Abstract
Irritable bowel syndrome (IBS) is characterized by chronic, recurrent abdominal pain and altered bowel habits and is currently defined by symptom criteria and the absence of detectable organic disease. The underlying pathophysiology remains incompletely understood. Despite considerable efforts by the scientific community and the pharmaceutical industry to develop novel pharmacological treatments aimed at chronic visceral pain, the traditional approach to identifying and evaluating novel drugs for this target have largely failed to translate into effective IBS treatments. However, several novel drugs aimed at normalizing bowel movements have produced clinical effects, not only on the primary target, but also on pain and discomfort. While some of the commonly used experimental animal models for the pain dimension of IBS have some face and construct validity, the predictive validity of most of the models is either unknown, or has been disappointing. A reverse translational approach is proposed, which is based on identification and characterization of brain endophenotypes in patients, followed by translation of these endophenotypes for pharmacological studies in rodent models.
Keywords: functional gastrointestinal disorders, intestinal transit, neuroimaging, visceral pain
Functional gastrointestinal disorders (FGID), including irritable bowel syndrome (IBS), are complex, polygenic, symptom-based disorders that frequently overlap with other complex conditions, including persistent generalized pain disorders such as interstitial cystitis/painful bladder syndrome, fibromyalgia and psychiatric disorders, all sharing a poorly defined pathophysiology. Even though the pathophysiology of IBS in humans remains incompletely understood, various animal models have been proposed that claim to model either the entire disease process or cardinal features of the disorder (e.g., chronic visceral hyperalgesia, stress hyperresponsiveness, intestinal transit, altered fecal pellet output). For example, various interventions have been used to produce either acute or chronic visceral hyperalgesia in animals, as assessed by the response to an acute pain stimulus. The most commonly used interventions include acute and chronic visceral inflammation, mucosal irritation and various types of stressors, including perinatal stress and acute and chronic stress in the adult animal [1]. More recently, transgenic mouse models have been proposed (such as the serotonin transporter knockout mouse) that are thought to mimic some aspects of the human syndrome [2]. There are multiple ways to measure the outcome in a model, including physiologic and reflex responses (e.g., gastrointestinal [GI] transit time, frequency and consistency of stool, visceromotor reflex), spontaneous behaviors (licking, posturing and so on), operant behaviors (learned escape, place aversion, and so on), pain-directed complex behaviors (anxiety, attention, social avoidance, and so on), or brain responses (functional brain imaging). These various animal models have helped to identify an increasing number of possible molecular targets on visceral afferent neurons, epithelial cells, immune cells, enterochromaffin cells, enteric neurons and central stress circuits [3], which have been used to develop highly selective candidate compounds. If shown to be effective and safe in the animal models, these compounds are then tested in Phase I studies for their pharmacokinetics and safety, and in Phase IIa clinical trials for their ability to normalize visceral hypersensitivity or altered colonic function. Eventually, the assessment of symptoms and their improvement with therapeutic interventions in Phase III in patients depends on subjective patient reports, requiring a large number of patients in different participating centers. Even though this approach to drug development for FGIDs appears to be rational at first glance, it is expensive and has generally produced disappointing results. Similar frustrations have been experienced in drug development for other symptom-based disorders, such as chronic pain and many psychiatric conditions [4–6].
In this article, we will first critically review evidence related to the correlation between readouts for intestinal transit and visceral pain obtained in preclinical and clinical models, and the limited predictive validity of existing models for IBS drug effectiveness. We will then focus on experimental models for visceral pain, propose a reverse translational strategy and address the potential benefit of new rodent models using functional brain imaging.
Transit time as a readout in experimental human & animal models
The measurement of gut transit is a clinically relevant readout to assess GI function primarily related to motility and secretion [7,8]. Although the identification of IBS subgroups (IBS diarrhea predominant or constipation predominant) is not based on gut transit measurements, there is some correlation between transit times and predominant bowel habit [9,10]. However, similar to visceral sensitivity testing assessed by barostat, transit time does not appear to be a strong predictor of overall IBS symptom severity [11]. Similarly, symptoms associated with stool frequency or ease of stool passage have been shown to be poor predictors of IBS symptoms or health-related quality of life (HRQoL) measures [11]. Thus, gut transit is a good surrogate marker for stool form and, therefore, may be a useful tool to evaluate drugs that affect primarily bowel habit in IBS (in particular in the subset of patients with demonstrated abnormalities in GI transit), but is not a satisfactory surrogate marker for overall IBS severity, abdominal pain and HRQoL.
Changes in GI transit or fecal pellet output in rodent models are often observed in response to stressors, and many drug effects (e.g., corticotropin-releasing factor 1 [CRF1] receptor antagonist, neurokinin 1 [NK1] receptor antagonist) have been evaluated on stress-induced acceleration of transit or increased fecal pellet output [12]. By contrast, in the majority of human transit studies, compounds have been evaluated in healthy control subjects, or in IBS patients in the absence of any acute stressor. This is important, as drugs aimed at stress-induced changes of GI function have consistently failed to show effects on baseline measures in the rodent models [12].
In summary, while there is a poor correlation between transit and symptoms, preclinical models have been relatively successful at translating objective GI transit measurements between animals and humans [8]. It is important to emphasize that despite the limited correlation between symptoms and GI transit in humans, several recently developed drugs aimed at accelerating intestinal transit and/or at normalizing bowel movements (including tegaserod, lubiprostone and linaclotide) have shown beneficial effects on abdominal pain/discomfort in Phase III clinical trials. Based on these findings, one may speculate that at least part of the pain/discomfort reported by IBS patients is not related to a primary visceral hypersensitivity, but may be secondary to discomfort and symptom-related anxiety associated with unsatisfactory bowel movements.
Visceral sensitivity in experimental human models
Perceptual responses to mechanical (and to a much lesser degree electrical or chemical) stimulation are common measures of visceral sensitivity in clinical studies, and mechanical rectosigmoid hypersensitivity has been referred to as a ‘biological’ marker of IBS [13], showing relative specificity to the IBS patient population. Results from a large number of studies comparing groups of IBS patients with healthy controls indicate that graded barostat-mediated distension is a reliable and valid approach for testing perception of visceral sensation and changes in perception to an acute aversive stimulus. However, to be useful as a biomarker or surrogate marker for IBS, abnormal visceral testing results (‘visceral hypersensitivity’) should be observed in all patients, should be syndrome specific and should be helpful in discriminating medications that do or do not have a positive impact on either specific or global IBS symptoms. Regrettably, there is no evidence for a strong correlation between this acutely evoked response and the presence and severity of spontaneous abdominal pain or global IBS symptoms. In fact, some drugs that produce a positive change on visceral sensitivity testing in human barostat studies fail to show beneficial effects on spontaneous IBS symptoms and vice versa (Table 1) [14–38]. For example, while the κ-opioid antagonist fedotozine or the synthetic somatostatin analogue octreotide have shown significant beneficial effects in human barostat studies, the positive impact of this class of compounds on IBS symptoms could not be verified in Phase II clinical trials. On the other hand, other compounds targeting the serotonergic system showed no clear effect on the perception of visceral stimuli in clinical experimental studies, but were found to have positive effects on global IBS symptoms.
Table 1.
Drug effects on visceral sensitivity in irritable bowel syndrome.
