Abstract
Maternal infections are implicated in a variety of complications during pregnancy, including pregnancy loss, prematurity, and increased risk of neurodevelopmental disorders in the child. Here, we show in mice that even mild innate immune activation by low-dose lipopolysaccharide in early pregnancy causes hemorrhages in the placenta and increases the risk of pregnancy loss. Surviving fetuses exhibit hypoxia in the brain and impaired fetal neurogenesis. Maternal Toll-like receptor 4 signaling is a critical mediator of this process, and its activation is accompanied by elevated proinflammatory cytokines in the placenta. We evaluated the role of tumor necrosis factor-α (TNF-α) signaling and show that TNF receptor 1 (TNFR1) is necessary for the illness-induced placental pathology, accompanying fetal hypoxia, and neuroproliferative defects in the fetal brain. We also show that placental TNFR1 in the absence of maternal TNFR1 is sufficient for placental pathology to develop and that a clinically relevant TNF-α antagonist prevents placental pathology and fetal loss. Our observations suggest that the placenta is highly sensitive to proinflammatory signaling in early pregnancy and that TNF-α is an effective target for preventing illness-related placental defects and related risks to the fetus and fetal brain development.
Maternal infections during pregnancy have been associated with a variety of gestational complications, including pregnancy loss (particularly in the second trimester),1,2 preterm birth,3 and poor neurological outcome in the child.4,5 Infections that invade the uterus can directly damage the developing fetus or compromise placental function.1 Systemic maternal illness that does not involve the fetus can also threaten fetal viability by inducing high fever or maternal respiratory distress.1 Both of these scenarios have been linked with poor neurological outcome in the child. In particular, uterine infection and chorioamnionitis near the time of delivery can cause cerebral palsy or cystic periventricular leukomalacia,4 and systemic infection or immune events earlier in pregnancy are implicated in neuropsychiatric disorders such as schizophrenia6 and autism spectrum disorders.7,8 Animal studies have further shown that activation of the maternal inflammatory response is alone sufficient to induce placental damage,9 overt pregnancy loss,9,10 preterm birth,11 or behavioral alterations in offspring.12–14
The adverse effects of infection on pregnancy and fetal development are thought to be mediated in large part by proinflammatory cytokines. Activation of the innate immune system by microbes or pathogen-associated microbial products such as lipopolysaccharide (LPS) lead to robust cytokine expression by immune cells, mediated in large part by Toll-like receptor (TLR) signaling.15 These cytokines have direct access to the placenta via maternal blood, and signaling may be propagated across the placental barrier through stimulation of inflammation within the placenta.16 Direct passage of maternal cytokines through the placenta is also possible, at least for a subset of cytokines.17 Inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) are readily detected in maternal serum, placenta, and fetus after activation of the innate immune response in pregnant animals.18–20 Of these, TNF-α and IL-1β are known to have detrimental effects on the placenta.9,21–23 TNF-α in particular is known for its cytotoxic effects, which are mediated largely through TNF receptor 1 (TNFR1) via its intracellular death domain, which activates the caspase apoptosis pathway.24 Blocking TNF-α during an LPS response in late pregnancy is able to prevent the resulting fetal loss,10 and administration of recombinant TNF-α alone is sufficient to cause cell death in the placenta and fetal death.9,21
We have used a mouse model of maternal immune activation with LPS to examine the effects of mild immune challenge on placentas, fetal survival, and fetal brains. We have found that the placenta is extremely sensitive to LPS in early pregnancy. Even small doses that fail to evoke sickness behaviors in pregnant mice are still sufficient to elicit placental pathology and an accompanying transient hypoxia in the developing fetus at embryonic day 12.5 (E12.5). In evaluating the signaling cascades that mediate these effects, we have demonstrated that TLR4 signaling in the maternal immune system is required for LPS to affect the placenta. TNFR1 signaling is also required for the placental response to LPS and associated effects on the developing fetal brain. Furthermore, TNFR1 in the fetus and placenta are sufficient to mediate LPS-induced placental pathology. Finally, we show that attenuation of TNF-α signaling with soluble TNFR2-IgG fusion protein25 prevents placental defects caused by mild maternal immune activation in early pregnancy.
Materials and Methods
Animals
All animal studies were performed in accordance with NIH guidelines for the humane use of animals and all procedures were reviewed and approved by the Stanford Animal Care and Use Committee. C57BL/6J, C57BL/10J, C57BL/10ScNJ (TLR4−/−), C57BL/6-Tnfrsf1atm1Imx/J (TNFR1−/−), B6.129S2-Tnfrsf1btm1Mwm/J (TNFR2−/−), and B6.129S6-Tnftm1Gkl/J (TNFα−/−) mice were purchased from Jackson Laboratories (Bar Harbor, ME).
Timed Pregnancies and Treatments
Timed pregnancies were generated by housing pairs of females with a single male overnight. Animals were separated the next day, and noon on this day was classified as E0.5 for these studies. At E12.5 or E14.5, pregnant mice were treated with intraperitoneal LPS from Escherichia coli (Sigma-Aldrich, St. Louis, MO) at doses between 30 and 300 μg/kg body weight, prepared in saline. Except as otherwise noted, all chemicals were purchased from Sigma-Aldrich. The TNFR2-IgG fusion protein25 (Etanercept; Pfizer, New York, NY) was purchased from the Stanford University Hospital pharmacy.
Placental Histology
Placentas were dissected and fixed overnight in 10% neutral buffered formalin, followed by dehydration into ethanol, paraffin embedding, and sectioning at 6 μm. H&E staining was performed according to standard protocols. For quantification, images of an entire cross-section were taken at ×10 magnification. The spongiotrophoblast layer and the area within the layer with unusual pink eosinophilic staining were outlined and pixel areas were determined using ImageJ software version 1.42 (NIH, Bethesda, MD). Data are expressed as the percentage of the spongiotrophoblast layer showing this characteristic staining.
