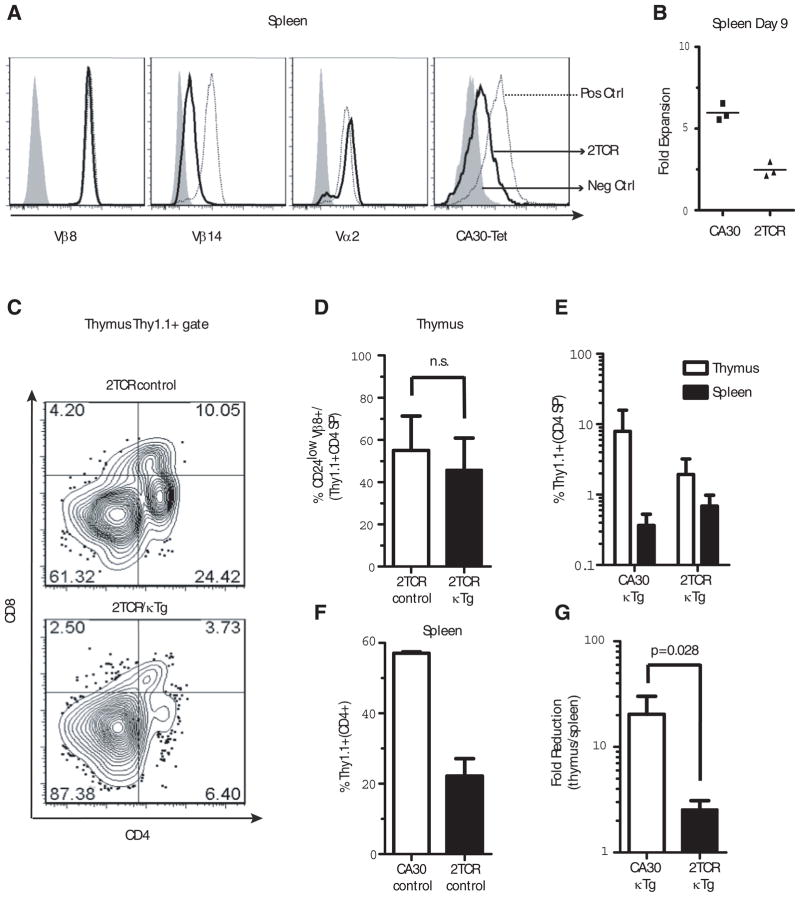
To determine if expression of a second TCR by autoreactive CA30 thymocytes would protect them from thymic deletion, we bred a second TCR transgene from the F508 TCR Tg mouse into CA30 donors to produce dual TCR mice (2TCR). The F508 TCR is encoded by Vα2/Vβ14 transgenes and binds an immunogenic peptide called 3K in the context of I-Ab (21). Somewhat unexpectedly, 2TCR T cells expressed surface Vβ8 and Vα2 at levels that were comparable to those of pure single receptor CA30 or F508 T cells respectively. This was accompanied by some, albeit poor, expression of the Vβ14 gene in 2TCR cells, which can be seen in comparative stains of F508 and 2TCR T cells (Figure 6A). This suggests that 2TCR T cells expressed slightly more total TCR than did CA30 T cells. Similar results were observed with 2TCR thymocytes (data not shown). Importantly, 2TCR T cells stained poorly with the Vκ36–71 FR1-I-Ak tetramer, indicating reduced expression of the CA30 TCR (Figure S2). We can infer that most of this reduction was likely due to competition by the F508 Vα2 for chain pairing with the CA30 Vβ8.2 because Vα2 was expressed at high levels on 2TCR T cells (Figure 6A panel 3).
Figure 6. Expression of a second TCR rescues mature CA30 CD4 SP thymocytes.