| Drug | Dose | IBS study subjects |
Study design | Barostat methodology |
Effect on Sensitivity in IBS |
Effect on motor function |
Effective on IBS symptoms |
Conclusions from Phase II & III trials |
|---|---|---|---|---|---|---|---|---|
| Opiates | ||||||||
| Fentanyl [14] | Two doses (HD, LD), bolus + infusion |
10 IBS (6 F) | DB, PC, crossover |
Phasic pressure | HD and LD: ↑ DiscTh, ↑ SensTh HD: ↑ PainTh |
None | NR | NR |
| Fedotozine −κ agonist [15] |
100 mg single dose |
14 IBS (8 F) | PC, crossover | Phasic pressure | ↑ PainTh, ↑ SensTh | None | NR | Small but significant change in pain in small trial [16] |
| Asimodoline −κ agonist [17] |
0.5 mg single dose | 20 IBS (20 F), all hypersensitive |
DB, PC, crossover |
Phasic pressure | No change in PainTh, ↓ sensory ratings |
None | NR | NR |
| 5-HT | ||||||||
| Alosetron [18] | Either 0.25 mg b.i.d. or 4 mg b.i.d. × 7 days |
22 IBS (9 F) | PC, parallel group study |
Phasic pressure | No effect on PainTh (pressure) |
↑ compliance | NA | Effective for global symptom relief [19] |
| Alosetron [20] | Either 1 mg alosetron or 4 mg alosetron b.i.d. × 4 weeks |
25 IBS (19 F) | PC, parallel group study |
Phasic pressure | No effect on rectal sensory scores |
↑ rectal compliance |
NA | NR |
| Ondansetron [21] | One dose at 0.15 mg/kg |
12 IBS (6 F) | DB, PC, parallel group study |
Phasic pressure | No effect on sensitivity |
↑ rectal compliance |
NA | No effect in pilot study [22] |
| Ondansetron [23] | 16 mg 3 times/day | 6 IBS | PC, crossover | Ramp volume and electrical stimulation |
↑ SensTh | None | ↓ number of episodes of abdominal pain |
NR |
| Ondansetron [24] | Single dose of 0.15 mg/kg |
5 IBS (3 F) | PC, parallel group study |
Phasic pressure | No change in sensitivity |
None | NR | NA |
| Granisetron [25] | Either 40 ug/kg or 160 µg/kg |
12 IBS (8 F) | DP, PC, crossover |
Ramp volume | ↑ DiscTh, ↑ UrgeTh | None | Participants noted constipation |
NR |
| Tegaserod [26] | 6 mg b.i.d. | 49 IBS (49 F | DB, PC, parallel group study |
Phasic sigmoid pressure |
No effect | ↑ sigmoid accommodation |
No symptom change |
Effective for global symptoms and constipation [27] |
| Anitridepressants | ||||||||
| Amitriptyline [28] | 10 mg hours × 2 weeks and then 25 mg hours for the following 4 weeks |
12 IBS (7 F) | Parallel group study? |
Phasic pressure | ↑ PainTh | None | Symptom reduction |
Some effectiveness in meta-analysis and in high-quality clinical trial [29] |
| Fluoxetine [30] | 20 mg/day × 6 weeks |
40 IBS | DB, PC, parallel group study |
Phasic pressure and volume ramp |
No change in sensitivity. ↓ abdominal pain in hypersensitivity patients |
None | ↓ number of patients reporting significant abdominal of pain |
May be effective. Positive effect on pain in small trial [30] |
| Other CNS | ||||||||
| Gabapentin [31] | 600 mg/day × 5 days |
43 IBS-D (no sex data) |
DB, PC, parallel group study |
Phasic pressure | ↑ PainTh, DiscTh | ↑ compliance | NR | NR |
| Pregabalin [32] | 50–200 mg t.i.d. titration over 21 days |
26 IBS (19 F) | DB, PC, parallel group study |
Ramp volume | ↑ PainTh | ↑ rectal compliance |
Nonsignificant ↓ in abdominal pain |
NR |
| Octreotide [33] | 1.25 µg/kg(s.c.) | 10 IBS | DB, PC, crossover |
Phasic pressure | ↑ PainTh, DiscTh | None | NR | Preliminary evidence suggests some effectiveness on IBS symptoms [34] |
| Octreotide [35] | 100 µg (s.c.) | 8 IBS-D | DB, PC, crossover |
Ramp volume | ↑ tolerance | ↑ compliance | NR | NR |
| Octreotide [36] | 0.175–0.820 µg/kg bolus followed by 0.41–1.5 µg/min (i.v.) infusion OR 1.5 µg/kg (s.c.) |
7 IBS (4 F) 9 IBS (9 F) |
DB, PC, crossover (2 studies) |
Phasic pressure alone and after sigmoid stimulation |
↑ DiscTh, ↓ sensitization from sigmoid stimulation |
None | NR | NR |
| Talnetant [37] | 25 and 100 mg | 102 healthy controls (60 F) |
DB, PC, parallel group study |
Phasic rectal pressure |
No effect | No effect | NA | Not different from placebo [38] |
The table highlights the inconsistent correlation between results of visceral sensitivity testing in experimental barostat studies and spontaneous IBS symptoms.
↑: Increased: ↓. Decreased: 5-HT: Serotonin: b.i.d.: Twice a day: DB: Double-blind: DiscTh: Discomfort threshold: F: Female: HD: High dose: IBS: Irritable bowel syndrome: IBS-D: Diarrhea-predominant irritable bowel syndrome: iv.: Intravenous: LD: Low dose: M: Male: NA: Not applicable: NR: Not reported: PainTh: Pain threshold: PC: Placebo-controlled: s.c: Subcutaneous: SensTh: Sensory threshold: t..i.d.: Three-times a day: UrgeTh: Urgency threshold.
In summary, these findings illustrate that while acute visceral perception testing procedures have been used in a wide range of preclinical and clinical studies to evaluate the potential benefits of candidate drugs as visceral analgesics/antihyperalgesics, and as potential medication for treating IBS symptoms, the weak predictability in discriminating compounds that may have a positive impact on either specific or global IBS symptoms suggests that these human tests may not be suitable as cost-effective drug development strategies for IBS.
Visceral sensitivity in animal models
Reflexive or behavioral nociceptive responses to acute colo-rectal distension (CRD) have become the standard readout for the assessment of visceral sensitivity in rodents, and the visceromotor response to distension is the most commonly used index of visceral pain response in rats [39]. The popularity of this measure is related to the fact that it has been assumed to be homologous to the subjective response to colorectal distension in humans, to the relative ease to perform and automate it, and the reproducibility across laboratories. However, based on the lack of a consistent clinical viscero-analgesic effect of a series of compounds that initially showed robust analgesic and anti-hyperalgesic effects in experimental animal models, predictive validity of these animal models has proven to be disappointing (Table 2) [14–16,18,19,33,36,40–80,201]. For example, fedotozine, which showed robust visceroanalgesic and antihyperalgesic effects in several animal models, failed to show positive effect on visceral sensitivity testing in humans. Similarly, alosetron (a 5HT3 receptor antagonist) and tegaserod (a 5HT4 receptor agonist and 5HT2b antagonist) exhibited visceral antihyperalgesic effects in preclinical testing using the model of CRD in sensitized animals, but produced no change in visceral sensitivity in human visceral pain testings. Pregabalin, an anticonvulsant and an α(2)δ ligand used in the treatment of seizures and pain syndromes, showed equivocal results on visceral sensitivity in rodent models [81,82], but was shown to normalize the increased perception threshold to rectal distension in IBS patients with rectal hypersensitivity [32].
Table 2.
Effect of irritable bowel syndrome candidate compounds on preclinical (rodents) and clinical readouts of altered visceral sensitivity and gastrointestinal transit.
| Receptor targeted |
Compound | Preclinical | Clinical | |||||
|---|---|---|---|---|---|---|---|---|
| Motility |
Visceral analgesial anti-hyperalgesia |
Anxiety | Transit | Perception |
Brain imaging for visceral pain |
IBS symptoms (Phase II or III) |
||
| κ1-opioid | Fedotozine (agonist) |
↑ transit after ileus induced by laparotomy or irritation [42,43] |
Reduced visceral hypersensitivity in a model of colonic irritation [44,45] Antinociceptive effect on duodenal pain reflexes in rats [46] |
NR | NR | ↓ gastric sensitivity to to distension in healthy humans [47] Relieved hypersensitivity CRD in IBS patients [15] |
NR | Relief of abdominal pain and bloating in IBS patients compared with control. Effect on transit not reported [16] |
| μ-opioid | Fentanyl (agonist) |
↓ Gl transit [48–50] |
Prevented the sensitizing response associated to repetitive CRD in mice [51,52] |
Fentanyl attenuated fear-potentiated startle in rats [53] Anxiolytic effect of central μ-opioid agonist on pain-induced anxiety [54] |
Slowed Gl transit [55] |
Attenuated the perception of phasic rectal distension in IBS patients [14] |
NR | NR |
| 5-HT3 | Alosetron (antagonist) |
Reduction of colonic motility [56] |
Centrally mediated visceral anti- hyperalgesic effect [57,58] |
NR | Reduction of Gl transit [59] |
↑ colonic compliance Lack of true visceroanalgesic effect [18] |
Changes in central modulation of gut function and pain [60] |
Global improvement of symptoms in male and female patients with IBS-D [19] |
| 5-HT4, 5-HT2b | Tegaserod (5-HT4agonist, 5-HT2b antagonist) |
Enhanced Gl motor function [61] |
Reduction in visceral sensitivity [62,63] |
NR | Acceleration of Gl transit [64] |
Generally no evidence for visceroanalgesic effect [65,66] |
Modulation of central processing of visceral afferent information [67] |
Effective in the treatment of constipation- predominant IBS symptoms [68] |
| 5HT1A | Robalzotan tartrate monohydrate |
Inhibition of micturition [69] |
Reduction of the visceromotor response to CRD but no change in the pseudoaffective cardiovascular autonomic response [69] |
NR | No evidence for changes in bowel movements [70] |
No evidence for changes in abdominal discomfort or pain [70] |
NR | Not more effective than placebo in providing adequate relief from IBS symptoms [70] |
| Somatostatin | Octreotide (agonist) |
Reduction of Gl transit time [71] |
Visceroanalgesic effect [72] |
NR | Reduction of Gl transit time [73] |
Visceroanalgesic & antihyperalgesic effect during rectal distension [33,36,74–76] |
NR | Overall symptom improvement [77] |
| CCK1 | Dexloxiglumide (antagonists) |
Accelerates transit time [78] |
↓ sensitivity to CRD in rats with inflamed colon [79] |
NR | Accelerates transit time [80 |
NR | NR | NR: therapeutic effect not confirmed [201] |
| NK3 | Talnetant (antagonist) |
Inhibits motility, reduces excitatory reflex induced by stretch in the colon [81] |
Anti-hyperalgesic effect [81] |
Anxiolytic effect [82] |
NR | Under evaluation (unpublished) |
NR | NR |
↑: Increased; ↓: Decreased; 5-HT: Serotonin; CRD: Colorectal distension; Gl: Gastrointestinal; IBS: Irritable bowel syndrome; IBS-D: Diarrhea-predominant irritable bowel syndrome; NR: Not reported
However, it is important to emphasize that some of the compounds that showed positive effects on visceral hypersensitivity in rodents but failed to show visceral analgesic effects in human testing did produce positive results on IBS symptoms, presumably via mechanisms other than visceral analgesia. This demonstrates the limitations of the conventional approach of trying to translate between rodent and human pain studies.
