Abstract
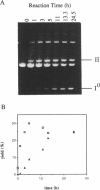
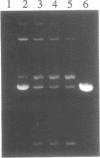
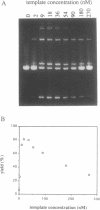
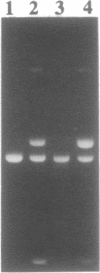
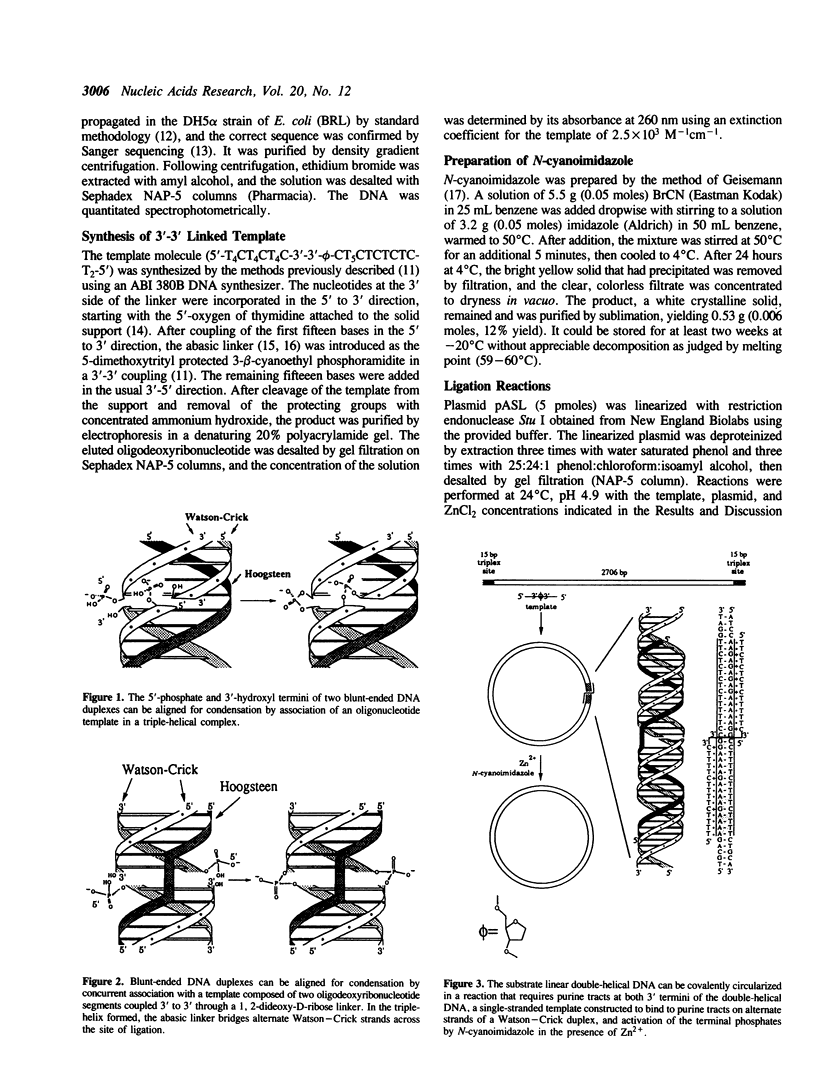
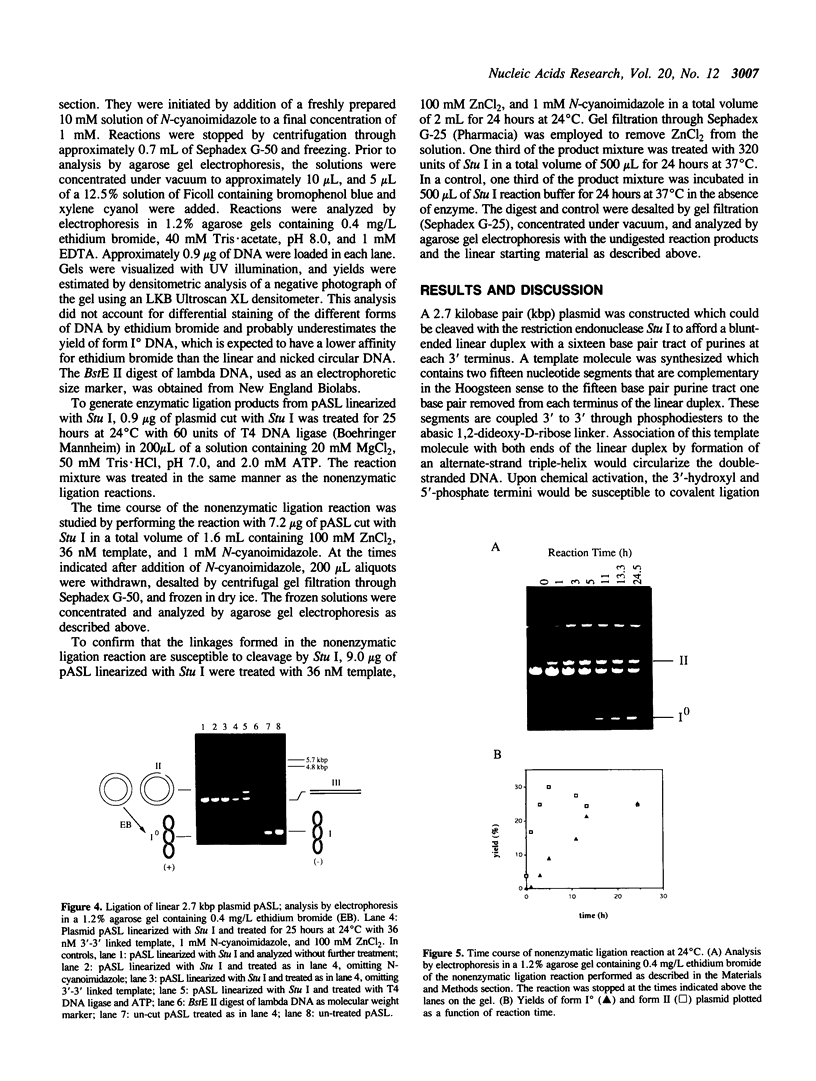
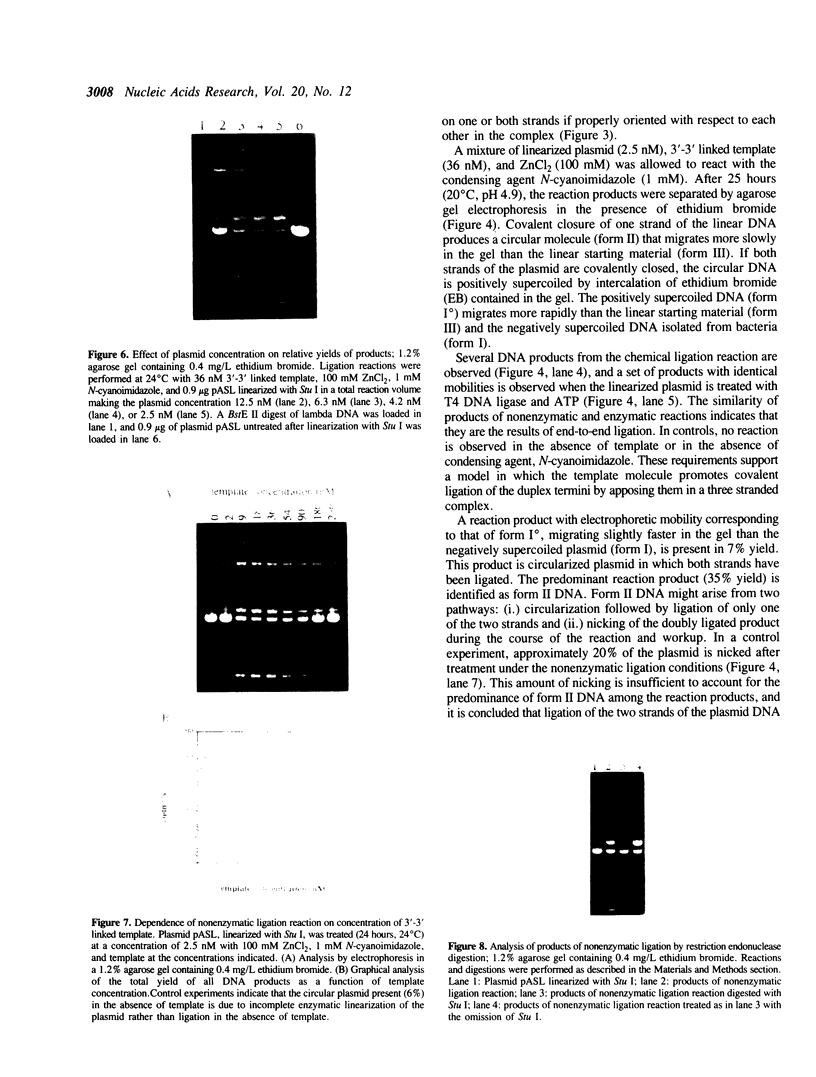
Nonenzymatic ligation of double-stranded DNA has been performed using an alternate-strand binding oligodeoxyribonucleotide template to juxtapose the duplex termini in a triple helical complex. The template associates with the duplex termini by Hoogsteen hydrogen bonding to alternate strands on opposite sides of the ligation site. Intermolecular and intramolecular ligation of linearized plasmid DNA are observed in the reaction, which depends on the template oligodeoxyribonucleotide and a condensing agent, N-cyanoimidazole. Intramolecular ligation products include those in which both strands are covalently closed in a circle. Ligation of the two strands is sequential and occurs at comparable rates for the first and second strands ligating. The covalent linkages formed in the reaction can be cleaved by the restriction endonuclease Stu I, supporting their identification as phosphodiesters.