A, Expression of various αβTCR chains by CA30/F508 2TCR T cells (dark line), positive controls (dotted line, CA30 1st and 4th panels, F508 2nd and 3rd panels) and negative controls (shaded histogram, F508 1st and 4th panels, CA30 2nd and 3rd panels). One of 15 mice. B, Diminished primary immune response by 2TCR T cells. 106 total lymph node cells (2TCR or CA30) were transferred into B6AF1 recipients that were immunized i.p. 1 day later with Vκ36–71 FR1 peptide (10 μg) + LPS (7 μg) or with PBS. At day 9, Thy1.1+ CD4+ cells were quantified and are shown as fold-increase in peptide immunized mice versus PBS-immunized mice. One of 2 experiments. C, Frequency of 2TCR CD4 SP thymocytes in representative experimental (2TCR/κTg) and control (2TCR/B6AF1) chimeras. D, Mean frequencies of mature 2TCR CD4 SP thymocytes in mixed chimeras containing κTg B cells (~6% engraftment) or B6AF1 B cells (control). E, Percent CA30 T cells among CD4+ cells in the thymus and spleen of BM chimeras. F, Poor engraftment of 2TCR cells relative to CA30 T cells. Both sets of chimeras made with B6AF1 BM. Percent of Thy1.1+ T cells in spleens of CA30 or 2TCR chimeric control mice (reconstitution ratio CA30 or 2TCR/B6AF1 80:20). G, Fold-reduction from thymus to periphery in 2TCR CD4+ and CA30 CD4+ cells in chimeras with κTg B cells. Values calculated using frequencies of total CD4+ T cells in periphery or total CD4 SP thymocytes that were Thy1.1+. Columns are mean plus SEM (panels C–G, n=4, n.s. = not significant).
As a measure of TCR signaling potential and function, we compared the ability of 2TCR and CA30 T cells to respond to the Vκ36–71 FR1 peptide. As expected, there was a reduced in vitro proliferative response to the Vκ36–71 FR1 peptide by naive 2TCR T cells relative to CA30 T cells (Figure S3A). This was particularly evident at the lowest concentrations of peptide, indicating a higher threshold concentration of peptide required for proliferation of 2TCR T cells. Results of in vivo tests were similar. Nine days after adoptive transfer of 2TCR or CA30 T cells (Thy1.1+) and immunization with the Vκ36–71 FR1 peptide, CA30 T cells proliferated more extensively than 2TCR T cells (Figure 6B). At the same time, there was no difference in the percent of IL-2 producing cells among recovered Thy1.1 CD4+ T cells from the two groups, suggesting that the 2TCR T cells were functionally normal (Figure S3B). In addition, no phenotypic differences were observed between naïve 2TCR and CA30 T cells with respect to expression of CD44, CD62L and CD25 (Figure S3C & D), indicating that, like CA30 T cells, 2TCR T cells were in a resting state. These results were consistent with the interpretation that expression of a second TCR diminished functional responses to antigen due to diminished signaling mediated by the CA30 TCR, without obvious effects on T cell physiology.
To determine if a “diluted” CA30 TCR on 2TCR T cells would promote thymocyte maturation and migration to the periphery, we generated mixed chimeras with donor 2TCR or CA30 and κTg BM (95:5 ratio). Figure 6C illustrates 2TCR thymocyte progression in mixed BMC with (lower panel) or without κTg B cells (upper panel). Somewhat unexpectedly, expression of a second TCR did not rescue Thy1.1+ T cells at the double positive stage. However, at the CD4 SP stage, a substantial proportion of Thy1.1+ thymocytes (~50%) advanced to maturity as assessed with CD24. This frequency was similar to that (~60%) of control mice reconstituted with 2TCR and B6AF1 BM (95:5 ratio) (Figure 6D).