The role of endophenotypes in bench-to-bedside translation
While the face validity (i.e., how well does a model mimic clinical features of IBS patients) of some rodent models of visceral pain has been good, the predictive validity (i.e., how well do drug studies performed in the model predict effectiveness in humans) has been disappointing. For example, adult rats having been exposed to the maternal-separation paradigm as pups show evidence for stress-induced fecal pellet output, stress-induced visceral hyperalgesia and anxiety-like behavior, all findings homologous to those reported in IBS patients [83]. However, several drugs that showed effectiveness in this model (e.g., antagonists for the CRF1 or NK1 receptors) have failed to show effectiveness in human models or in clinical trials. The problem of bench-to-bedside translation, however, does not simply originate in a failure of the animal models. Improved definition and classification of clinical states based on biological abnormalities are needed. Such improvement is dependent on the identification of robust endophenotypes in humans with adequate effect sizes for cardinal symptoms or global end points, which can be modeled in a transverse translational approach in rodents.
We propose a novel reverse translational approach, which begins with the identification and in-depth characterization of neurobiological endophenotypes in IBS patients or subsets of such patients. This approach does not aim to identify a rodent model of a complex, unique human disorder, but aims to use the rodent model to pharmacologically characterize the homologue of an endophenotype that has previously been characterized in patients. In contrast to biomarkers, which are thought to be specific for a particular disorder, endophenotypes are dimensional constructs that play a role across categorical disease definitions [84,85]. For example, in the case of IBS, the endophenotype of enhanced responsiveness of a stress and emotional arousal circuit is likely to be found in anxiety disorders, and in other stress-sensitive disorders. Similarly, the endophenotype of ineffective cerebral cortico-limbic inhibition is likely to be found in many, often overlapping disorders characterized by physical or emotional discomfort. It has been suggested that clusters of endophenotypes may be more similar among subsets of patients with different disorders, rather than being seen in all patients of a given disorder [86]. Rodent models of such endophenotypes are important to identify potential molecular targets, dose ranging and possible side-effect profiles of candidate compounds. As implied by the endophenotype concept, successful drug development based on this approach would be expected to be useful for subsets of patients with different syndromes, but not necessarily for all patients of a given syndrome.
Given the high incidence of mood and anxiety symptoms in IBS patients, as well as the growing acceptance of the importance of central pain amplification in the pathophysiology of IBS [87,88], the development of rodent homologues of such brain endophenotypes should be important. However, the question remains, what level of inquiry of the involved brain endophenotypes in humans is most promising for the preclinical assessment of candidate IBS drugs? A behavioral change, while capturing a broad spectrum of dysfunction, may lack specificity, while a neuromolecular change may not generalize across a disease that is likely multifactorial in origin and that is characterized by subtypes with overlapping symptoms. Pseudoaffective responses (e.g., electromyography, pain behavior) themselves do not allow for the elucidation of the underlying systems-level processes by which molecular, cellular and genetic profiles bias behavior and nociception. Neuroimaging can complement such association studies by identifying the biological effects of a compound at the brain endophenotype level, such as the level of integrated neural systems and circuits.
The utility of neuroimaging in CNS drug development
Our understanding of the functional and structural reorganization of the brain in response to chronic pain, and how the brain responds to pharmacological treatment, has been significantly changed as a result of developments in neuroimaging of the CNS. The key findings of these studies can be summarized as follows:
In several chronic pain conditions, regional changes in gray matter density have been demonstrated with anatomical imaging. Even though the underlying neuroanatomical changes remain to be determined, these findings have great potential to function as endophenotypes for persistent pain conditions, or even as biomarkers for individual syndromes;
Alterations in brain state and response of the brain circuits to drugs have been demonstrated with PET and functional MRI (fMRI);
Changes in neurotransmitter levels (glutamate, aspartate, glycine and γ-amino butyric acid [GABA]) have been shown with magnetic resonance spectroscopy (MRS) [89].
Borsook et al. [89] have insightfully described how the use of fMRI, in particular, may help speed drug development for CNS indications at a number of levels that include:
Evaluation of differential efficacy of drugs within and across pharmacological subtypes;
Identification of potential for CNS side effects;
Opportunities to define drug dosing and benefits of drug combinations;
Potential for surrogate models using healthy subjects for drug evaluation;
Setting up a potential method for re-evaluating failed drug candidates;
An objective method to select and stratify patient populations to enable pro of-of-concept clinical investigations [90].
The potential applications of neuroimaging provide many opportunities for bidirectional translation between humans and rodents, which may help speed drug development for chronic visceral pain states, including IBS. An underlying assumption in this proposition is that pharmacologic subtypes and/or side effect profiles show specific brain mapping ‘signatures’ that are similar in humans and animals.
One of the potential strengths of integrating neuroimaging during the drug development process is its potential to translate findings of alterations in neural circuits across species, enabling a more focused use of animal models in research. Neuroimaging may also serve as a useful proxy measure of pain responses that, in the animal, cannot be elicited verbally. While pain imaging of the CNS has been extensively explored in human subjects, neuroimaging technologies applied to rodents to study endophenotypes of persistent pain in animals is still in its infancy. A significant gap remains in ‘bedside-to-bench’ translation of well-studied brain-mapping abnormalities in IBS patients (reviewed in [91]).
The choice of imaging modalities in animal models
While structural imaging has the potential to greatly increase our understanding of the functional neuroanatomy of chronic pain conditions, the current lack of understanding of the mechanisms underlying such structural changes, and the temporal characteristics of these changes, makes it currently impractical to use such end points for drug development in rodents. In this regard, functional brain mapping and chemical imaging may represent more suitable approaches. Ideally, such imaging in animals would be performed under conditions that approximate those used in human subjects – that is, nonsedated animals with minimal interference with the subject’s natural behavior. At the same time, the ideal imaging modality would optimize spatial and temporal resolution, allow for serial measurements across time, while providing 3D views of brain function. No method simultaneously meets all these criteria.
Past research on brain responses to noxious visceral stimulation in animals has relied predominantly on the measurement of early response genes, in particular c-Fos expression [92–95], with a broad variability reported between laboratories [92,96,97]. Unlike human imaging studies evaluating brain responses during acute CRD, c-Fos studies typically use prolonged exposure (>30 min) to high-intensity visceral stimuli, which may lead to the integration of a variety of nonspecific behaviors over the duration of pain exposure, including acute sensitization of the visceral afferent system. Furthermore, analysis is often limited to a few selected brain regions, lacking the whole-brain level analysis achieved in human studies. Thus, it is not surprising that translation of findings between human and animal brain mapping has been diffcult. It is noteworthy that studies examining increases in c-Fos expression in response to CRD in the lumbosacral region of the spinal cord have reported more consistent results within animals [92,98–100], but parallels to human imaging have not been explored extensively. Other region-specific analyses of neuronal responses to CRD have been carried out using in vivo electrophysiological recording [101,102], and such brain electrical recordings may prove useful once specific brain regions of vulnerability have been determined. Spatial resolution, with microPET and advanced image reconstruction software, remains at best approximately 1.2 mm at the center of the field of view. This represents approximately 7% and 13% of the width of the rat and mouse brain, respectively, and is poorly suited for the detection for all but the broadest changes in regional cerebral blood flow (rCBF) or metabolism in rodent models. In specific instances, however, such broad changes may suffice for the testing of specific compounds, as has been demonstrated for opiates [103]. Functional MRI and single photon emission computed tomography (SPECT, nanoSPECT), though they provide whole brain analysis and adequate temporal and/or spatial resolution, require sedation of the animal, limiting the types of brain responses that can be examined [97]. We have advocated in the past the use of autoradiographic methods of perfusion mapping, as this method can be applied in awake, nonrestrained animals and yield information at the circuit level across the entire brain, with a spatial resolution (~100 µm) appropriate for the rat or mouse models, and a temporal resolution (seconds–minutes) sufficient for capturing acute brain changes. Nevertheless, autoradiographic methods, although they provide 3D spatial information, contain no information about dynamic cerebral changes. Therefore, studies of disease progression or response to treatment using intra-animal comparisons cannot be performed. In addition, because of the need for extensive cryosectioning of the brain, the method lends itself less well to high-throughput screening than perhaps MRI, nanoSPECT or microPET.
