Abstract
Although ionizing radiation induces germline mutations in animals, human studies of radiation-exposed populations have not detected an effect. We conducted a case-control study of sporadic bilateral retinoblastoma, which results from a new germline RB1 mutation, to investigate gonadal radiation exposure of parents from medical sources before their child's conception. Parents of 206 cases from 9 North American institutions and 269 controls participated; fathers of 184 cases and 223 friend and relative controls and mothers of 204 cases and 260 controls provided information in telephone interviews on their medical radiation exposure. Cases provided DNA for RB1 mutation testing. Of common procedures, lower GI series conferred the highest estimated dose to testes and ovaries. Paternal history of lower GI series was associated with increased risk of retinoblastoma in the child (matched odds ratio (OR)=3.6, 95% confidence interval (CI) 1.2, 11.2, 2-sided P=0.02), as was estimated total testicular dose from all procedures combined (OR for highest dose=3.9, 95% CI 1.2, 14.4, P =0.02). Maternal history of lower GI series was also associated with increased risk (OR=7.6, 95% CI 2.8, 20.7, P <0.001) as was estimated total dose (OR for highest dose=3.0, 95% CI 1.4, 7.0, P =0.005). The RB1 mutation spectrum in cases of exposed parents did not differ from that of other cases. Some animal and human data support our findings of an association of gonadal radiation exposure in men and women with new germline RB1 mutation detectable in their children, although bias, confounding, and/or chance may also explain the results.
Keywords: germline mutation, ionizing radiation, retinoblastoma, case-control studies, pediatric cancer
Introduction
Ionizing radiation and numerous other physical and chemical exposures are well documented germ-cell mutagens in animals 1,but studies of exposed human populations, i.e. atomic bomb survivors and cancer patients treated with radiation and mutagenic drugs, have not detected an effect 2, 3. A consensus has emerged that humans are not resistant to the germline mutagenic effects of radiation exposure but that technological and other limitations of previous research explain the discrepancy between animal and human studies4.
An alternative approach to investigating the effect of radiation on germline mutation is to study conditions that nearly always result from germline mutation and assess past exposure. Sporadic bilateral retinoblastoma (bilateral retinoblastoma not inherited from a parent), which results from a new germline mutation in the RB1 gene, is a good candidate for study. A small previous study observed associations that did not reach statistical significance with medical radiation exposure for both mothers and fathers based on crude exposure assessment 5. We conducted a larger, more rigorous study of sporadic bilateral retinoblastoma to further investigate the role of parents’ exposure to ionizing radiation from medical sources.
Materials and Methods
Institutional review boards of all participating institutions approved the study. Participants verbally consented to the telephone interview and gave written consent for the use of DNA.
Eligible patients were diagnosed with sporadic bilateral retinoblastoma from January 1998 – May 2006 and treated at one of nine participating institutions: Children's Hospital of Philadelphia, Wills Eye Institute (Philadelphia), Memorial Sloan-Kettering Cancer Center (New York), University of Illinois – Chicago, Children's Memorial Hospital (Chicago), Children's Hospital of Los Angeles, St. Jude Children's Research Hospital (Memphis), Hospital for Sick Children (Toronto), and Children's Hospital and Regional Medical Center (Seattle).
Patients with retinoblastoma often travel from out of state or out of region to the treating institution, making referral patterns complex. Therefore, for our study with hospital-based case ascertainment, we used controls selected from the case child's friends and relatives who were in the same or adjacent age group as the case (0-1, 2-4, 5-6, 7-9, and 10-12 years). The study focused on paternal exposures since 85% of new RB1 mutations occur on the father's allele 6, 7. Therefore, for relatives, we chose families in which the father was not a biological relative of the case father so that the control group consisting of both friends and relatives was homogeneous in terms of the absence of a biological relationship between case and control fathers. We asked the parents to enumerate friends and relatives with children from whom we chose those who fit the criteria. For each case, we attempted to recruit one to two friends and one relative.
Additional eligibility criteria for both cases and controls were residence in North America, at least one parent who spoke English or Spanish, and at least one biological parent available for participation, i.e., child not adopted or in foster care.