In the periphery of 2TCR/κTg mice, Thy1.1+ 2TCR cells constituted about 0.8% of all CD4 T cells, which is modestly higher than the frequency observed for the CA30/κTg chimeras (~0.4%), in which a similar degree of reconstitution by κTg B cells was attained (6–7%) (Figure 6E). However, 2TCR T cells were far less competitive than CA30 T cells, as assessed by their low percentages in the thymus and periphery of control mice in which no κTg B cells were present (Figure 6F and data not shown). CD4 SP CA30 (Thy1.1+) thymocytes constituted ~8% of all CD4 SP thymocytes in CA30/κTgchimeras, while this value was ~2% for the 2TCR/κTg chimeras (Figure 6E). Therefore, in going from the thymic CD4 SP stage to the periphery, there was a ~20-fold reduction in the fraction of CA30 T cells in CA30/κTg chimeras (8% to 0.4%) while the reduction was only ~2.5-fold in the case of the 2TCR/κTg chimeras (Figure 6G). Collectively these results indicate that expression of a second TCR by CA30 T cells enhanced their maturation in the thymus at the CD4 SP stage, which was reflected by increased frequencies of CA30 T cells in the periphery. This, together with preceding results indicating poor Vα allelic exclusion in mature CA30 CD24low thymocytes and peripheral CA30 T cells of CA30/κTg chimeras, support the conclusion that autoreactive CA30 T cells escaped thymic deletion in part due to expression of a second TCRα chain. While we cannot formally rule out the possibility that mature 2TCR thymocytes and T cells occupied larger niches due to selection mediated by a second receptor, this possibility seems inconsistent with the observation that in hosts lacking the κTg self-antigen, 2TCR thymocytes competed more poorly with wildtype thymocytes than did CA30 thymocytes, and this was reflected by reduced frequencies of 2TCR T cells in the periphery.
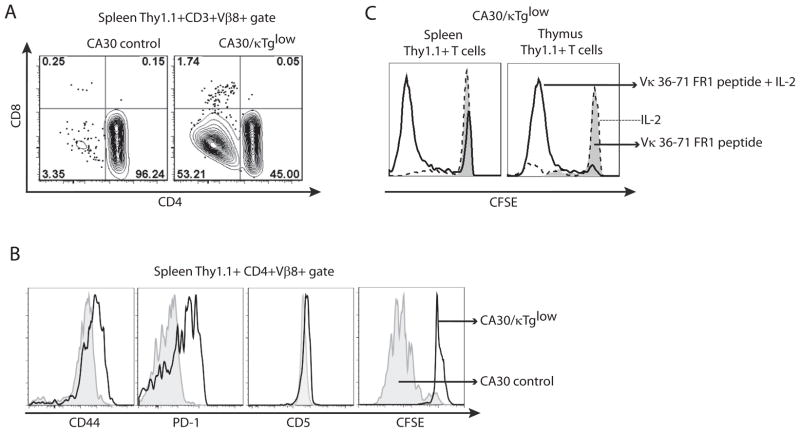
Unresponsive state of BCR peptide-specific T cells in the periphery
Peripheral CA30 T cells (CD3+, Thy1.1+, Vβ8+) in CA30/κTg chimeras segregated on the basis of CD4 expression (Figure 7A). Compared to those in the control CA30/B6AF1 chimeras, the peripheral CD4+ CA30 T cells in CA30/κTg chimeras expressed high levels of CD44 and PD-1 and had modestly increased levels of CD5 (Figure 7B). These surface molecules are considered to be indicators of antigen experience. However, when CD4+ CA30 T cells were stimulated in vitro with the Vκ36–71 FR1 peptide, they failed to proliferate, as assessed by CFSE dilution (Figure 7B) or by incorporation of H3-thymidine (data not shown). The unresponsive state was overcome by addition of rIL-2 at a high dose (50 U/ml), indicating that the cells were anergic (Figure 7C). CD4 SP thymocytes from CA30/κTg mice behaved similarly, indicating that the anergic state was induced during T cell development in the thymus (Figure 7C). Mature Thy 1.1+ thymocytes and peripheral Thy 1.1+ CD4 T cells from 2TCR cells in the 2TCR/κTg chimeras displayed a similar antigen experienced/anergic phenotype (Figure S4).
Figure 7. Peripheral CA30 T cells are antigen experienced and anergic.
A, CD4+ and CD4− populations of CA30 T cells in the periphery of mixed BM chimeras generated with κTg BM or control B6AF1 BM. One of ~15 mice. B, Expression of activation markers by, and proliferation of, CA30 CD4+ T cells from experimental and control chimeras. First 3 panels, one of ~15 mice. CFSE panel, one of 3 mice. C, In vitro proliferation of CD4+ CA30 T cells and CD4+ thymocytes from CA30/κTg chimeras when rIL-2 is provided. CFSE profiles of CD4+ CA30 T cells from CA30/κTglow chimeras cultured in the presence of Vκ36–71 FR1 peptide (1 μM) alone or with rIL-2 (50 U/ml). One of 2 experiments with similar results.