Luminex Bead Array
Maternal serum and placentas were harvested 2, 6, or 24 hours after treatment with saline or LPS (60 μg/kg) at E12.5. Placentas were homogenized in lysis buffer using a mechanical homogenizer. Total protein was assessed using protein reagent from Bio-Rad (Hercules, CA). Cytokine/chemokine levels were assessed using a Luminex bead array from Affymetrix (Santa Clara, CA) according to the manufacturer's instructions, with the following incubation times: antibody beads, 2 hours at room temperature followed by overnight at 4°C; detection antibody, 2 hours at room temperature; and streptavidin-PE, 30 minutes at room temperature. Standard curves and reports were prepared with MiraiBio MasterPlex QT software (Hitachi Solutions America, South San Francisco, CA). Molecules showing significant increases in both the placenta and maternal serum are reported.
Immunohistochemical Analysis
Pimonidazole (Hypoxyprobe-1; HPI, Burlington, MA) was injected intraperitoneally into pregnant mice at 100 mg/kg body weight 15 minutes before saline or LPS injection. Two hours later, fetuses were harvested and decapitated. Heads were fixed overnight in 4% paraformaldehyde, equilibrated in 30% sucrose, and rapidly frozen in HistoPrep (Fisher Scientific, Pittsburgh, PA) in 25.20.5 mm Tissue Tek cryomolds (VWR, Radnor, PA). Whole heads were sectioned on a Microm HM505E cryostat (Fisher Scientific) to 16 μm and then were mounted on glass slides. Slides were first rinsed twice with Tris-buffered saline (TBS) and then were blocked in TBS plus 0.3% Triton-X 100 plus 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature. Tissues were then incubated with primary antibody to pimonidazole (HPI clone 4.3.11.3; 1:50) and phosphohistone H3 (pHH3; 1:400; Cell Signaling Technologies, Danvers, MA) in staining buffer (TBS plus 0.3% Triton-X 100 plus 1% normal donkey serum) overnight at 4°C. After three washes with TBS, slides were incubated with secondary donkey anti-mouse and donkey anti-rabbit conjugated to Cy3 or fluorescein isothiocyanate (Jackson ImmunoResearch) diluted 1:500 in staining buffer for 4 hours at room temperature. Slides were washed twice in TBS and then incubated with DAPI at 0.5 μg/mL for 10 minutes at room temperature. Slides were then washed twice with TBS, fixed with 4% paraformaldehyde for 10 minutes at room temperature, and washed three more times in TBS. Coverslips were mounted using polyvinyl alcohol and 1,4 diazabicyclo[2.2.2]octane in glycerin.
Confocal Microscopy
Confocal microscopy was performed on a Zeiss 510 confocal microscope (Thornwood, NY) with gain and offset adjusted to eliminate under- and oversaturated pixels. Settings were then held constant and images were collected from control and experimental tissues that had been stained in parallel. Image analysis was performed using ImageJ 1.42q software. For pimonidazole, the area of positive pixels for pimonidazole and DAPI were quantified separately for each image, using identical thresholding criteria. Data are expressed as a ratio of pimonidazole-positive pixels to DAPI-positive pixels, to normalize for total tissue area within each image frame. The number of pHH3-positive cells was counted manually and normalized to the tissue area using DAPI. Data are expressed as density of cells or the percentage of the control saline values for each strain.
Results
Subclinical Immune Activation Causes Significant Fetal Loss in Early Pregnancy
To determine the effects of maternal immune signaling during pregnancy, a mild maternal innate immune response was elicited in pregnant mice by activating Toll-like receptor 4 (TLR4) signaling with low doses of LPS. To establish timing and dose, animals were challenged with increasing amounts of LPS injected intraperitoneally at E8.5, E10.5, E12.5, or E14.5. Previous work has shown that susceptibility to LPS-induced fetal loss decreases between E8 and E11, but we were interested in extending this to later times in gestation.10 We found that early pregnancies are very sensitive to innate immune activation, with doses as low as 60 μg/kg of LPS causing complete miscarriage at E8.5 (Figure 1A). The ability of LPS to induce fetal loss gradually decreased through E12.5, although the low dose of 60 μg/kg was still sufficient to induce some fetal loss at this time. At E14.5, a much higher dose (300 μg/kg) of LPS was required to generate similar fetal loss, indicating that pregnancies in mice become much more resistant to LPS after E12.5. The low doses of LPS that elicit fetal loss at these early times in pregnancy are not sufficient to trigger classic sickness behaviors (such as lethargy, piloerection, or poor grooming) in the pregnant animals; the only effect noted was slight maternal weight loss (Figure 1B). We continued these studies at E12.5 with the 60 μg/kg dose, which elicited some fetal loss but at which most of the litters (81.6%) had at least some surviving fetuses 24 hours after challenge.
Figure 1.
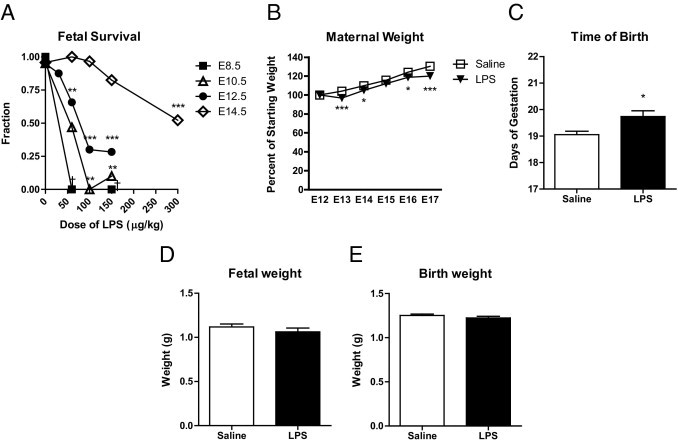
Dose-dependent risk of fetal loss but lack of growth restriction or premature birth after maternal LPS administration in a mouse model. A: The number of surviving fetuses was counted 24 hours after treatment with LPS at different embryonic ages and doses (n = 3 to 40 pregnancies per group). The effect of treatment on survival was significant at E10.5 (P = 0.0022), E12.5 (P < 0.0001), and E14.5 (P < 0.0001) by one-way analysis of variance. Analysis of variance could not be performed for E8.5 treatments because of lack of variance within groups. **P < 0.01; ***P < 0.001 by Dunnett's multiple comparison post hoc test. B: Pregnant mice were treated with saline (n = 13) or LPS (60 μg/kg) (n = 17) on E12.5. Maternal weights were monitored daily and were expressed as a percentage of starting weight at the time of treatment. LPS-treated maternal weights are slightly but significantly lower than for saline-injected control animals (P < 0.0001 by two-way analysis of variance). *P < 0.05; ***P < 0.001 by Bonferroni post hoc test). C: Pregnant mice were treated with saline (n = 18) or LPS (60 μg/kg) (n = 21) on E12.5 and the time of birth was recorded. The length of gestation in LPS-injected animals was increased by approximately 0.5 days. *P = 0.014 by two-tailed t-test. D and E: Pregnant mice were treated with saline (n = 36) or LPS (60 μg/kg) (n = 31) on E12.5. Fetuses were collected and weighed at E17.5 or at birth. Fetal growth was unaffected in LPS-treated pregnancies (E17.5: P = 0.30 by two-tailed t-test; birth: P = 0.27 by two-tailed t-test). Data are reported as means ± SEM.