Homology between the rodent & human brain
Despite their differences, remarkable similarities exist between normal rats and humans at the level of brain anatomy, neurotrans-mitters and their respective receptors, and nociceptive processing. Our own work examining functional activation during acute noxious visceral stimuli in rats and humans has shown that many of the sensory, limbic and paralimbic brain regions that show significant changes in the rodent are analogous to those reported in normal human subjects [104,105]. Nevertheless, differences in neuroanatomy have been reported. Rats and mice appear to lack the lamina I spinothalamocortical pathway to the dorsal posterior insula by way of the posterior part of the ventromedial nucleus, as well as a pathway from lamina I to medial dorsal thalamus to the anterior cingulate cortex [106]. In addition, pain is a multifaceted problem, with pain perception engaging not only sensory and motor processes, but also emotional and cognitive ones. To what extent the emotional and cognitive components of human pain perception can be modeled in the animal remains open to debate. That emotional and cognitive input can modulate pain perception in the rat has been suggested by the accentuation of pain responses by acute and chronic stress [107,108]. Furthermore, work in the rat has shown that the opioid system of the anterior cingulate, a region thought to be involved in the affective–motivational dimension of pain, may selectively process the aversive quality of noxious mechanical stimulation, with little effect on the physical paw withdrawal [109]. Though indeed ‘rats are not monkeys are not humans’ [106], the use of select functional neuroimaging end points in animals may provide an improved means for the bidirectional translation of endophenotypes relevant to persistent pain between animal models and human disease conditions. Reverse translation of some of these endophenotypes into rodents may provide an important tool for evaluating pharmacological effects of candidate compounds, which cannot be performed in humans.
Brain imaging of noxious visceral stimulation in normal human subjects & animals
In human subjects, increases in rCBF to noxious somatosensory stimuli are consistently observed in the insula, in secondary somatosensory cortex (S2), in the anterior cingulate cortex (ACC), and with slightly less consistency in the contralateral thalamus and in primary somatic areas (S1) [110]. Studies of noxious visceral stimulation in normal human subjects (typically acute CRD) have also identified the insular cortex as the single most consistently activated brain region, with the posterior insula being a primary projection area for visceral afferent information, while mid- and anterior insula subregions are considered higher association areas for these bodily signals, where they are integrated with affective and cognitive inputs. A majority of studies have also reported activation in response to CRD of the dorsal ACC, S1/S2, prefrontal cortical regions and, to a lesser extent, posterior parietal cortex and thalamus (reviewed in [91]).
Functional brain mapping in normal rats during acute CRD has also clearly identified the insula as a region that is activated [105]. Using perfusion mapping of the brain, we found greater activation in the anterior insula in male than in female rats, similar to what had previously been reported in healthy human subjects [111], as well as in IBS patients [112,113]. Significant differences in response to CRD were also noted in the cingulate and somatosensory cortex and thalamus, although sex differences remain. Of note, in the insula and dorsal ACC of the animals, changes in rCBF showed a positive correlation with both electromyographic (EMG) and behavioral pain scores [114]. The clusters showing significant correlations with either EMG or pain score were noticeably smaller than those showing group differences in rCBF within a given region. Furthermore, not all brain regions that showed group differences correlated with EMG or pain score. This suggests that EMG and behavioral measures are likely to reflect only part of the animal’s response to the noxious visceral stimulus.
Activation of the insula was also noted during re-exposure to an environmental context previously paired with acute, noxious visceral stimulation [115]. In this study, the insula was activated in rats during avoidance of stepping down from a platform, a behavior that previously had been conditioned over 2 days (18 trials/day) to acute CRD. This suggests the importance of fear-conditioned responses in cerebral nociceptive circuits that persist independently of actual visceral stimulation. Similar insular activation relative to controls has also been reported in IBS subjects during anticipatory fear of CRD [116].
Relevant known alterations of brain circuits in IBS subjects that need to be examined in animal models of FGIDs include:
A hyperresponsiveness of the homeostatic afferent network to distension;
A hyperresponsiveness of the emotional arousal network during expectation;
Compromised engagement of cortico–limbic–pontine systems during delivery of aversive visceral stimuli [91].
With regards to the emotional arousal network (locus coeruleus complex, amygdala, infralimbic [‘infragenual’] and ventral cingulate cortex [‘supragenual’]), we have found a hyperresponsiveness of this network during acute CRD in female compared with male rats [105]. Also noted was a diminished response in females compared with males of cortical circuits that modulate activity in limbic and paralimbic areas. These results highlight the importance of sex as a factor in defining an animal model, and that female rats may model aspects of the functional brain response observed in IBS subjects (a majority of whom are women) more closely than male rats. Recent findings also point to the importance of strain differences in both the visceromotor response, as well as in the extent of prefrontal cortical activation [117,118].
Animal models typically do not take into account the biopsychosocial and environmental interactions that represent major components of the patient’s pain complaints and responses to treatment. Based on the understanding that environmental infuences affect pain behavior in FGIDs, a strong clinical argument can be made for inclusion of measures of visceral hyperalgesia, in particular stress-induced visceral hyperalgesia. Visceral hyperalgesia is accentuated during periods of acute and chronic stress in IBS subjects, as well as in rodent models [108]; there is an increased incidence of life stressors in IBS subjects compared with controls [119–122]; and IBS subjects show an exaggerated stress hormone response and visceral perceptual alterations compared with controls [123–125]. It has been proposed that such increased history of early life stress, and alterations in the stress response, may trigger not only long-term changes in cognitive processing and mood, but also in visceral functions and visceral sensitivity to noxious stimuli [124,126,127]. Results from our laboratory in the rat model suggest that stress-induced visceral hyperalgesia is accompanied by exaggerated insula activation during CRD [128]. These three observations in the insula – activation during acute CRD, during recall of fear-conditioned CRD and in association with a stress-related model of visceral hyperalgesia – suggest that this brain region, and associated networks, is a promising candidate for an improved animal-to-human translation during drug development, despite well-known neuroanatomical differences between the human and the rodent insula. Possibilities exist in the future for applying connectivity analysis to animal brain mapping to understand cerebral responses at the network level [129], as has been begun to be undertaken in IBS human subjects [130].
Expert commentary
While animal model-based drug development efforts for diseases, such as cancer or inflammatory bowel disease, have shown significant promise, similar efforts in complex, symptom-based disorders, such as IBS, using rodent models have generally been disappointing. Traditional drug development for FGIDs has been partially successful in the development of novel treatments aimed at modulating biological targets such as slow transit or alterations in intestinal secretion. A substantial number of compounds aimed at these biological end points have been developed and have shown promise to be clinically effective in IBS, chronic diarrhea and chronic constipation [8]. By contrast, this approach has largely failed for the subjective, multidimensional aspect of FGIDs that is characterized by persistent pain or discomfort. Similar frustration using this approach has been experienced in psychiatry [5] and in pain research [6], putting into question the validity of animal models for such disorders in general.
The drug development for well-defined ‘organic’ disorders with agreed upon pathophysiology (including certain motility disorders, such as slow-transit constipation) has been successful since it is based on modeling objective, disease-specific, biological end points (‘biomarkers’) such as tumor regression, inflammation or colonic transit, which are highly correlated with the human disease. However, even though intestinal transit measures and the effect of candidate compounds on such readouts show good correlation between preclinical and clinical models, the correlation between such measures in humans and clinical symptoms is not very strong, since a large number of IBS patients have normal transit. For example, it is unclear why constipation-predominant IBS patients with normal colonic transit should benefit from a drug that is aimed at accelerating colonic transit.
As previously proposed for other human pain conditions [89,90,131,132], we propose that brain endophenotypes that influence central pain modulation (rather than the entire disease process) can be modeled in rodents in a reverse translational approach. Current research strongly suggests that brain imaging approaches in awake or minimally sedated rodents may provide improved quantifiable readouts, which can be translated directly to human brain imaging findings. In the future, transgenic and knockout mouse models hold promise for improving our understanding of specific molecular mechanisms that contribute to the respective phenotype.
Brain mapping has started to be invoked in drug development for pain conditions in human and animal studies over the past decade (reviewed in [90]), although at this early stage, standards for the value of neuroimaging in drug development remain to be defined. In addition, questions remain regarding whether a drug’s response ‘signature’ at the level of brain mapping may differ by disease state and chronicity, and whether neuroimaging should focus on resting state function or brain responses to acute experimental challenges. Functional brain mapping in rodents will likely complement behavioral measurements in animal models of visceral pain. While the optimal imaging strategy may differ between pain disorders and their chronicity, functional neuroimaging in rodents is beginning to validate the relevance of animal models to human conditions at the brain level [133–136].