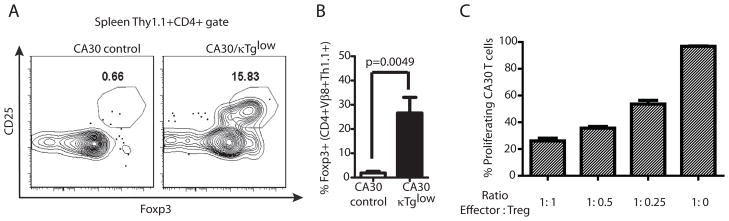
Anergic CA30 T cells have a regulatory phenotype
Prior studies have demonstrated that autoreactive thymocytes sometimes develop into natural regulatory T cells that seed the periphery (26–29). There are also reports that Treg do not proliferate in response to cognate peptide-MHC II, unless IL-2 is provided at a high concentration (30). These similarities with our model prompted us to determine whether anergic peripheral CA30 T cells expressed hallmarks of Treg. As seen in Figure 8, approximately 25% of peripheral CD4+ CA30 T cells derived from CA30/κTg chimeras expressed CD25 and Foxp3, which are characteristic of Treg. This stands in contrast to CD4+ CA30 T cells from control CA30/κTg chimeras, which were negative for both molecules (~0.7%). The presence of such cells in CA30/κTg mice suggested that they might be capable of enforcing self-tolerance to Ig and BCR-derived peptides in the periphery.
Figure 8. Evidence for regulatory CA30 T cells in the periphery of CA30/κTglow BM chimeras.
A, High frequency of CA30 T cells express CD25 and Foxp3. B, Mean frequencies and SEM of Foxp3+ cells among CD4+ Thy1.1+ Vβ8+ splenocytes (n=9, combined results of 3 experiments). C, CD4+ CD25+ CA30 T cells from CA30/κTg mice inhibit proliferation of CA30 T cells to Vκ36–71 FR1 peptide. CD4+ CD25+ CA30 T cells were mixed with naive CFSE-labeled CA30 T cells at indicated ratios and cultured for 7d with Vκ36–71 FR1 peptide and APC (n=3). Non proliferating CA30 T cells were determined in cultures without peptide and CD4+ CD25+ CA30 T cells. One of 2 experiments with similar results.
To test for potential regulatory function, we cultured purified CFSE-labeled CD4 CA30 T cells with or without Thy 1.1+ CD4 T cells from CA30/κTg chimeras at various ratios and evaluated proliferation in response to the Vκ36–71 FR1 peptide. Results of Figure 8C show that proliferation was strongly suppressed in cultures containing peripheral CA30 T cells derived from CA30/κTg BM chimeras.
Discussion
The repertoire of CD4 T cells attains a state of self-tolerance to BCR V regions as part of a larger tolerance program ensuring that T cell help to B cells is restricted through linked recognition of antigen. Prior studies with intact BCR and TCR double transgenic mice have shown that such tolerance occurs in the thymus by deletion at/or preceding the CD4 SP stage of development (14, 18, 19). In contrast, using mixed bone marrow chimeras to achieve physiological numbers of B cells expressing a tolerogenic BCR, we observed at least 3 checkpoints at which reactive CD4 T cells were rendered tolerant. We found that the extent of thymocyte deletion preceding the CD4 SP stage was dependent on the proportion of B cells in the repertoire that expressed the Vκ epitope seen by the T cells. This is consistent with the massive thymocyte deletion observed in prior studies involving “monoclonal” mice, where virtually all B cells expressed a single tolerogenic BCR (14, 18). In chimeric mice containing physiological numbers of relevant B cells, early-stage thymocyte deletion was followed by a second deletional checkpoint at the CD4+ SP stage, where few cells reached maturity. A third censorship point was revealed by the anergic state of those thymocytes that expressed a second receptor, reached maturity and migrated to the periphery. At least some of these cells had developed regulatory capabilities.