To determine whether the mild LPS challenge used in the present study would restrict fetal growth or cause premature birth, pregnant animals were injected with LPS (60 μg/kg) at E12.5 and either fetuses were collected and weighed near term (E17.5) or pregnancies were allowed to proceed to term and the length of gestation and birth weights were recorded. There was no evidence of premature birth (Figure 1C); rather, length of gestation showed a slight but significant increase, from 19.1 ± 0.1 to 19.7 ± 0.2 days (P = 0.014) (Figure 1C). Although others have observed that prolonged administration of LPS can lead to intrauterine growth restriction,22 the single small doses used in the present study had no effect on body weight in surviving fetuses (Figure 1, D and E), suggesting that the effect on the fetus is transient.
Mild Immune Challenge at E12.5 Results in Placental Pathology, Reduced Oxygen Levels in the Fetal Brain, and Reduced Fetal Neural Progenitor Cell Proliferation
The sensitivity of the pregnancy to immune activation at E12.5 led us to examine the placentas and fetuses in greater detail. At 24 hours after LPS injection at E12.5, placental vasculature was notably dilated, and small areas of hemorrhage were visible at the rim of the placenta (Figure 2, A–C). Histologically, placentas showed vascular engorgement with significant trapping of red blood cells within the labyrinth of the placenta as early as 6 hours after treatment (Figure 2, F and G). By 24 hours, the vascular congestion had partially receded, but the placentas showed prominent tracts of tissue necrosis in the spongiotrophoblast layer, highlighted by diffuse eosin staining and pycnotic nuclei (Figure 2, E and H–J).
Figure 2.
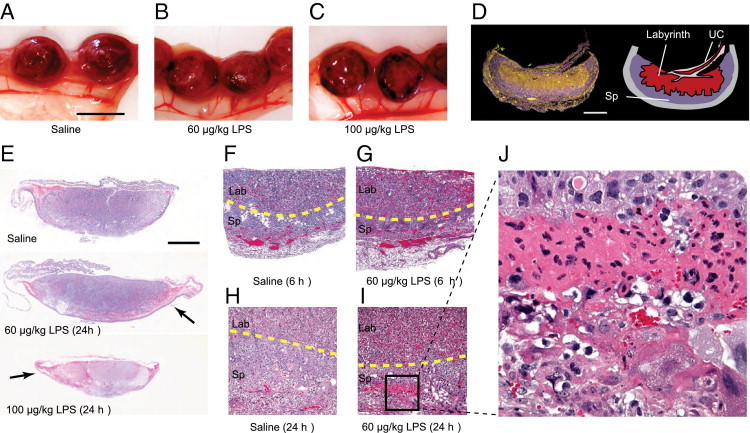
Placental pathology after mild maternal immune challenge at E12.5. A–C: Placentas were harvested from pregnant mice 24 hours after E12.5 saline or LPS treatment. Placentas show typical dark areas of hemorrhage in LPS-challenged pregnancies. Scale bar = 1 cm. D: Dark-field image of a saline mouse placental cross section showing reflective red blood cells (yellow) within the umbilical cord (UC) and labyrinth layer where the maternal-fetal blood exchange occurs. The spongiotrophoblast (Sp) layer appears darker. Scale bar = 100 μm. E–J: Uteri and placentas were harvested from pregnant mice at 6 hours or 24 hours after E12.5 treatment with saline or LPS (60 or 100 μg/kg). Vascular engorgement is visible in placentas stained with H&E at 6 hours (F and G) or 24 hours (E and H–J) after LPS treatment (60 μg/kg) within the labyrinth (Lab) layer. Tracts of eosinophilic necrotic tissue are also seen within the spongiotrophoblast (Sp) layer 24 hours after LPS challenge, indicated by arrows (E) and a boxed area (I), which is shown also at higher magnification (J). Yellow dotted lines mark the border between the spongiotrophoblast and labyrinth layers. Scale bar = 100 μM (E). Original magnification: ×10 (E); ×100 (F–I); ×400 (J).
To determine whether placental pathology influenced fetal perfusion and oxygen supply to the developing fetal brain, pregnant animals were injected with pimonidazole, a compound that can cross the placenta and form adducts with proteins in hypoxic tissues, defined as tissue in which O2 partial pressures are <1.5%.26,27 Pimonidazole (100 mg/kg, i.p.) was injected 15 minutes before the saline or LPS (60 μg/kg) challenge, to equilibrate within maternal and fetal tissues; fetal cortex was evaluated for pimonidazole adducts 2 hours after LPS injection (Figure 3, A–C). Strong pimonidazole staining was observed in the fetal brain after LPS challenge. When quantified, the fraction of fetal cortex area positive for pimonidazole was significantly increased, compared with saline controls (P = 0.0040) (Figure 3E).
Figure 3.