Five-year view
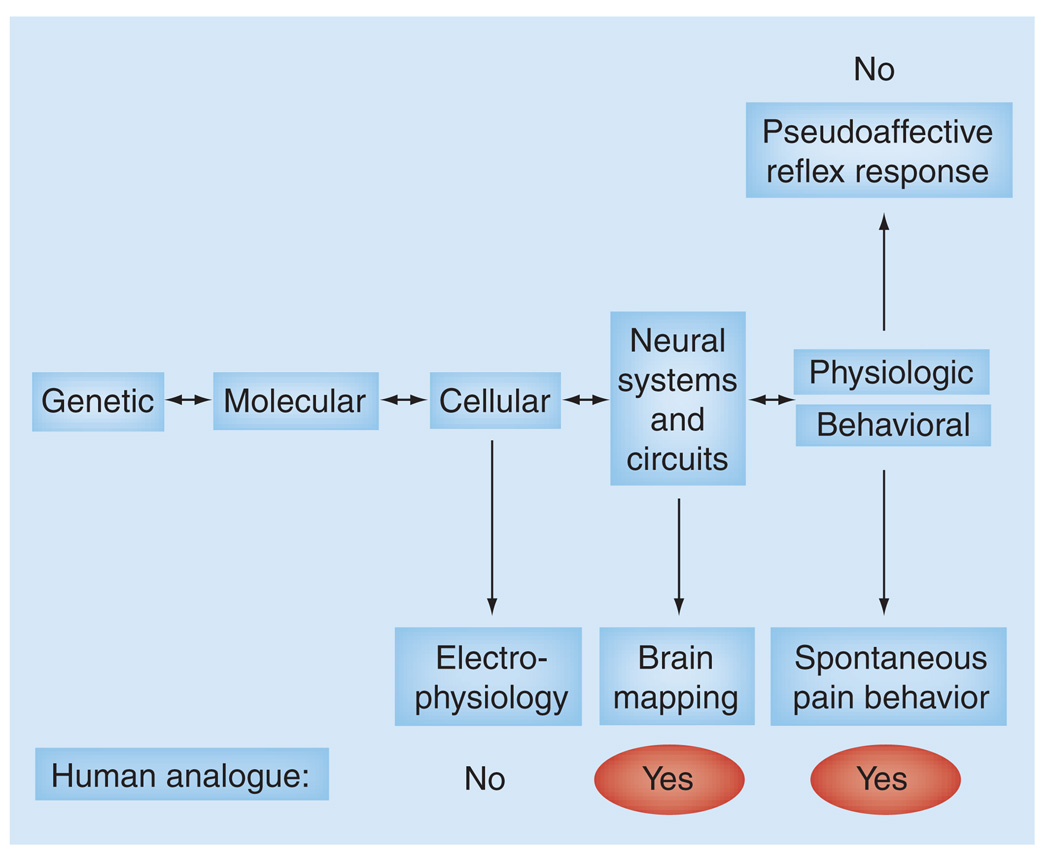
Currently there is a need to clearly define, characterize and validate human endophenotypes that allow better prediction of relevant outcome measures in clinical trials, and to develop preclinical homologues of these human endophenotypes. Current measures of pain in animals are mainly focused on evoked acute pain responses and do not correspond to the spontaneous, on going or recurrent pain found in IBS. New functional and behavioral assays are needed that model such chronic pain conditions. This includes the replacement of reflexive outcome measures with homologous measures, such as brain imaging approaches, and the replacement of measurements of evoked pain responses with measurements of spontaneous pain behavior. Spontaneous behaviors might include such measures as spontaneous locomotor activity, gait, posture, guarding, flinching, social behavior, anxiety, body weight and food intake (Figure 1) . In a recent study, a 5HT1A receptor antagonist was found to inhibit the visceromotor response to CRD, while demonstrating no effect on the cardiovascular pseudo affective response. These results in animals contrast with a lack of clinical efficacy to reduce abdominal pain in IBS patients and illustrate the need to use multiple readouts for the measurement of pain to increase the predictive value of animal studies [67].
Figure 1. Spectrum of endophenotypes for translating nociceptive responses from humans to rodents.
While spontaneous pain behavior and pseudoaffective reflex responses are individually lacking in this regard, it has been suggested that the use of combined multiple physiologic and behavioral readouts may improve the process of translating findings from humans to rodents.
Key issues.
Perceptual responses to mechanical (and to a lesser degree electrical or chemical) stimulation are common measures of visceral sensitivity in clinical studies. The weak predictability of such acute visceral perception testing in discriminating compounds that may have a positive impact on either specific or global irritable bowel syndrome (IBS) symptoms suggests that these human tests may not be suitable as cost-effective drug development strategies for IBS.
In rodent models of functional gastrointestinal disorders (FGID), reflexive or behavioral nociceptive responses to acute colorectal distension have become the standard readout for the assessment of visceral sensitivity; however, the predictive validity of these animal models has proven to be disappointing.
While there is a poor correlation between transit and symptoms, preclinical models have been relatively successful at translating objective gastrointestinal transit measurements between animals and humans.
The problem of bench-to-bedside translation, however, does not simply originate in the limitations of the animal models. Improved definition and classification of clinical states based on biological abnormalities are needed. Such improvement is dependent on the identification of robust endophenotypes in humans with adequate effect sizes for cardinal symptoms or global end points, which can be modeled in a transverse translational approach in rodents.
Rather than trying to develop rodent models with good face validity for the human FGIDs, it may be more productive to translate robust human endophenotypes into homologous rodent readouts.
Functional imaging may provide one means of identifying endophenotypes that can translate from the human to the animal. Imaging-based endophenotypes promise to provide improved end points to define treatment and drug development for FGIDs. Proof-of-concept studies with effective (and ineffective) candidate drugs are required to test this hypothesis.
Acknowledgments
The work discussed in this paper is supported by NIH grants DK 48351, DK 64539, DA026597.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122(7):2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M. Is there a SERT-ain association with IBS? Gut. 2004;53(10):1396–1399. doi: 10.1136/gut.2004.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer EA, Naliboff BD, Chang L. Evolving pathophysiological model of functional gastrointestinal disorders: implications for treatment. Eur. J. Surg. Suppl. 2002;168 Suppl. 587:3–9. [PubMed] [Google Scholar]
- 4.Langley CK, Aziz Q, Bountra C, et al. Volunteer studies in pain research - opportunities and challenges to replace animal experiments: the report and recommendations of a Focus on Alternatives workshop. Neuroimage. 2008;42(2):467–473. doi: 10.1016/j.neuroimage.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Hohoff C. Anxiety in mice and men: a comparison. J. Neural Transm. 2009;116(6):679–687. doi: 10.1007/s00702-009-0215-z. [DOI] [PubMed] [Google Scholar]
- 6.Mogil JS, Simmonds K, Simmonds MJ. Pain research from 1975 to 2007: a categorical and bibliometric meta-trend analysis of every research paper published in the journal, Pain. Pain. 2009;142(1–2):48–58. doi: 10.1016/j.pain.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Kellow JE, Azpiroz F, Delvaux M, et al. ROME III: The Functional Gastrointestinal Disorders. VA, USA: Degnon Associates; 2006. Principles of applied neurogastroenterology: Physiology/motility-sensation; pp. 89–160. [Google Scholar]
- 8.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin. Pharmacol. Ther. 2010;87(6):748–753. doi: 10.1038/clpt.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horikawa Y, Mieno H, Inoue M, Kajiyama G. Gastrointestinal motility in patients with irritable bowel syndrome studied by using radioopaque markers. Scand. J. Gastroenterol. 1999;34(12):1190–1195. doi: 10.1080/003655299750024698. [DOI] [PubMed] [Google Scholar]
- 10.Cann PA, Read NW, Brown C, Hobson N, Holdsworth CD. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983;24(5):405–411. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchoucha M, Devroede G, Dorval E, Faye A, Arhan P, Arsac M. Different segmental transit times in patients with irritable bowel syndrome and ‘normal’ colonic transit time: is there a correlation with symptoms? Tech. Coloproctol. 2006;10(4):287–296. doi: 10.1007/s10151-006-0295-9. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Bueno L, De Ponti F, Fioramonti J, Lydiard RB, Tack J. Pharmacological and pharmacokinetic aspects of functional gastrointestinal disorders. Gastroenterology. 2006;130(5):1421–1434. doi: 10.1053/j.gastro.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Mertz H. Review article: visceral hypersensitivity. Aliment. Pharmacol. Ther. 2003;17(5):623–633. doi: 10.1046/j.1365-2036.2003.01447.x. [DOI] [PubMed] [Google Scholar]
- 14.Lembo T, Naliboff BD, Matin K, et al. Irritable bowel syndrome patients show altered sensitivity to exogenous opioids. Pain. 2000;87(2):137–147. doi: 10.1016/S0304-3959(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 15.Delvaux M, Louvel D, Lagier E, Scherrer B, Abitbol JL, Frexinos J. The κ agonist fedotozine relieves hypersensitivity to colonic distension in patients with irritable bowel syndrome. Gastroenterology. 1999;116(1):38–45. doi: 10.1016/s0016-5085(99)70226-x. [DOI] [PubMed] [Google Scholar]
- 16.Dapoigny M, Abitbol JL, Fraitag B. Efficacy of peripheral κ-agonist fedotozine versus placebo in treatment of irritable bowel syndrome: a multicenter dose-response study. Dig. Dis. Sci. 1995;40(10):2244–2249. doi: 10.1007/BF02209014. [DOI] [PubMed] [Google Scholar]
- 17.Delvaux M, Beck A, Jacob J, Bouzamondo H, Weber FT, Frexinos J. Effect of asimadoline, a κ opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004;20(2):237–246. doi: 10.1111/j.1365-2036.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 18.Delvaux M, Louvel D, Mamet JP, Campos-Oriola R, Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 1998;12(9):849–855. doi: 10.1046/j.1365-2036.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT 3 receptor antagonist. Aliment. Pharmacol. Ther. 1999;13(9):1149–1159. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 20.Thumshirn M, Coulie B, Camilleri M, Zinsmeister AR, Burton DD, Van Dyke C. Effects of alosetron on gastrointestinal transit time and rectal sensation in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2000;14(7):869–878. doi: 10.1046/j.1365-2036.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- 21.Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. Visceral perception in irritable bowel syndrome. Dig. Dis. Sci. 1995;40(4):819–827. doi: 10.1007/BF02064986. [DOI] [PubMed] [Google Scholar]