We conducted telephone interviews with one or both parents of cases and controls to obtain information on medical radiation and other exposures before the index child's conception. Eleven individuals conducted the interviews from 2002-2007 with 2 individuals doing 80% of the interviews. The interviewers were not blinded to case-control status. To improve recall of medical radiation, we asked about symptoms and conditions that are indications for imaging procedures that use ionizing radiation as well as the imaging procedures themselves (diagnostic radiographs, other radiological procedures, computerized axial tomography (CT), nuclear medicine scans, and radiation treatment). For example, using the structured questionnaire, the interviewers asked about ulcers, thyroid problems, blood in vomit or stool, and inflammatory bowel disease as well as upper gastrointestinal (GI) series, thyroid scan, treatment with radioactive iodine, and lower GI series. Parents provided the number of procedures and the calendar years or their ages at their first and last such procedure. (The relevant questionnaire sections are provided as online supplements.) Parents who were not able or willing to complete the full interview were offered a shortened version of the questionnaire that did not include the section on medical radiation.
Mutation detection
Mutation analysis was performed for 172 of the cases as previously described 8 in the Genetic Diagnostic Laboratory (GDL) at the University of Pennsylvania. The 27 exons and flanking intronic regions of the RB1 gene were amplified using genomic DNA. PCR products were subjected to cycle sequencing (ABI, CA) and analyzed on an ABI 3100 capillary sequencer. Any observed variation in the RB1 sequence was confirmed by sequencing the PCR product generated from a second, independently isolated sample of DNA. When a novel missense mutation was identified, a set of 50 anonymous control samples was analyzed for the same mutation. For samples without coding sequence mutations, multiplex ligation-dependent probe amplification (kit P047, MRC Holland, Netherlands), a PCR based protocol for detection of inter-exon and intra-exon deletions, was performed and the results analyzed using Coffalyzer software. Quantitative PCR using primers designed in the GDL independently confirmed these results. For 16 of the cases, RB1 mutation analysis was performed at Retinoblastoma Solutions as described previously 9. Mutation analysis for 2 cases was performed elsewhere.
When a mutation was detected, the parents were screened for the same mutation. When a parent also carried the mutation, the child had familial retinoblastoma and was ineligible for the study. For 169 of the 206 case families, both parents and the child provided samples. For 21, the child and one parent provided samples. Sixteen families did not provide samples. Cases for whom some or all samples were missing were assumed to have sporadic bilateral retinoblastoma based on parental medical history. Of the cases with complete sets of samples, 6.5% were found to have familial retinoblastoma or were mosaic for the mutation. Thus, we estimate that about 2 (6.5%) of the 37 cases without a complete set of samples did not have a new germline RB1 mutation.
Cases were categorized by mutation type: transition at a CpG site, other transition, transversion, small insertion or deletion, large deletion or rearrangement, or no mutation detected.
Radiation dose estimates
To estimate testicular and ovarian doses, we used PCXMC software 10 for radiographs and fluoroscopy and ImPACT software 11 for CTs, based on current common techniques such as x-ray energy, exposure time, and typical views. For example, the estimate for a lower GI series was based on seven minutes of fluoroscopy with 25 additional spot films. Doses from nuclear medicine scans and I131 treatment for hyperthyroidism were derived using published dose estimates and typical administered dosages 12-14. Published dose estimates were used for myelograms 15 and radiation therapy for breast cancer 16.
Total gonadal doses were estimated as the sum of doses from all procedures, except those with estimated gonadal doses of less than 1 mGy. Procedures included in the questionnaire but excluded from the estimation of total dose because of negligible testicular dose were: knee, thigh, and lower spine x-rays; knee and lower spine CTs; bladder, thyroid, lung, liver, spleen, and gall bladder nuclear medicine scans; gastric emptying scans; cardiac catheterizations, angiograms and angioplasties; intravenous pyelograms (IVPs); and upper GI series. Procedures excluded because of negligible ovarian dosages were: knee and thigh x-rays; knee CTs; bladder, thyroid, liver and spleen nuclear medicine scans; gastric emptying scans; and hysterosalpingograms.
Statistical analysis
Demographic and other characteristics of cases and controls were compared by chi-squared tests for categorical variables and t-tests for continuous variables.
We assessed the association between medical radiation exposure and risk of retinoblastoma in two ways. We used logistic regression to compare all cases and all controls (‘Complete population”) as the most inclusive analysis. However, as a substantial proportion of cases did not have controls and the cases with controls and those without controls differed demographically, the comparison of all cases and controls might be biased. Therefore, we also conducted analyses restricted to the matched case-control sets (‘Case-control sets’) using conditional logistic regression.