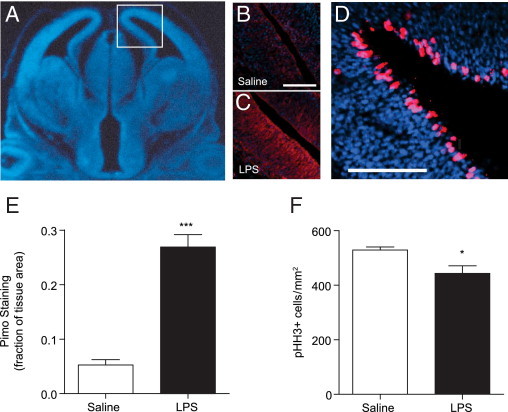
Hypoxia and impaired proliferation in the fetal brain. Pregnant mice were treated with pimonidazole 15 minutes before saline or LPS (60 μg/kg) at E12.5. Two hours after saline or LPS treatment, fetuses were harvested and brains were processed and stained with an antibody against pimonidazole adducts or phosphohistone H3 (pHH3) and DAPI to mark all nuclei. A: A coronal section of the fetal brain stained with DAPI (blue) to mark nuclei; the boxed area indicates the approximate region magnified in the accompanying images (B–D). B and C: Representative images of saline- and LPS-treated fetal brain. Brains were stained with an antibody against pimonidazole adducts (red) and DAPI to mark nuclei (blue). Scale bars = 100 μm. D: An example of a saline-treated control brain stained with an antibody against pHH3 (red) and DAPI to mark nuclei (blue). Scale bar = 100 μm. E: Pimonidazole (pimo) staining was quantified as the proportion of pimo-positive pixels relative to DAPI-positive pixels. The amount tissue area in the cortex positively staining for pimonidazole increased dramatically after LPS administration (n = 9 saline and 12 LPS brains from 3 to 4 pregnancies per group). ***P < 0.0001 by two-tailed Student's t-test. F: The number of pHH3+ metaphase cells was counted per unit tissue area using DAPI. The density of pHH3+ metaphase cells was reduced in fetal brain tissues from pregnancies challenged with LPS (n = 9 saline and 12 LPS brains from 3 to 4 pregnancies per group). *P = 0.0199 by two-tailed Student's t-test. Data are reported as mean ± SEM.
The elevated placental sensitivity at midgestation corresponds with early stages of cortical development in the fetus, a time when neural progenitor cells are undergoing rapid proliferative expansion and differentiation into neurons. To evaluate effects on neural progenitor cell mitotic index, E12.5 fetal brains were stained and scored for the abundance of pHH3-positive cells (Figure 3, D and F). Histone H3 is phosphorylated during M phase and marks mitotic cells in the ventricular zone and intermediate zone of the developing cortex. The placental pathology was accompanied by a small but significant decrease in the number of mitotic cells in LPS-treated fetuses (P = 0.028) (Figure 3F).
Cytokine Profiles in Maternal Circulation and Placenta
To evaluate cytokine abundance after low-dose LPS, maternal serum was collected and placentas were harvested and homogenized at 2, 6, and 24 hours after saline or LPS (60 μg/kg) treatment and were analyzed for cytokine and chemokine levels with a Luminex bead assay. IL-1β, IL-6, and TNF-α are considered key mediators of the innate proinflammatory response, and all three were significantly elevated in both the maternal serum and the placenta. IL-6 and TNF-α showed highly significant increases after LPS treatment in both the placenta and serum, with highest levels detected at 2 hours, two-way analysis of variance: IL-6, P = 0.070 (placenta overall; 2 hours significant by Bonferroni post hoc test) and P = 0.024 (serum); TNF-α, P = 0.0037 (placenta) and P = 0.015 (serum) (Figure 4, A and B). IL-1β also showed a significant overall increase in both serum and placenta for all of the time points combined, although post hoc tests did not show any single time point to be significantly increased in the placenta: IL-1β, P = 0.017 (placenta) and P < 0.0001 (serum) (two-way analysis of variance).
Figure 4.
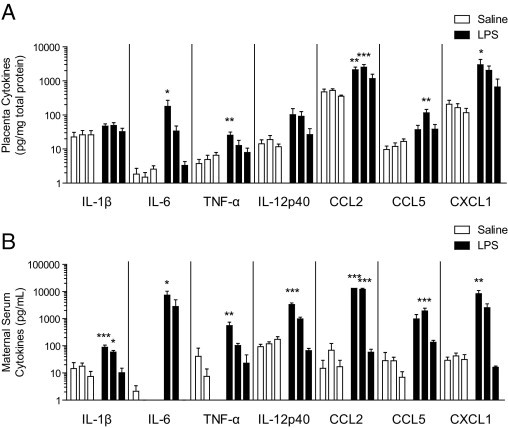
Cytokine levels in the placenta and maternal serum. Pregnant mice were injected with saline or LPS (60 μg/kg) at E12.5. Maternal serum and placentas were collected at 2, 6, or 24 hours after treatment and were analyzed for cytokine abundance using a Luminex bead array. Data are plotted as pg cytokine/mg total protein for placenta (A) or pg/mL of maternal serum (B), in order by time (2, 6, and 24 hours). A: Statistically significant effects of treatment in the placenta were examined by two-way analysis of variance as follows: IL-1β (P = 0.0167), IL-6 (P = 0.0702), TNF-α (P = 0.0037), IL-12 p40 (P = 0.0259), CXCL1 (P = 0.0076), CCL2 (P < 0.0001), and CCL5 (P = 0.0002). B: All seven molecules also showed statistically significant treatment effects in the maternal serum by two-way analysis of variance: IL-1β (P < 0.0001), IL-6 (P = 0.0238), TNF-α (P = 0.0146), IL-12 p40 (P < 0.0001), CXCL1 (P = 0.0043), CCL2 (P < 0.0001), and CCL5 P = 0.0008) (n = 4 to 6 serum, n = 6 to 8 placentas, from at least three pregnancies per time point for each group). Differences in individual time points were assessed by Bonferroni post hoc tests. *P < 0.05; **P < 0.01; ***P < 0.001. Data are reported as means ± SEM.