- 22.Steadman CJ, Talley NJ, Phillips SF, Zinsmeister AR. Selective 5-hydroxytryptamine type 3 receptor antagonism with ondansetron as treatment for diarrhea-predominant irritable bowel syndrome: a pilot study. Mayo Clinic Proc. 1992;67:732–738. doi: 10.1016/s0025-6196(12)60797-6. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg PA, Kamm MA, Setti-Carraro P, van der Sijp JR, Roth C. Modification of visceral sensitivity and pain in irritable bowel syndrome by 5-HT3 antagonism (ondansetron) Digestion. 1996;57(6):478–483. doi: 10.1159/000201377. [DOI] [PubMed] [Google Scholar]
- 24.Hammer J, Phillips SF, Talley NJ, Camilleri M. Effect of a 5-HT3-antagonist (ondansetron) on rectal sensitivity and compliance in health and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 1993;7(5):543–551. doi: 10.1111/j.1365-2036.1993.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 25.Prior A, Read NW. Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 1993;7:175–180. doi: 10.1111/j.1365-2036.1993.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 26.Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am. J. Gastroenterol. 2005;100 Suppl. 1:S5–S21. doi: 10.1111/j.1572-0241.2005.50613_2.x. [DOI] [PubMed] [Google Scholar]
- 27.Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2004;(1):CD003960. doi: 10.1002/14651858.CD003960.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Poitras P, Riberdy Poitras M, Plourde V, Boivin M, Verrier P. Evolution of visceral sensitivity in patients with irritable bowel syndrome. Dig. Dis. Sci. 2002;47(4):914–920. doi: 10.1023/a:1014729125428. [DOI] [PubMed] [Google Scholar]
- 29.Rajagopalan M, Kurian G, John J. Symptom relief with amitriptyline in the irritable bowel syndrome. J. Gastroenterol. Hepatol. 1998;13:738–741. doi: 10.1111/j.1440-1746.1998.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensistivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled study. Clin. Gastroenterol. Hepatol. 2003;1(3):219–228. doi: 10.1053/cgh.2003.50032. [DOI] [PubMed] [Google Scholar]
- 31.Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2005;22(10):981–988. doi: 10.1111/j.1365-2036.2005.02685.x. [DOI] [PubMed] [Google Scholar]
- 32.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation a2d ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56(9):1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradette M, Delvaux M, Staumont G, Fioramonti J, Bueno L, Frexinos J. Octreotide increases thresholds of colonic visceral perception in IBS patients without modifying muscle tone. Dig. Dis. Sci. 1994;39(6):1171–1178. doi: 10.1007/BF02093780. [DOI] [PubMed] [Google Scholar]
- 34.Klooker TK, Beaumont H, Kuiken SD, Lei A, Boeckxstaens GE. Octreotide as potential treatment for patients with non-constipated irritable bowel syndrome. Gastroenterology. 2005;128 4 Suppl. 2:A93–A94. [Google Scholar]
- 35.Hasler WL, Soudah HC, Owyang C. A somatostatin analogue inhibits afferent pathways mediating perception of rectal distention. Gastroenterology. 1993;104(5):1390–1397. doi: 10.1016/0016-5085(93)90347-f. [DOI] [PubMed] [Google Scholar]
- 36.Schwetz I, Naliboff B, Munakata J, et al. Anti-hyperalgesic effect of octreotide in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004;19(1):123–131. doi: 10.1111/j.1365-2036.2004.01774.x. [DOI] [PubMed] [Google Scholar]
- 37.Dukes GE, Dewit OE, Sanger GJ, et al. Lack of effect of the NK3 receptor antagonist, talnetant SB223242 on symptoms of IBS: results of 2 randomized, double-blind, placebo-controlled dose ranging trials. Gastroenterology. 2007;132 4 Suppl. 2 [Google Scholar]
- 38.Houghton LA, Atkinson W, Lockhart C, Whorwell PJ, Keevil B. Sigmoid-colonic motility in health and irritable bowel syndrome: a role for 5-hydroxytryptamine. Neurogastroenterol. Motil. 2007;19(9):724–731. doi: 10.1111/j.1365-2982.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 39.Gebhart GJ, Sengupta JN, Gaginella TS. Handbook of Methods in Gastrointestinal Pharmacology. FL, USA: CRC Press; 1996. Evaluation of visceral pain; pp. 359–374. [Google Scholar]
- 40.De Winter BY, Boeckxstaens GE, De Man JG, Moreels TG, Herman AG, Pelckmans PA. Effects of m- and κ-opioid receptors on postoperative ileus in rats. Eur. J. Pharmacol. 1997;339(1):63–67. doi: 10.1016/s0014-2999(97)01345-9. [DOI] [PubMed] [Google Scholar]
- 41.Friese N, Chevalier E, Angel F, et al. Reversal by κ-agonists of peritoneal irritation-induced ileus and visceral pain in rats. Life Sci. 1997;60(9):625–634. doi: 10.1016/s0024-3205(96)00647-9. [DOI] [PubMed] [Google Scholar]
- 42.Langlois A, Diop L, Friese N, et al. Fedotozine blocks hypersensitive visceral pain in conscious rats: action at peripheral κ-opioid receptors. Eur. J. Pharmacol. 1997;324(2–3):211–217. doi: 10.1016/s0014-2999(97)00089-7. [DOI] [PubMed] [Google Scholar]
- 43.Langlois A, Diop L, Rivišre PJ, Pascaud X, Junien JL. Effect of fedotozine on the cardiovascular pain reflex induced by distension of the irritated colon in the anesthetized rat. Eur. J. Pharmacol. 1994;271(2–3):245–251. doi: 10.1016/0014-2999(94)90780-3. [DOI] [PubMed] [Google Scholar]
- 44.Diop L, Riviere PJ, Pascaud X, Junien JL. Peripheral κ-opioid receptors mediate the antinociceptive effect of fedotozine (correction of fetodozine) on the duodenal pain reflex in rat. Eur. J. Pharmacol. 1994;271(1):65–71. doi: 10.1016/0014-2999(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 45.Coffn B, Bouhassira D, Chollet R, et al. Effect of the κ agonist fedotozine on perception of gastric distension in healthy humans. Aliment. Pharmacol. Ther. 1996;10(6):919–925. doi: 10.1046/j.1365-2036.1996.109280000.x. [DOI] [PubMed] [Google Scholar]
- 46.Pol O, Ferrer I, Puig MM. Diarrhea associated with intestinal infammation increases the potency of μ and δ opioids on the inhibition of gastrointestinal transit in mice. J. Pharmacol. Exp. Ther. 1994;270(1):386–391. [PubMed] [Google Scholar]
- 47.Pol O, Valle L, Sanchez-Blazquez P, Garzon J, Puig MM. Antibodies and antisense oligodeoxynucleotides to μ-opioid receptors, selectively block the effects of μ-opioid agonists on intestinal transit and permeability in mice. Br. J. Pharmacol. 1999;127(2):397–404. doi: 10.1038/sj.bjp.0702570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topcu I, Ekici NZ, Isik R, Sakarya M. The effects of tramadol and fentanyl on gastrointestinal motility in septic rats. Anesth. Analg. 2006;102(3):876–881. doi: 10.1213/01.ane.0000196506.28780.94. [DOI] [PubMed] [Google Scholar]
- 49.Arvidsson S, Larsson M, Larsson H, Lindstrom E, Martinez V. Assessment of visceral pain-related pseudo-affective responses to colorectal distension in mice by intracolonic manometric recordings. J. Pain. 2006;7(2):108–118. doi: 10.1016/j.jpain.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Larsson M, Arvidsson S, Ekman C, Bayati A. A model for chronic quantitative studies of colorectal sensitivity using balloon distension in conscious mice - effects of opioid receptor agonists. Neurogastroenterol. Motil. 2003;15(4):371–381. doi: 10.1046/j.1365-2982.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 51.Fendt M, Mucha RF. Anxiogenic-like effects of opiate withdrawal seen in the fear-potentiated startle test, an interdisciplinary probe for drug-related motivational states. Psychopharmacology. 2001;155(3):242–250. doi: 10.1007/s002130100709. [DOI] [PubMed] [Google Scholar]
- 52.Narita M, Kaneko C, Miyoshi K, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31(4):739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 53.Corazziari E. Role of opioid ligands in the irritable bowel syndrome. Can. J. Gastroenterol. 1999;13 Suppl. A:A71–A75. doi: 10.1155/1999/598659. [DOI] [PubMed] [Google Scholar]
- 54.Gershon MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004;20 Suppl. 7:3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 55.Bradesi S, Lao L, McLean PG, et al. Dual role of 5-HT(3) receptors in a rat model of delayed stress-induced visceral hyperalgesia. Pain. 2007;130(1):56–65. doi: 10.1016/j.pain.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 56.Miranda A, Peles S, McLean PG, Sengupta JN. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. Pain. 2006;126(1–3):54–63. doi: 10.1016/j.pain.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Houghton LA, Foster JM, Whorwell PJ. Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers. Aliment. Pharmacol. Ther. 2000;14(6):775–782. doi: 10.1046/j.1365-2036.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- 58.Mayer EA, Berman SM, Derbyshire SWG, et al. The effect of the 5-HT 3 receptor antagonist alosetron on regional brain activation in IBS patients is not dependent on activation of nociceptive visceral afferents: a H 2 15 O PET study. Gastroenterology. 2001;120:A67. [Google Scholar]