While our work reveals multiple checkpoints to eliminate or contain CD4 T cells that might otherwise provide help to B cells via BCR-derived peptides, it is unclear how large of a role the nondeletional tolerance checkpoints play in wildtype animals. Our chimeras contained large numbers of CA30 precursor cells, which conceivably may compete for tolerogenic self-antigen in ways that may not be true of wildtype mice. And even with large precursor numbers, tolerance in the thymus was impressively efficient, suggesting that in wildtype animals few if any BCR peptide-specific T cells ever reach the periphery. However, the efficiency of thymic deletion may be variable among clones and likely depends upon a number of factors, such as the affinity of the TCR for BCR peptide-MHC, the affinity of the BCR peptide for MHC and the abundance of B cells and perhaps other APC that present the cognate BCR peptide. In this regard, the CA30 clone is strongly reactive to the Vk36–71 FR1 peptide, and consistently predominates in CD4 T cell responses in mice immunized with whole mAb36–71, which may explain why this clone is prone to efficient thymic deletion in chimeras. In addition, the CA30 TCR is expressed at an unusually early stage of development (DN) because the TCR transgenes are driven by a CD2 promoter. DN cells in chimeras with large numbers of CA30 precursors may have an opportunity to encounter the Vκ36–71 FR1 peptide at an earlier stage of development than normal.
It is conceivable that there is an additional late tolerance mechanism, as represented by the DN CA30 T cells observed in the periphery. Down-modulation of CD4 and CD8 co-receptors has been observed in other transgene models of αβ T cell tolerance and has been proposed as a potential censorship mechanism for self-reactive cells (31, 32). Alternatively, peripheral DN T cells could be artifacts of premature expression of the transgenic αβTCR at the DN stage of thymocyte development as mentioned above (33). Some authors have argued that prematureαβTCR expression may preempt co-receptor expression and MHC-selection in the thymus and allow peripheral migration of αβ analogues of γδ T cells (34, 35). Because we cannot conclusively distinguish between these alternatives, the DN peripheral CA30 T cells were not incorporated into our tolerance scheme.
We found that the CA30 clone was severely underrepresented among peripheral CD4 T cells relative to its representation among CD4 SP thymocytes. In most chimeras, this proportional reduction from the thymus to the periphery was greater than 10-fold and could be largely accounted for by deletion at the mature CD4+ SP thymocyte stage: among wildtype CD4+ thymocytes, ~67% were mature (CD24low), whereas this value was ~25% in the mixed BM chimeras. Those CA30 T cells that escaped to the periphery apparently did so due to expression of a second TCRα chain, as inferred from the relatively high frequency (7%) of peripheral CA30 T cells expressing a nontransgenic Vα2 for which a diagnostic antibody was available. This was not seen in peripheral CA30 T cells from control chimeras lacking κTg B cells. This is reminiscent of observations made in other TCR transgene models of tolerance where expression of a second TCRα chain was associated with a state of peripheral tolerance (36, 37). For example, using moth cytochrome c-specific CD4 T cells crossed with antigen Tg mice, Girgis et al. found that cytochrome c-specific T cells were rendered anergic to cognate peptide. Anergy was the consequence of suboptimal TCR signaling on peripheral T cells due to dilution of the specific TCR by an endogenous TCRα chain (36). Due to the A/J genetic background of our CA30 and κTg lines, this interpretation could not readily be tested using RAG-deficient animals. However, other groups have reported increased frequencies of anergic/regulatory T cells in TCR/Ag double-transgenic RAG+/+ mice relative to those of RAG−/− mice.
Rather than using RAG−/− mice, we found support for the Vα-rescue interpretation by using double TCR transgenic (CA30 × F508)F1 mice (2TCR) as a source of donor BM in mixed 2TCR/κTg chimeras (38). In these chimeras, the frequencies of mature CA30 CD4+ thymocytes were increased relative to those of mixed CA30/κTg BM chimeras. It is noteworthy that expression of a second TCR did not diminish the degree of thymic deletion at the first checkpoint preceding the CD4 SP stage, as the frequencies of CD4 SP thymocytes were comparable in 2TCR/κTg and CA30/κTg mixed chimeras. Instead, the second TCR increased the proportion CA30 CD4 SP thymocytes that survived to maturity (CD24low). The most straightforward interpretation of this observation is that the signaling threshold for deletion at this second checkpoint is higher than that for deletion at the first because expression of a second TCR promoted survival of thymocytes only at the second checkpoint. This is consistent with results of prior studies employing model peptides and antigens, where the antigen concentration threshold for negative selection was found to be lower for immature thymocytes than for mature thymocytes (39–41).