Additional cytokines and chemokines significantly elevated (two-way analysis of variance) were IL-12 subunit p40, CCL2 (MCP-1), CCL5 (RANTES), and CXCL1 (KC), as follows: IL-12 p40, P = 0.026 (placenta) and P < 0.0001 (serum); CCL2, P < 0.0001 (placenta) and P < 0.0001 (serum); CCL5, P = 0.0002 (placenta) and P < 0.0001 (serum); and CXCL1, P = 0.0076 (placenta) and P = 0.0043 (serum) (Figure 4). We detected the highest levels for most of these molecules at 2 hours, the exceptions being CCL5 in the placenta and serum and CCL2 in the placenta, which were highest at 6 hours after treatment. Other cytokines and chemokines assayed were not significantly increased in the placenta and serum (see Supplemental Table S1 at http://ajp.amjpathol.org).
Maternal TLR4 Is Necessary for the Placental Response to LPS
Because of the close connection between the placenta and maternal blood, we were unable to tell by the cytokine profiles whether the placenta or maternal immune system is directly responding to the LPS challenge. The placenta does express TLRs,28 so it is possible that there is direct recognition and response to the LPS in this organ. To determine whether placental TLR expression is sufficient to respond to LPS, we bred female mice that are TLR4 deficient with WT males, generating TLR4+/− fetuses in a TLR4−/− maternal environment, and evaluated placental pathology. The amount of tissue necrosis was quantified as a percentage of the spongiotrophoblast layer. Fetuses in the TLR4−/− environment exhibited no pathology after LPS treatment, demonstrating that maternal recognition is necessary for and precedes the vascular response, hemorrhage, and tissue necrosis within the placenta.
TNFR1 Is Necessary for Illness-Induced Fetal Loss, Placental Pathology, and Accompanying Effects on Fetal Perfusion and Neurogenesis
Of the cytokines increased by LPS in the placenta, TNF-α and IL-1β have been reported to have roles in placental pathology and miscarriage caused by infection.9,21–23 In our low-dose LPS model, we found the upregulation of TNF-α was more prominent than that of IL-1β, and we subsequently examined whether TNF-α signaling is necessary for the effects on placenta and fetus. TNF-α-null mice29 were highly protected from LPS-induced fetal loss (Figure 5B). This protection appeared to be mediated entirely by TNFR1, given that TNFR1−/− mice30 are protected from fetal loss but TNFR2−/−30 animals are fully susceptible (Figure 5B).
Figure 5.
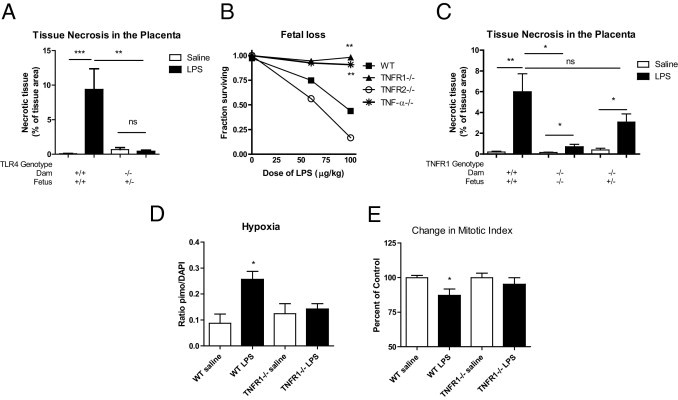
TLR4 and TNF-α signaling through TNFR1 is necessary for fetal loss and placental pathology. A: WT (C57BL/10J) females were bred with WT males to produce WT fetuses or TLR4−/− females were bred with WT males to produce TLR4+/− fetuses in at TLR4−/− maternal environment. Pregnant mice were injected with saline or LPS (60 μg/kg) at E12.5. Placentas were harvested at 24 hours after treatment and stained with H&E. The percent area of the spongiotrophoblast layer that had a necrotic, hypereosinophilic appearance was measured. WT mice showed an increase in necrosis in the spongiotrophoblast layer with LPS, but fetuses in a TLR4−/− environment did not (n = 6 to 9 placentas from at 2 to 3 pregnancies per group). P = 0.0002 by one-way analysis of variance. **P < 0.01; ***P < 0.001 by Bonferroni post hoc test. B: Pregnant mice were injected with saline or LPS at different doses at E12.5. Fetal loss in WT mice (C57BL/6) or mice deficient in TNF-α, TNFR1, or TNFR2 was assessed at 24 hours after treatment. TNF-α−/− and TNFR1−/−, but not TNFR2−/−, mice were protected from LPS-induced fetal loss (n = 4 to 7 saline, 6 to 11 LPS for all strains). There was a significant effect of strain (P < 0.0001), dose (P = 0.0002), and interaction (P = 0.0239) by two-way analysis of variance. **P < 0.01 versus WT by Bonferroni post hoc test. C: WT females were bred with WT males to produce WT fetuses, TNFR1−/− females were bred with TNFR1−/− males to produce TNFR1−/− fetuses, and TNFR1−/− females were bred with WT males to produce TNFR1+/− fetuses in a TNFR1−/− maternal environment. Pregnant mice were injected with saline or LPS (μg/kg) at E12.5. Placentas were harvested and analyzed as described for panel A. LPS-treated mice showed significant levels of necrotic tissue within the placenta in all genotypes: WT (**P = 0.0067; n = 14 saline, 18 LPS), TNFR1−/− (*P = 0.0279; n = 14 saline, 10 LPS), and TNFR1+/− (*P = 0.0183; n = 6 saline, 9 LPS) (two-tailed t-tests). A two-way analysis of variance revealed significant effects of strain (P = 0.0473), and treatment (P = 0.0033). TNFR1−/− LPS placentas were highly protected from pathology, compared with WT LPS placentas, but TNFR1+/− LPS placentas did not differ from WT LPS placentas (**P < 0.01, Bonferroni post hoc test; n = at least 6 placentas per group). D: Pregnant mice were treated with pimonidazole 15 minutes before saline or LPS (60 μg/kg) treatment. Two hours after saline or LPS, fetuses were harvested and pimonidazole staining was quantified as the proportion of pimo-positive pixels relative to total tissue area. Pimonidazole staining increased after LPS administration in WT but not in TNFR1−/− mice (P = 0.0067 by one-way analysis of variance; n = 6 to 9 brains from 2 to 3 pregnancies per group). *P < 0.05 versus WT saline by Bonferroni post hoc test. E: The number of pHH3+ metaphase cells was also scored and compared with saline controls for each strain. The density of pHH3+ metaphase cells was reduced in fetal brain tissues from pregnancies challenged with LPS in WT mice (*P = 0.028, two-tailed t-test; n = 12 saline, 18 LPS) but not in TNFR1−/− mice (P = 0.41; n = 9 saline, 9 LPS). Data are reported as means ± SEM.