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferris J. P., Huang C. H., Hagan W. J., Jr N-cyanoimidazole and diimidazole imine: water-soluble condensing agents for the formation of the phosphodiester bond. Nucleosides Nucleotides. 1989;8(3):407–414. doi: 10.1080/07328318908054184. [DOI] [PubMed] [Google Scholar]
- Kanaya E., Yanagawa H. Template-directed polymerization of oligoadenylates using cyanogen bromide. Biochemistry. 1986 Nov 18;25(23):7423–7430. doi: 10.1021/bi00371a026. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Naylor R., Gilham P. T. Studies on some interactions and reactions of oligonucleotides in aqueous solution. Biochemistry. 1966 Aug;5(8):2722–2728. doi: 10.1021/bi00872a032. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal P., Feigon J. NMR studies of triple-strand formation from the homopurine-homopyrimidine deoxyribonucleotides d(GA)4 and d(TC)4. Biochemistry. 1989 Sep 19;28(19):7859–7870. doi: 10.1021/bi00445a048. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklenár V., Feigon J. Formation of a stable triplex from a single DNA strand. Nature. 1990 Jun 28;345(6278):836–838. doi: 10.1038/345836a0. [DOI] [PubMed] [Google Scholar]
- Sokolova N. I., Ashirbekova D. T., Dolinnaya N. G., Shabarova Z. A. Chemical reactions within DNA duplexes. Cyanogen bromide as an effective oligodeoxyribonucleotide coupling agent. FEBS Lett. 1988 May 9;232(1):153–155. doi: 10.1016/0014-5793(88)80406-x. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- de los Santos C., Rosen M., Patel D. NMR studies of DNA (R+)n.(Y-)n.(Y+)n triple helices in solution: imino and amino proton markers of T.A.T and C.G.C+ base-triple formation. Biochemistry. 1989 Sep 5;28(18):7282–7289. doi: 10.1021/bi00444a021. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Ramsing N. B., Germann M. W., Elhorst W., Kalisch B. W., von Kitzing E., Pon R. T., Clegg R. C., Jovin T. M. Parallel stranded DNA. Science. 1988 Jul 29;241(4865):551–557. doi: 10.1126/science.3399890. [DOI] [PubMed] [Google Scholar]