Results of studies with the 2TCR mice must be interpreted with caution, however, because 2TCR cells express a second Vβ, and because there is a lack of diversity in the second TCRα chain, neither of which is physiological. The CA30 TCR in 2TCR T cells was probably more effectively “diluted” than that of CA30 T cells expressing only an additional endogenous TCRα chain. Nevertheless, CA30 T cells and 2TCR T cells of tolerogenic hosts had similar phenotypes and were functionally unresponsive to peptide stimulation in vitro, unless provided with high doses of rIL-2 (Figure S4). And the results obtained with 2TCR mice were predicted based on poor allelic exclusion by mature CA30 thymocytes and peripheral CA30 T cells in CA30/κTg chimeras, as inferred by high frequency expression of an endogenous Vα and poor tetramer staining.
Cells with a Treg phenotype (CD25+, Foxp3+) were enriched among peripheral CD4+ CA30 T cells in mixed chimeras, suggesting that they were positively selected to enter the peripheral pool. They comprised ~15% of CD4 CA30 T cells, which was approximately 15 times higher than the percent observed in control chimeras lacking κTg B cells. Still, a majority of CD4 CA30 T cells did not have the Treg phenotype. This heterogeneity among CD4 CA30 T cells could be due to several causes discussed in related prior studies that include: homeostatic constraints on the size of the Treg pool, differences in signal strength among CA30 T cells expressing diverse endogenous TCRα chains, or constraints on the APC niche required for Treg development (42–48). In addition, it is possible that the CD25− Foxp3− population of CA30 T cells possess regulatory activity, as CD25− Treg cells have been seen in another transgene model of tolerance (49). In this vein, several reports have shown that the most biologically potent Treg expressed high levels of CD44 (50–54). In our study, all of the CD4+ CA30 T cells in the periphery of the mixed BM chimeras were CD44hi.
Treg that are specific for germline-encoded BCR peptides may provide a tier of peripheral tolerance by quenching CD4 T cell responses to germline-encoded BCR peptides. It is also plausible that germline peptide-specific T cells inhibit other T cells that react with somatically-generated BCR peptides defining CDR3 of the BCR. These CDR3 sequences may be sufficiently rare that they are largely invisible to the developing thymocyte repertoire, such that ignorant CDR3-reactive T cells in the periphery may first encounter their cognate antigen in expanded clones of activated B cells. If not neutralized shortly after activation, CDR3-reactive T cells would be licensed by changes in CXCR5 and CXCR7 expression to enter GC reactions. Regardless of the precise role of BCR peptide-specific Treg, it is clear from this study that multiple checkpoints in tolerance are in place to functionally or physically eliminate an important category of T cells that might otherwise provide BCR peptide-directed help to B cells, in violation of the principle of linked antigen recognition.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH R01AI033613, AI073945 and T32 AI007405.
We thank Drs. Philippa Marrack and Megan MacLeod for the F508 TCR Tg mice, Dr. John Kappler, James B. St. Clair, Greg Kirchenbaum and Dr. Wenzhong Guo for critical review of the manuscript and Dr. Judith Spiegel for proof reading the manuscript.
Footnotes
List of abbreviations: BCR (B cell antigen receptor), BM (bone marrow), DN (double negative), GC (germinal center), Ig (immunoglobulin), mAb (monoclonal antibody), r (recombinant), SP (single positive), TCR (T cell receptor), TFH (follicular helper), Treg (regulatory T cells) and V (variable).
Disclosures
The authors declare they have no financial conflicts of interest.