We further examined whether loss of TNFR1 protected the placenta from hemorrhage and tissue necrosis. TNFR1−/− mice were protected from placental tissue necrosis caused by maternal immune activation (Figure 5C). To explore the role of TNF-α signaling in maternal versus placental compartments, we bred TNFR1−/− female mice with wild-type (WT) males to generate fetuses and placentas that are TNFR1 competent (TNFR1+/−) in a TNFR1−/− maternal environment. After maternal injection of LPS at E12.5, these placentas showed similar pathology to WT placentas (Figure 5C), indicating that TNF-α acting on TNFR1 within the placenta is sufficient for the TLR-evoked placental pathology and that TNFR1 signaling in the maternal compartment is not necessary for these effects.
To determine whether loss of TNFR1 is sufficient to prevent LPS-induced fetal hypoxia, pimonidazole adduct formation in the fetal cortex was compared between WT and TNFR1−/− mice (Figure 5D). In contrast to WT mice, TNFR1−/− mice showed no increase in pimonidazole staining, demonstrating that TNF-α signaling is necessary for the hypoxia experienced in the fetal brain after maternal LPS exposure. Similarly, TNFR1−/− mice are protected from the reduction in neural progenitor cell proliferation after LPS exposure seen in WT mice (Figure 5E).
Pharmacological Targeting of TNF-α Prevents Placental Pathology
To determine whether pharmacological attenuation of TNF-α signaling would protect the placenta, animals were administered a soluble TNFR2-IgG1 Fc fusion protein (TNFR2-IgG; marketed as Etanercept)25 and were injected with LPS. Fetal survival and placental pathology was assessed at 24 hours. TNFR2-IgG protected mice from fetal loss caused by LPS (Figure 6A), and the amount of tissue necrosis in the placenta was also significantly reduced (Figure 6B).
Figure 6.
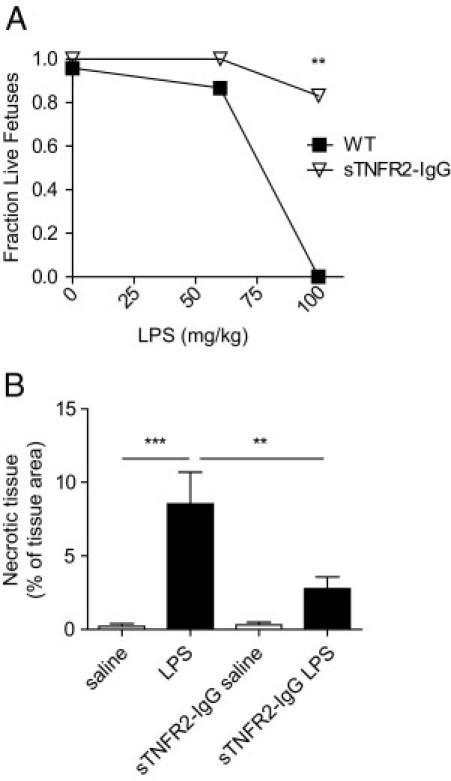
Blockade of TNF-α signaling prevents fetal loss and placental pathology. TNFR2-IgG (5 mg/kg) was administered intraperitoneally to pregnant mice at E12.5, 1 hour before treatment with saline or LPS. A: Fetal loss was assessed at 24 hours after treatment. TNFR2-IgG-treated mice were protected from LPS-induced fetal loss (P = 0.015 treatment effect by two-way analysis of variance; n = 4 to 6 pregnancies). **P < 0.01 versus WT by Bonferroni post hoc test. B: Placentas were harvested at 24 hours after saline or LPS (60 μg/kg) treatment at E12.5 and stained with H&E. The percent area of the spongiotrophoblast layer that had a necrotic, hypereosinophilic appearance was measured. WT LPS-treated mice showed an increase in necrotic tissue that was reduced by treatment with TNFR2-IgG (P = 0.001 by one-way analysis of variance; n = 6 to 10 placentas per group). **P < 0.01; ***P < 0.001 by Bonferroni post hoc test. Data are reported as means ± SEM.
Discussion
We have found in mice that mild inflammatory signaling at E12.5 is sufficient to elicit vasodilation, hemorrhage, and consequent tissue necrosis in the placenta. This model involves TLR4-mediated activation of an innate maternal immune response after maternal LPS administration. Doses of LPS that are sufficient to elicit a placental response and pathology are very low and do not evoke sickness behavior in the pregnant mouse, nor do they cause premature birth or intrauterine growth restriction. Previous studies have shown LPS can induce preterm birth; however, these tended to administer higher doses of LPS later in pregnancy.31,32 Similarly, intrauterine growth restriction has been associated with later, chronic treatment with LPS.22 In comparison, our model is an early and mild challenge, which causes transient placental vasodilation and hemorrhage that ultimately result in small tracts of necrosis and permanent tissue injury in the placenta. By E14.5, the placenta is much more resistant to immune challenge, implying that earlier times in pregnancy may be particularly relevant to illness-evoked alterations in fetal development.