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for individual procedures with an estimated gonadal dose of at least 1 mGy and a total of at least 10 exposed subjects as well as for estimated total gonadal dose analyzed as a continuous variable and as a categorical variable. Without specific hypotheses about doses, the cutpoints for the categorical dose variable were arbitrary by necessity and based on the data. We chose a round number somewhat below the lower GI series dose (which had the highest testicular and ovarian doses of the common procedures) as the cutpoint for the high category and defined the middle category as from 1 mGy to the cutpoint. The lower GI series dose and cutpoint for the high category were 90 mGy and 50 mGy for fathers, respectively and 36 mGy and 25 mGy for mothers, respectively. The effect of total dose levels (none, low, high) was investigated using indicator variables.
The ORs presented here are adjusted for child's birth year (a finer categorization of age group, a matching factor) and the possible confounders of race/ethnicity (non-Hispanic white, other), and education level (not a college graduate, college graduate). Father's interview by proxy, parental age at birth of child, and income were considered as possible confounders, but did not change the results appreciably and were not included in the final models. Results from models including medical conditions and symptoms as possible confounders are presented.
As the numbers of exposed subjects were small particularly for the analyses of case-control sets, we attempted exact conditional logistic regression when the number of exposed cases or controls was 10 or fewer for a procedure or dose level. The exact model with both parents’ dose levels and the three confounders could not be run using the exact procedure because the most powerful computer available to us was insufficient for this computationally intensive analysis. For the successful exact analyses, the ORs and P values were very similar to those from standard conditional logistic regression, which are the ones presented.
STATA/IC version 10.0 was used to perform conditional logistic regression and SAS version 9.2 for exact conditional logistic regression. Other analyses were performed using SPSS version 16.0. All P values were 2-sided. Statistical significance was defined as P < 0.05.
Results
Recruitment and characteristics of cases and controls
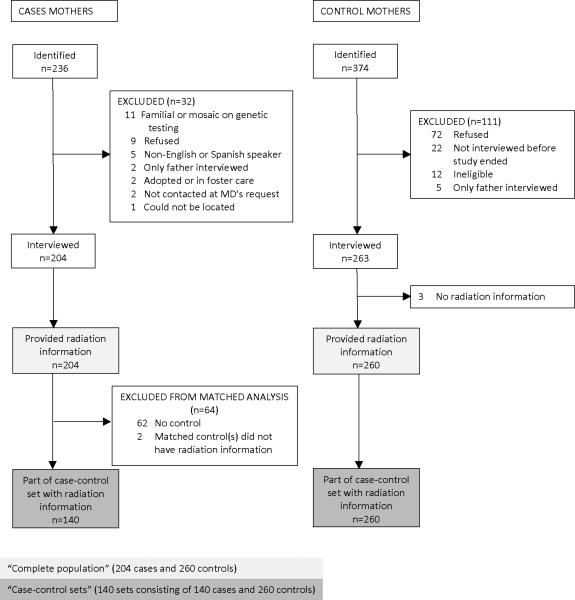
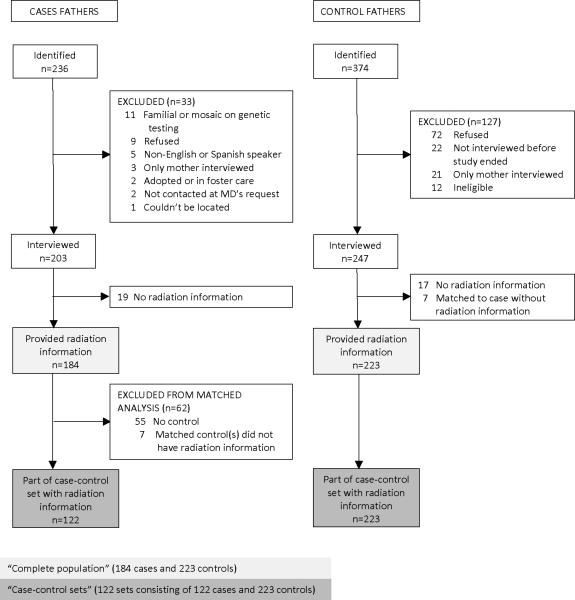
Recruitment of case and control parents is summarized in Figures 1 and 2. Participating institutions identified 236 patients. Patients were excluded for the following reasons: biological parent not available due to foster care or adoption (n=2), neither parent spoke English or Spanish (n=5), inability to locate (n=1), physician requested no contact (n=2), parents refused (n=9), mutation testing showed an inherited RB1 mutation or mosaicism (n=11). The mother (n=204) and/or father (n=203) of the remaining 206 patients were interviewed for the study.