References
- 1.Griffiths GM, Berek C, Kaartinen M, Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984;312:271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- 2.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wysocki L, Manser T, Gefter ML. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986;83:1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casson LP, Manser T. Random mutagenesis of two complementarity determining region amino acids yields an unexpectedly high frequency of antibodies with increased affinity for both cognate antigen and autoantigen. The Journal of experimental medicine. 1995;182:743–750. doi: 10.1084/jem.182.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder CM, Zhang X, Wysocki LJ. Negligible class II MHC presentation of B cell receptor-derived peptides by high density resting B cells. J Immunol. 2002;168:3865–3873. doi: 10.4049/jimmunol.168.8.3865. [DOI] [PubMed] [Google Scholar]
- 6.Rudensky AY, Mazel SM, Blechman JM, Yurin VL. Immunoglobulin-specific T-B cell interaction. IV. B cell presentation of idiotypic determinant(s) of monoclonal anti-surface immunoglobulin antibody to idiotope-recognizing helper T clones. Eur J Immunol. 1990;20:1691–1696. doi: 10.1002/eji.1830200811. [DOI] [PubMed] [Google Scholar]
- 7.Munthe LA, Kyte JA, Bogen B. Resting small B cells present endogenous immunoglobulin variable-region determinants to idiotope-specific CD4(+) T cells in vivo. Eur J Immunol. 1999;29:4043–4052. doi: 10.1002/(SICI)1521-4141(199912)29:12<4043::AID-IMMU4043>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Bikoff EK, Yu H, Eckhardt LA. T cell recognition of endogenous IgG2a expressed in B lymphoma cells. Eur J Immunol. 1988;18:341–348. doi: 10.1002/eji.1830180304. [DOI] [PubMed] [Google Scholar]
- 9.Benaroch P, Bordenave G. T cell-induced Ig allotypic suppression in mice. II. Both CD4+ CD8- and CD4- CD8+ T cell subsets from sensitized Igha mice are required to induce suppression of Igh-1b allotype expression. J Immunol. 1989;142:1–7. [PubMed] [Google Scholar]
- 10.Weiss S, Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991;64:767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- 11.Weiss S, Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartnes K, Hannestad K. Engagement of the B lymphocyte antigen receptor induces presentation of intrinsic immunoglobulin peptides on major histocompatibility complex class II molecules. European Journal of Immunology. 1997;27:1124–1130. doi: 10.1002/eji.1830270512. [DOI] [PubMed] [Google Scholar]
- 13.Mitchison NA. The carrier effect in the secondary response to hapten-protein conjugates. V. Use of antilymphocyte serum to deplete animals of helper cells. Eur J Immunol. 1971;1:68–75. doi: 10.1002/eji.1830010204. [DOI] [PubMed] [Google Scholar]
- 14.Snyder CM, Aviszus K, Heiser RA, Tonkin DR, Guth AM, Wysocki LJ. Activation and tolerance in CD4(+) T cells reactive to an immunoglobulin variable region. J Exp Med. 2004;200:1–11. doi: 10.1084/jem.20031234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munthe LA, Os A, Zangani M, Bogen B. MHC-restricted Ig V region-driven T-B lymphocyte collaboration: B cell receptor ligation facilitates switch to IgG production. J Immunol. 2004;172:7476–7484. doi: 10.4049/jimmunol.172.12.7476. [DOI] [PubMed] [Google Scholar]
- 16.Eyerman MC, Zhang X, Wysocki LJ. T cell recognition and tolerance of antibody diversity. J Immunol. 1996;157:1037–1046. [PubMed] [Google Scholar]
- 17.Guo W, Smith D, Guth A, Aviszus K, Wysocki LJ. T cell tolerance to germline-encoded antibody sequences in a lupus-prone mouse. J Immunol. 2005;175:2184–2190. doi: 10.4049/jimmunol.175.4.2184. [DOI] [PubMed] [Google Scholar]
- 18.Bogen B, Dembic Z, Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993;12:357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauritzsen GF, Hofgaard PO, Schenck K, Bogen B. Clonal deletion of thymocytes as a tumor escape mechanism. Int J Cancer. 1998;78:216–222. doi: 10.1002/(sici)1097-0215(19981005)78:2<216::aid-ijc16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Rueff-Juy D, Faure M, Drapier AM, Cazenave PA. Role of maternal Ig in the induction of C kappa-specific CD8+ T cell tolerance. J Immunol. 1998;161:721–728. [PubMed] [Google Scholar]