The lesions in the placenta we observe at E12.5 are accompanied by impaired fetal perfusion and notable hypoxia in the fetal brain. This period of gestation in mice also corresponds to a developmental window during which the fetal brain is undergoing peak periods of neurogenesis.33 Concurrent with defects in placental function and hypoperfusion of the fetus, we find that the neural progenitor cell mitotic index is reduced in the developing cortex. This is consistent with prior work showing that hypoxia alone is sufficient to perturb neurodevelopment and to cause neurological disease.34 It is also consistent with the observation in humans that even mild infections or immune events, such as autoimmune episodes, allergy, or asthma in the first or second trimester have been linked to neurodevelopmental diseases in the child.35–37 Animal studies have further confirmed that immune activation of the mother is sufficient to induce long-term behavioral consequences in the offspring, in a variety of studies with different gestational times and stimuli.12–14,38,39 Although we have not confirmed that the transient changes observed in the present study are sufficient to permanently alter brain function, the data do suggest that an immune-mediated placental mechanism may be relevant to the epidemiological links between mild maternal illness in early to midgestation and neurodevelopmental diseases such as autism and schizophrenia.6–8
The effects of immune activation on the placenta in this model are initially mediated by maternal TLR4 signaling in response to LPS. LPS is produced by Gram-negative bacteria, and the model is therefore most relevant to the types of infections caused by exposure to these organisms in the environment. Given the sensitivity of the pregnancy at early times in gestation, even common exposures, such as the ingestion of contaminated food, may be relevant to pregnancy outcome. TLR4 signaling cascades and cytokine responses are analogous to those triggered by other pathogen-associated microbial products and respective TLRs,15,28 and the placental response observe in the present study could be more generalizable to viral and fungal infections (although this remains to be determined).
The TLRs may act in this model by signaling directly in placental tissues40 or through the action of circulating maternal cytokines that trigger the vascular changes within the placenta. Although TLRs are expressed in the placenta,28 we found that maternal TLR4 signaling is essential to the development of pathology in the placenta. Furthermore, we have found that loss of TNF-α signaling protects the placenta and also protects the fetal brain from hypoxia and from the accompanying decrease in progenitor cell proliferation. This is in accord with prior work showing that TNF-α is important in infection-induced fetal loss21 and is consistent with cytotoxic properties that would fit well with our present observations of tissue injury and cell death in the placenta. TNFR1 signaling in the mother is not required for these effects, because a TNFR1+/− placenta and fetus are still vulnerable in TNFR1−/− mothers. Together, these results suggest a model in which the maternal immune system is activated by an infectious stimulus and produces cytokines that act in the placenta to cause tissue damage. The consequent effects within the fetal brain do not necessarily require the infection to invade the uterus.
To our knowledge, this is the first report to differentiate effects of cytokine signaling on the overall maternal immune response versus specific signaling within the placenta. One study reported that an IL-1 receptor antagonist is able to rescue the effects of LPS on the placenta and adult motor function, using a chronic, high dose of LPS late in pregnancy (200 μg/kg every 12 hours from E18 to E20 in rats), but failed to determine whether IL-1 was acting directly in the placenta or whether the treatment affected the overall maternal immune response.23 In contrast, in our model, we saw little IL-1 induction, possibly because of the decreased dose or earlier gestational age. In a separate study, treatment with anti-IL-6 also prevented the altered behavior of offspring induced by E9 treatment with polyinosinic-polycytidylic acid, a TLR3 agonist, but similarly it is unclear whether IL-6 is acting in the placenta, fetus, or maternal immune system.41 It seems plausible that any method of attenuating the maternal innate cytokine response could be protective. To date, however, TNF-α is the only cytokine that has been confirmed to act directly in the placenta during the critical periods in early development when the placenta is most sensitive.
With clinical applications in mind, we have found that a clinically approved drug, TNFR2-IgG (marketed as Etanercept), is able to protect the placenta, even at these early stages in pregnancy when the placenta is unexpectedly sensitive to proinflammatory signaling. Etanercept is considered a class B drug during pregnancy by the US Food and Drug Administration. Human studies during pregnancy have been limited, but the drug appears to be relatively safe; however, there is some controversy as to whether the rates of some rare congenital defects are increased.42,43 In these studies, women were administered Etanercept throughout the course of their pregnancies, whereas a very limited course or even a single dose (as used here) may be sufficient to prevent or attenuate placental defects during infection. Etanercept has also been suggested as a potential therapy for recurrent spontaneous abortion and failure of in vitro fertilization, which can be caused by excessive inflammatory responses in the mother.44,45 Our results suggest that the use of TNF-α inhibitors in pregnancy might be protective against infection-induced pregnancy loss or other inflammatory disorders of the placenta. Furthermore, these drugs may be effective in preventing the consequent hypoxia and changes in fetal neurogenesis, and may therefore be germane to reducing or preventing alterations in neurodevelopment that contribute to later neuropsychiatric disorders in the child.
Footnotes
Supported by grants from the March of Dimes, Autism Speaks, and the Blume Foundation (T.D.P.); by fellowship support from the Lucile Packard Foundation and NIH F32 NS060427-01A1 and NIH 5T90DK070103-04 (P.A.C.); and by fellowship support from the Howard Hughes Medical Foundation (A.L.D.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.02.042.
Current address of A.L.D., Department of Pediatrics, University of Colorado, Denver, Colorado.