Figure 1.
Recruitment of case and control mothers for a study of sporadic bilateral retinoblastoma
Figure 2.
Recruitment of case and control fathers for a study of sporadic bilateral retinoblastoma
Although we aimed to recruit 1 relative and 1 to 2 friend controls per case, some families were unable or refused to nominate any controls. The other case families each nominated 1 to 3 friends and relatives for a total of 374 potential controls. Of the 374, mothers of 263 (70%) and fathers of 247 (66%) fathers completed interviews. The majority of those not interviewed actively or passively refused; 12 control children were ineligible (Figures 1 and 2). We recruited at least one control parent for 146 (71%) of the 206 case families. For 42 cases with no controls, the case family had no eligible friend or relative, refused to nominate any controls, or provided no contact information; for 18 cases with no controls, the nominated controls actively or passively refused interviews or were ineligible.
Of the 203 case fathers, 19 did not provide information on medical radiation, 55 had no interviewed control, and 7 had one or more controls but none with medical radiation information. Of the 247 interviewed control fathers, 17 did not provide radiation information and 7 were controls of cases without radiation information. Thus, 122 case fathers and 223 control fathers formed 122 matched case-control sets with radiation information. All 204 interviewed case mothers provided radiation information but 62 did not have any controls and 2 did not have a control with radiation information. All but 3 of the 263 control mothers provided radiation information, resulting in 140 case and 260 control mothers who formed 140 matched sets. For a small proportion of participants, the other parent provided the information (proxy interview, Table 1).
Table 1.
Demographic and other characteristics of retinoblastoma cases and controls
| Fathers† |
Mothers‡ |
|||||
|---|---|---|---|---|---|---|
| All cases (n=184) # (%) | Cases in case-control sets (n=122) # (%) | Controls (n=223) # (%) | All cases (n=204) # (%) | Cases in case-control sets (n=140) # (%) | Controls (n=260) # (%) | |
| Race/ethnicity † ‡ | ||||||
| Non-Hispanic | 125 (68) | 100 (82) | 192 (86) | 132 (65) | 104 (74) | 208 (80) |
| White | ||||||
| Non-Hispanic | 18 (10) | 6 (5) | 7 (3) | 28 (14) | 16 (11) | 21 (8) |
| African American | ||||||
| Hispanic | 24 (13) | 10 (8) | 16 (7) | 31 (15) | 14 (10) | 22 (8) |
| Other | 17 (9) | 6 (5) | 8 (4) | 13 (6) | 6 (4) | 9 (3) |
| Educational level † ‡ | ||||||
| No college degree | 108 (58) | 64 (52) | 88 (39) | 121 (59) | 70 (50) | 108 (42) |
| College degree or higher | 76 (41) | 58 (48) | 135 (61) | 83 (41) | 70 (50) | 152 (58) |
| Annual income § | ||||||
| < $25,000 | 23 (14) | 6 (5) | 15 (8) | 34 (21) | 15 (13) | 31 (14) |
| $25 - 35,000 | 21 (12) | 12 (11) | 15 (8) | 19 (12) | 13 (11) | 18 (8) |
| $35 - 50,000 | 32 (19) | 24 (21) | 33 (17) | 32 (20) | 24 (21) | 48 (21) |
| $50 - 75,000 | 39 (23) | 25 (22) | 49 (25) | 35 (21) | 26 (23) | 46 (20) |
| > $75,000 | 54 (32) | 46 (41) | 85 (43) | 43 (26) | 37 (32) | 85 (37) |
| Marital status at interview † ‡ | ||||||
| Married | 168 (91) | 117 (96) | 216 (97) | 164 (80) | 122 (87) | 239 (92) |
| Not married | 16 (9) | 5 (4) | 7 (3) | 40 (20) | 18 (13) | 21 (8) |
| Age at birth of child | ||||||
| < 20 | 0 (0) | 0 (0) | 0 (0) | 6 (3) | 3 (2) | 8 (3) |
| 20 -24 | 13 (7) | 3 (2) | 11 (5) | 25 (12) | 14 (10) | 29 (11) |
| 25 -29 | 45 (24) | 28 (23) | 62 (28) | 63 (31) | 41 (29) | 77 (30) |
| 30 - 34 | 56 (30) | 42 (34) | 76 (34) | 64 (31) | 50 (36) | 96 (37) |