- 59.Galligan JJ, Vanner S. Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol. Motil. 2005;17(5):643–653. doi: 10.1111/j.1365-2982.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 60.Jiao HM, Xie PY. Tegaserod inhibits noxious rectal distention induced responses and limbic system c-Fos expression in rats with visceral hypersensitivity. World J. Gastroenterol. 2004;10(19):2836–2841. doi: 10.3748/wjg.v10.i19.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang LX, Zhang Q, Qian W, Hou XH. Antinociceptive property of tegaserod in a rat model of chronic visceral hypersensitivity. Chin. J. Dig. Dis. 2005;6(1):21–25. doi: 10.1111/j.1443-9573.2005.00182.x. [DOI] [PubMed] [Google Scholar]
- 62.Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 63.Naliboff BD, Chang L, Crowell MD, et al. Tegaserod increases sigmoid accommodation in female irritable bowel syndrome (IBS) patients. Gastroenterology. 2004;126 4 Suppl. 2:A101. [Google Scholar]
- 64.Camilleri M. Review article: tegaserod. Aliment. Pharmacol. Ther. 2001;15(3):277–289. doi: 10.1046/j.1365-2036.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 65.Kilpatrick L, Labus J, Berman SM, et al. A course of tegaserod treatment modulates CNS processing of visceral afferent information. Gastroenterology. 2006;130 4 Suppl. 2:A289–A290. [Google Scholar]
- 66.Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment. Pharmacol. Ther. 2001;15(10):1655–1666. doi: 10.1046/j.1365-2036.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 67.Lindstrom E, Ravnefjord A, Brusberg M, Hjorth S, Larsson H, Martinez V. The selective 5-hydroxytryptamine 1A antagonist, AZD7371 [3(R)-(N,N-dicyclobutylamino)-8-fuoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide (R,R)-tartrate monohydrate] (robalzotan tartrate monohydrate), inhibits visceral pain-related visceromotor, but not autonomic cardiovascular, responses to colorectal distension in rats. J. Pharmacol. Exp. Ther. 2009;329(3):1048–1055. doi: 10.1124/jpet.109.152330. [DOI] [PubMed] [Google Scholar]
- 68.Drossman DA, Danilewitz M, Naesdal J, Hwang C, Adler J, Silberg DG. Randomized, double-blind, placebo-controlled trial of the 5-HT1A receptor antagonist AZD7371 tartrate monohydrate (robalzotan tartrate monohydrate) in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2008;103(10):2562–2569. doi: 10.1111/j.1572-0241.2008.02115.x. [DOI] [PubMed] [Google Scholar]
- 69.Smedh U, Kaplan JM, Bjorkstrand E, Uvnas-Moberg K. Dual effects of somatostatin analog octreotide on gastric emptying during and after intragastric fll. Am. J. Physiol. 1999;277(5 Pt 2):R1291–R1296. doi: 10.1152/ajpregu.1999.277.5.R1291. [DOI] [PubMed] [Google Scholar]
- 70.Su X, Burton MB, Gebhart GF. Effects of octreotide on responses to noxious colorectal distension in the rat. Gut. 2001;48(5):676–682. doi: 10.1136/gut.48.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyer BM, Werth BA, Beglinger C, et al. Role of cholecystokinin in regulation of gastrointestinal motor functions. Lancet. 1989;2(8653):12–15. doi: 10.1016/s0140-6736(89)90255-9. [DOI] [PubMed] [Google Scholar]
- 72.Chey WD, Beydoun A, Roberts DJ, Hasler WL, Owyang C. Octreotide reduces perception of rectal electrical stimulation by spinal afferent pathway inhibition. Am. J. Physiol. Gastrointest. Liver Physiol. 1995;269(6 Pt 1):G821–G826. doi: 10.1152/ajpgi.1995.269.6.G821. [DOI] [PubMed] [Google Scholar]
- 73.Mertz H, Walsh JH, Sytnik B, Mayer EA. The effect of octreotide on human gastric compliance and sensory perception. Neurogastroenterol. Motil. 1995;7:175–185. doi: 10.1111/j.1365-2982.1995.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 74.Hasler WL, Soudah HC, Owyang C. Somatostatin analog inhibits afferent response to rectal distension in diarrhea-predominant irritable bowel patients. J. Pharmacol. Exp. Ther. 1994;268(3):1206–1211. [PubMed] [Google Scholar]
- 75.Klooker TK, Kuiken SD, Lei A, Boeckxstaens GE. Effect of long-term treatment with octreotide on rectal sensitivity in patients with non-constipated irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007;26(4):605–615. doi: 10.1111/j.1365-2036.2007.03398.x. [DOI] [PubMed] [Google Scholar]
- 76.Varga G, Balint A, Burghardt B, D’Amato M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br. J. Pharmacol. 2004;141(8):1275–1284. doi: 10.1038/sj.bjp.0705769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonnafous C, Bueno L, Griffin PH, Schneier H, Rovati LC, D’Amato M. Influence of dexloxiglumide on visceromotor and pain response induced by rectal distension in rats. Gastroenterology. 2002;122(4):A527. [Google Scholar]
- 78.Cremonini F, Camilleri M, McKinzie S, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am. J. Gastroenterol. 2005;100(3):652–663. doi: 10.1111/j.1572-0241.2005.41081.x. [DOI] [PubMed] [Google Scholar]
- 79.Sanger GJ. Neurokinin NK1 and NK3 receptors as targets for drugs to treat gastrointestinal motility disorders and pain. Br. J. Pharmacol. 2004;141(8):1303–1312. doi: 10.1038/sj.bjp.0705742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salome N, Stemmelin J, Cohen C, Griebel G. Selective blockade of NK2 or NK3 receptors produces anxiolytic- and antidepressant-like effects in gerbils. Pharmacol. Biochem. Behav. 2006;83(4):533–539. doi: 10.1016/j.pbb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 81.Diop L, Raymond F, Fargeau H, Petoux F, Chovet M, Doherty AM. Pregabalin (CI-1008) inhibits the trinitrobenzene sulfonic acid-induced chronic colonic allodynia in the rat. J. Pharmacol. Exp. Ther. 2002;302(3):1013–1022. doi: 10.1124/jpet.302.3.1013. [DOI] [PubMed] [Google Scholar]
- 82.Ravnefjord A, Brusberg M, Larsson H, Lindstrom E, Martinez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br. J. Pharmacol. 2008;155(3):407–416. doi: 10.1038/bjp.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122(7):2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 84.Sabb FW, Bearden CE, Glahn DC, Parker DS, Freimer N, Bilder RM. A collaborative knowledge base for cognitive phenomics. Mol. Psychiatr. 2008;13(4):350–360. doi: 10.1038/sj.mp.4002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology. 2010;138(4):1276–1285. doi: 10.1053/j.gastro.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23(12):605–613. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 87.North CS, Hong BA, Alpers DH. Relationship of functional gastrointestinal disorders and psychiatric disorders: Implications for treatment. World J. Gastroenterol. 2007;13(14):2020–2027. doi: 10.3748/wjg.v13.i14.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J. Pain. 2006;7(8):529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 89.Borsook D, Moulton EA, Schmidt KF, Becerra LR. Neuroimaging revolutionizes therapeutic approaches to chronic pain. Mol. Pain. 2007;3:25. doi: 10.1186/1744-8069-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borsook D, Bleakman D, Hargreaves R, Upadhyay J, Schmidt KF, Becerra L. A ‘BOLD’ experiment in defning the utility of fMRI in drug development. Neuroimage. 2008;42(2):461–466. doi: 10.1016/j.neuroimage.2008.04.268. [DOI] [PubMed] [Google Scholar]
- 91.Mayer EA, Aziz Q, Coen S, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol. Motil. 2009;21(6):579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci. Lett. 1996;215(3):165–168. doi: 10.1016/0304-3940(96)12978-5. [DOI] [PubMed] [Google Scholar]
- 93.Stam R, Ekkelenkamp K, Frankhuijzen AC, Bruijnzeel AW, Akkermans LM, Wiegant VM. Long-lasting changes in central nervous system responsivity to colonic distention after stress in rats. Gastroenterology. 2002;123(4):1216–1225. doi: 10.1053/gast.2002.36029. [DOI] [PubMed] [Google Scholar]
- 94.Monnikes H, Ruter J, Konig M, et al. Differential induction of c-Fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors. Brain Res. 2003;966(2):253–264. doi: 10.1016/s0006-8993(02)04197-5. [DOI] [PubMed] [Google Scholar]
- 95.Martinez V, Wang L, Tache Y. Proximal colon distension induces Fos expression in the brain and inhibits gastric emptying through capsaicin-sensitive pathways in conscious rats. Brain Res. 2006;1086(1):168–180. doi: 10.1016/j.brainres.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 96.Monnikes H, Ruter J, Konig M, et al. Differential induction of c-Fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors. Brain Res. 2003;21(966):253–264. doi: 10.1016/s0006-8993(02)04197-5. [DOI] [PubMed] [Google Scholar]