Supplementary data
References
- 1.Goldenberg R.L., Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 2.Michels T.C., Tiu A.Y. Second trimester pregnancy loss. Am Fam Physician. 2007;76:1341–1346. [PubMed] [Google Scholar]
- 3.Bergström S. Infection-related morbidities in the mother, fetus and neonate. J Nutr. 2003;133(5 Suppl 2):1656S–1660S. doi: 10.1093/jn/133.5.1656S. [DOI] [PubMed] [Google Scholar]
- 4.Redline R.W. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452–457. doi: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 5.McDonald D.G., Kelehan P., McMenamin J.B., Gorman W.A., Madden D., Tobbia I.N., Mooney E.E. Placental fetal thrombotic vasculopathy is associated with neonatal encephalopathy. Hum Pathol. 2004;35:875–880. doi: 10.1016/j.humpath.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Brown A.S., Schaefer C.A., Wyatt R.J., Goetz R., Begg M.D., Gorman J.M., Susser E.S. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26:287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 7.Wilkerson D.S., Volpe A.G., Dean R.S., Titus J.B. Perinatal complications as predictors of infantile autism. Int J Neurosci. 2002;112:1085–1098. doi: 10.1080/00207450290026076. [DOI] [PubMed] [Google Scholar]
- 8.Beversdorf D.Q., Manning S.E., Hillier A., Anderson S.L., Nordgren R.E., Walters S.E., Nagaraja H.N., Cooley W.C., Gaelic S.E., Bauman M.L. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- 9.Silen M.L., Firpo A., Morgello S., Lowry S.F., Francus T. Interleukin-1 alpha and tumor necrosis factor alpha cause placental injury in the rat. Am J Pathol. 1989;135:239–244. [PMC free article] [PubMed] [Google Scholar]
- 10.Gendron R.L., Nestel F.P., Lapp W.S., Baines M.G. Lipopolysaccharide-induced fetal resorption in mice is associated with the intrauterine production of tumour necrosis factor-alpha. J Reprod Fertil. 1990;90:395–402. doi: 10.1530/jrf.0.0900395. [DOI] [PubMed] [Google Scholar]
- 11.Pararas M.V., Skevaki C.L., Kafetzis D.A. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis. 2006;25:562–569. doi: 10.1007/s10096-006-0190-3. [DOI] [PubMed] [Google Scholar]
- 12.Shi L., Fatemi S.H., Sidwell R.W., Patterson P.H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golan H.M., Lev V., Hallak M., Sorokin Y., Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Hava G., Vered L., Yael M., Mordechai H., Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- 15.Uematsu S., Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 16.Dammann O., Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Zaretsky M.V., Alexander J.M., Byrd W., Bawdon R.E. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- 18.Ashdown H., Dumont Y., Ng M., Poole S., Boksa P., Luheshi G.N. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 19.Cai Z., Pan Z.L., Pang Y., Evans O.B., Rhodes P.G. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Urakubo A., Jarskog L.F., Lieberman J.A., Gilmore J.H. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 21.Silver R.M., Lohner W.S., Daynes R.A., Mitchell M.D., Branch D.W. Lipopolysaccharide-induced fetal death: the role of tumor-necrosis factor alpha. Biol Reprod. 1994;50:1108–1112. doi: 10.1095/biolreprod50.5.1108. [DOI] [PubMed] [Google Scholar]
- 22.Xu D.X., Chen Y.H., Wang H., Zhao L., Wang J.P., Wei W. Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol Lett. 2006;163:20–29. doi: 10.1016/j.toxlet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Girard S., Tremblay L., Lepage M., Sébire G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010;184:3997–4005. doi: 10.4049/jimmunol.0903349. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S. A decision between life and death during TNF-alpha-induced signaling. J Clin Immunol. 2002;22:185–194. doi: 10.1023/a:1016089607548. [DOI] [PubMed] [Google Scholar]
- 25.Peppel K., Crawford D., Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arteel G.E., Thurman R.G., Yates J.M., Raleigh J.A. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72:889–895. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ream M., Ray A.M., Chandra R., Chikaraishi D.M. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol. 2008;295:R583–R595. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahams V.M. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37:427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- 29.Pasparakis M., Alexopoulou L., Episkopou V., Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peschon J.J., Torrance D.S., Stocking K.L., Glaccum M.B., Otten C., Willis C.R., Charrier K., Morrissey P.J., Ware C.B., Mohler K.M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 31.Salminen A., Paananen R., Vuolteenaho R., Metsola J., Ojaniemi M., utio-Harmainen H., Hallman M. Maternal endotoxin-induced preterm birth in mice: fetal responses in Toll-like receptors, collectins, and cytokines. Pediatr Res. 2008;63:280–286. doi: 10.1203/PDR.0b013e318163a8b2. [DOI] [PubMed] [Google Scholar]
- 32.Mijovic J.E., Zakar T., Zaragoza D.B., Olson D.M. Tyrphostins inhibit lipopolysaccharide induced preterm labor in mice. J Perinat Med. 2002;30:297–300. doi: 10.1515/JPM.2002.043. [DOI] [PubMed] [Google Scholar]
- 33.Rice D., Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rees S., Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev. 2005;81:753–761. doi: 10.1016/j.earlhumdev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Ciaranello A.L., Ciaranello R.D. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- 36.Atladóttir H.O., Pedersen M.G., Thorsen P., Mortensen P.B., Deleuran B., Eaton W.W., Parner E.T. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- 37.Croen L.A., Grether J.K., Yoshida C.K., Odouli R., Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 38.Fortier M.E., Luheshi G.N., Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Meyer U., Nyffeler M., Engler A., Urwyler A., Schedlowski M., Knuesel I., Yee B.K., Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patni S., Wynen L.P., Seager A.L., Morgan G., White J.O., Thornton C.A. Expression and activity of Toll-like receptors 1–9 in the human term placenta and changes associated with labor at term. Biol Reprod. 2009;80:243–248. doi: 10.1095/biolreprod.108.069252. [DOI] [PubMed] [Google Scholar]
- 41.Smith S.E., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter J.D., Ladhani A., Ricca L.R., Valeriano J., Vasey F.B. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635–641. doi: 10.3899/jrheum.080545. [DOI] [PubMed] [Google Scholar]
- 43.Berthelot J.M., De Bandt M., Goupille P., Solau-Gervais E., Lioté F., Goeb V., Azaïs I., Martin A., Pallot-Prades B., Maugars Y., Mariette X., CRI (Club Rhumatismes et Inflammation) Exposition to anti-TNF drugs during pregnancy: outcome of 15 cases and review of the literature. Joint Bone Spine. 2009;76:28–34. doi: 10.1016/j.jbspin.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Clark D.A. Should anti-TNF-alpha therapy be offered to patients with infertility and recurrent spontaneous abortion? Am J Reprod Immunol. 2009;61:107–112. doi: 10.1111/j.1600-0897.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 45.Winger E.E., Reed J.L. Treatment with tumor necrosis factor inhibitors and intravenous immunoglobulin improves live birth rates in women with recurrent spontaneous abortion. Am J Reprod Immunol. 2008;60:8–16. doi: 10.1111/j.1600-0897.2008.00585.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