- 21.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Guth A, Detanico T, Smith D, Tung KS, Bonorino C, Wysocki LJ. Spontaneous autoimmunity in mice that carry an IghV partial transgene: a required arginine in VHCDR3. Lupus. 2009;18:299–308. doi: 10.1177/0961203308097480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. Journal of immunological methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 24.Marshak-Rothstein A, Siekevitz M, Margolies MN, Mudgett-Hunter M, Gefter ML. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyall R, Nikolic-Zugic J. The majority of postselection CD4+ single-positive thymocytes requires the thymus to produce long-lived, functional T cells. J Exp Med. 1995;181:235–245. doi: 10.1084/jem.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. The Journal of experimental medicine. 1999;189:877–882. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunology. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 28.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature Immunology. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi O, Kontani K, Ikeda H, Kezuka T, Takeuchi M, Takahashi T. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology. 1994;82:365–369. [PMC free article] [PubMed] [Google Scholar]
- 30.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. Journal of immunology. 2005;175:3053–3059. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 31.Teh HS, Kishi H, Scott B, Von Boehmer H. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989;169:795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illes Z, Waldner H, Reddy J, Anderson AC, Sobel RA, Kuchroo VK. Modulation of CD4 co-receptor limits spontaneous autoimmunity when high-affinity transgenic TCR specific for self-antigen is expressed on a genetically resistant background. Int Immunol. 2007;19:1235–1248. doi: 10.1093/intimm/dxm094. [DOI] [PubMed] [Google Scholar]
- 33.Furmanski AL, Bartok I, Chai JG, Singh Y, Ferreira C, Scott D, Holland SJ, Bourdeaux C, Crompton T, Dyson J. Peptide-specific, TCR-alpha-driven, coreceptor-independent negative selection in TCR alpha-chain transgenic mice. J Immunol. 2010;184:650–657. doi: 10.4049/jimmunol.0902291. [DOI] [PubMed] [Google Scholar]
- 34.Russell JH, Meleedy-Rey P, McCulley DE, Sha WC, Nelson CA, Loh DY. Evidence for CD8-independent T cell maturation in transgenic mice. J Immunol. 1990;144:3318–3325. [PubMed] [Google Scholar]
- 35.von Boehmer H, Kirberg J, Rocha B. An unusual lineage of alpha/beta T cells that contains autoreactive cells. J Exp Med. 1991;174:1001–1008. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girgis L, Davis MM, Fazekas de St Groth B. The avidity spectrum of T cell receptor interactions accounts for T cell anergy in a double transgenic model. J Exp Med. 1999;189:265–278. doi: 10.1084/jem.189.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akkaraju S, Ho WY, Leong D, Canaan K, Davis MM, Goodnow CC. A range of CD4 T cell tolerance: partial inactivation to organ-specific antigen allows nondestructive thyroiditis or insulitis. Immunity. 1997;7:255–271. doi: 10.1016/s1074-7613(00)80528-2. [DOI] [PubMed] [Google Scholar]
- 38.Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, Dossett ML, Huseby ES, Ohlen C. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eck SC, Zhu P, Pepper M, Bensinger SJ, Freedman BD, Laufer TM. Developmental alterations in thymocyte sensitivity are actively regulated by MHC class II expression in the thymic medulla. J Immunol. 2006;176:2229–2237. doi: 10.4049/jimmunol.176.4.2229. [DOI] [PubMed] [Google Scholar]
- 40.Stephen TL, Tikhonova A, Riberdy JM, Laufer TM. The activation threshold of CD4+ T cells is defined by TCR/peptide-MHC class II interactions in the thymic medulla. J Immunol. 2009;183:5554–5562. doi: 10.4049/jimmunol.0901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc Natl Acad Sci U S A. 2009;106:10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 47.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 50.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 51.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Firan M, Dhillon S, Estess P, Siegelman MH. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood. 2006;107:619–627. doi: 10.1182/blood-2005-06-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darrasse-Jeze G, Bergot AS, Durgeau A, Billiard F, Salomon BL, Cohen JL, Bellier B, Podsypanina K, Klatzmann D. Tumor emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumors in mice. J Clin Invest. 2009;119:2648–2662. doi: 10.1172/JCI36628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.