- 97.Lazovic J, Wrzos HF, Yang QX, et al. Regional activation in the rat brain during visceral stimulation detected by c-Fos expression and fMRI. Neurogastroenterol. Motil. 2005;17(4):548–556. doi: 10.1111/j.1365-2982.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhai QZ, Traub RJ. The NMDA receptor antagonist MK-801 attenuates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and non-noxious colorectal distention. Pain. 1999;83(2):321–329. doi: 10.1016/s0304-3959(99)00116-5. [DOI] [PubMed] [Google Scholar]
- 99.Traub RJ, Stitt S, Gebhart GF. Attenuation of c-Fos expression in the rat lumbosacral spinal cord by morphine or tramadol following noxious colorectal distention. Brain Res. 1995;701(1–2):175–182. doi: 10.1016/0006-8993(95)00990-5. [DOI] [PubMed] [Google Scholar]
- 100.Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT 3 receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-Fos expression in the anaesthetised rat. Gut. 2000;46(4):474–480. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao J, Wu X, Owyang C, Li Y. Enhanced responses of the anterior cingulate cortex neurones to colonic distension in viscerally hypersensitive rats. J. Physiol. 2006;570(Pt 1):169–183. doi: 10.1113/jphysiol.2005.096073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J. Neurophysiol. 1996;76(4):2661–2674. doi: 10.1152/jn.1996.76.4.2661. [DOI] [PubMed] [Google Scholar]
- 103.Ohashi K, Ichikawa K, Chen L, Callahan M, Zasadny K, Kurebayashi Y. MicroPET detection of regional brain activation induced by colonic distention in a rat model of visceral hypersensitivity. J. Vet. Med. Sci. 2008;70(1):43–49. doi: 10.1292/jvms.70.43. [DOI] [PubMed] [Google Scholar]
- 104.Wang Z, Bradesi S, Maarek J-MI, et al. Regional brain activation in conscious, unrestrained rats in response to visceral pain. Pain. 2008;138(1):233–243. doi: 10.1016/j.pain.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Z, Guo Y, Bradesi S, et al. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145(1–2):120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Craig AD. A rat is not a monkey is not a human: comment on Mogil. Nature Rev. Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606-c1. Nat. Rev. Neurosci. 10(6), 466 (2009) [DOI] [PubMed] [Google Scholar]
- 107.Bradesi S, Eutamene H, Fioramonti J, Bueno L. Acute restraint stress activates functional NK1 receptor in the colon of female rats: involvement of steroids. Gut. 2002;50(3):349–354. doi: 10.1136/gut.50.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: A new model for sustained visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289(1):G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 109.LaGraize SC, Borzan J, Peng YB, Fuchs PN. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp. Neurol. 2006;197(1):22–30. doi: 10.1016/j.expneurol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 110.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol. Clin. 2000;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 111.Berman SM, Naliboff BD, Suyenobu B, et al. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291(2):R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 112.Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124(7):1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 113.Berman S, Munakata J, Naliboff BD, et al. Gender differences in regional brain response to visceral pressure in IBS patients. Eur. J. Pain. 2000;4(2):157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 114.Wang Z, Bradesi S, Maarek JM, et al. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain. 2008;138(1):233–243. doi: 10.1016/j.pain.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang ZBS, Charles J, Pang R, et al. Assessment of functional brain activation in expectation of visceral pain in a rat step-down passive avoidance model. Gastroenterology. 2008;134 4 Suppl. 1 A-120. [Google Scholar]
- 116.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. 2008;28(2):349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sivarao DV, Langdon S, Bernard C, Lodge N. Colorectal distension-induced pseudoaffective changes as indices of nociception in the anesthetized female rat: morphine and strain effects on visceral sensitivity. J. Pharmacol. Toxicol. Methods. 2007;56(1):43–50. doi: 10.1016/j.vascn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 118.Gibney SM, Gosselin RD, Dinan TG, Cryan JF. Colorectal distension-induced prefrontal cortex activation in the Wistar-Kyoto rat: implications for irritable bowel syndrome. Neuroscience. 2010;165(3):675–683. doi: 10.1016/j.neuroscience.2009.08.076. [DOI] [PubMed] [Google Scholar]
- 119.Irwin C, Falsetti SA, Lydiard RB, Ballenger JC, Brock CD, Brener W. Comorbidity of posttraumatic stress disorder and irritable bowel syndrome. J. Clin. Psychiatr. 1996;57(12):576–578. doi: 10.4088/jcp.v57n1204. [DOI] [PubMed] [Google Scholar]
- 120.Levy RL, Cain KC, Jarrett M, Heitkemper MM. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. J. Behav. Med. 1997;20(2):177–193. doi: 10.1023/a:1025582728271. [DOI] [PubMed] [Google Scholar]
- 121.Ross CA. Childhood sexual abuse and psychosomatic symptoms in irritable bowel syndrome. J. Child. Sex. Abus. 2005;14(1):27–38. doi: 10.1300/J070v14n01_02. [DOI] [PubMed] [Google Scholar]
- 122.Walker EA, Katon WJ, Roy-Byrne PP, Jemelka RP, Russo J. Histories of sexual victimization in patients with irritable bowel syndrome or inflammatory bowel disease. Am. J. Psychiatr. 1993;150:1502–1506. doi: 10.1176/ajp.150.10.1502. [DOI] [PubMed] [Google Scholar]
- 123.Walter SA, Aardal-Eriksson E, Thorell LH, Bodemar G, Hallbook O. Pre-experimental stress in patients with irritable bowel syndrome: high cortisol values already before symptom provocation with rectal distensions. Neurogastroenterol. Motil. 2006;18(12):1069–1077. doi: 10.1111/j.1365-2982.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 124.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53(8):1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dickhaus B, Mayer EA, Firooz N, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am. J. Gastroenterol. 2003;98(1):135–143. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- 126.Mayer EA, Naliboff BD, Chang L, Coutinho SV. Stress and the gastrointestinal tract: V. Stress and irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280(4):G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 127.Murray CD, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127(6):1695–1703. doi: 10.1053/j.gastro.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z, Pang RD, Guo Y, Bradesi S, Mayer EA, Holschneider DP. Functional brain mapping of visceral hypersensitivity induced by chronic water avoidance stress in rats; Presented at: Annual Meeting of the Society for Neuroscience; 13–17 November 2010; San Diego, CA, USA. (Abstract 682.11/UU3) [Google Scholar]
- 129.McIntosh AR, Gonzalez-Lima F. Large-scale functional connectivity in associate learning: Interrelations of the rat auditory, visual, and limbic systems. J. Neurophysiol. 1998;80(6):3148–3162. doi: 10.1152/jn.1998.80.6.3148. [DOI] [PubMed] [Google Scholar]
- 130.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41(3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Borsook D, Becerra L. Phenotyping central nervous system circuitry in chronic pain using functional MRI: considerations and potential implications in the clinic. Curr. Pain Headache Rep. 2007;11(3):201–207. doi: 10.1007/s11916-007-0191-7. [DOI] [PubMed] [Google Scholar]
- 132.Wise RG, Tracey I. The role of fMRI in drug discovery. J. Magn. Reson. Imaging. 2006;23(6):862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- 133.Hess A, Sergejeva M, Budinsky L, Zeilhofer HU, Brune K. Imaging of hyperalgesia in rats by functional MRI. Eur. J. Pain. 2007;11(1):109–119. doi: 10.1016/j.ejpain.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 134.Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth. Analg. 2006;103(1):208–216. doi: 10.1213/01.ane.0000221457.71536.e0. [DOI] [PubMed] [Google Scholar]
- 135.Moylan Governo RJ, Morris PG, Prior MJ, Marsden CA, Chapman V. Capsaicin-evoked brain activation and central sensitization in anaesthetised rats: a functional magnetic resonance imaging study. Pain. 2006;126(1–3):35–45. doi: 10.1016/j.pain.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 136.Tuor UI, McKenzie E, Tomanek B. Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magn. Reson. Imaging. 2002;20(10):707–712. doi: 10.1016/s0730-725x(02)00599-4. [DOI] [PubMed] [Google Scholar]
Website
- 201.Press release. Forest to Discontinue Development in US of Dexloxiglumide for Irritable Bowel Syndrome (IBS). 1 October 2003 www.prnewswire.com/news-releases/forest-to-discontinue-development-in-us-of-dexloxiglumide-for-irritable-bowel-syndrome-ibs-72334602.html