| 35 - 39 | 54 (29) | 37 (30) | 51 (23) | 42 (21) | 29 (21) | 42 (16) |
| 40 + | 16 (9) | 12 (10) | 23 (10) | 4 (2) | 3 (2) | 8 (3) |
| Proxy interview | ||||||
| Yes | 15 (8) | 8 (7) | 26 (12) | 2 (1) | 1 (1) | 3 (1) |
| No | 169 (92) | 114 (93) | 197 (88) | 202 (99) | 139 (99) | 256 (99) |
| Smoked in year before index pregnancy † ‡ | ||||||
| Yes | 59 (32)† | 28 (23) | 45 (20) | 49 (24) | 32 (23) | 40 (15) |
| No | 125 (68) | 94 (77) | 178 (80) | 155 (76) | 108 (77) | 220 (85) |
Differences between fathers of all cases and fathers of controls were statistically significant for race/ethnicity (P <0.001), educational level (P<0.001), marital status (P =0.02) and smoking (P =0.006). The difference between fathers of cases in case-control sets and fathers of controls was statistically significant for educational level (P =0.02).
Differences between mothers of all cases and mothers of controls were statistically significant for race/ethnicity (P=0.003), educational level (P <0.001), marital status (P <0.001) and smoking (P =0.02). No differences between mothers of cases in case-control sets and mothers of controls were statistically significant.
Income missing for 15 case fathers, 26 control fathers, 41 case mothers, and 32 control mothers
Control parents were more likely to be non-Hispanic white, have at least a college education, have a higher income, be married, and be a non-smoker compared to cases. However, when only the case-control sets were considered, case parents and control parents were similar in race/ethnicity, marital status, age at the index child's birth, and income (Table 1); however, they differed in father's educational level and mother's smoking.
Individual imaging procedures
For fathers, the ORs for hip/pelvic x-ray and abdominal x-ray were not significantly elevated, but the OR for lower GI series was 2.7 (95% CI 1.1, 6.8, P=0.03) in the analysis of the complete population and 3.6 (95% CI 1.2, 11.2, P=0.02) in the matched analysis of the case-control sets; 14 cases and 8 controls reported a lower GI series (Table 2). There were too few exposed fathers for analysis of other procedures. One control and two case fathers reported having had the lower GI series before the child's conception, but they reported having had it an age that they reached after the child's conception. If their reported ages were accurate, the procedure would have occurred 0.1 to 2 years after the child's conception. When these three fathers were excluded from the analysis, the ORs decreased less than 15% and were now of borderline significance. Another case father did not remember when he had his lower GI series. The remaining fathers reported their lower GI series from 0.6 to 30 years before the child's conception. The largest case excess occurred in the interval 1 to 9.9 years before the conception (4 case fathers and 1 control father).
Table 2.
Medical sources of gonadal radiation exposure of fathers of cases of retinoblastoma and controls prior to the index child's conception: frequencies and odds ratios
| Procedure† | All cases (n=184) | Cases in case-control sets (n=122) | Controls (n=223) | Complete population‡ |
Case-control sets§ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose to testes | # | % | # | % | # | % | OR | 95% CI | P | OR | 95% CI | P |
| Testicular scan | 0 | 0 | 0 | 0 | 1 | 0.4 | ||||||
| 2 mGy | ||||||||||||
| Bone scan | 4 | 2.1 | 1 | 0.8 | 4 | 1.8 | ||||||
| 2 mGy | ||||||||||||
| Nuclear stress test | 2 | 1.1 | 2 | 1.6 | 2 | 0.9 | ||||||
| 2 mGy | ||||||||||||
| PET scan | 0 | 0 | 0 | 0 | 1 | 0.4 | ||||||
| 4 mGy | ||||||||||||
| Myelogram | 1 | 0.5 | 1 | 0.8 | 2 | 0.9 | ||||||
| 4 mGy | ||||||||||||
| Hip/pelvic x-ray | 16 | 8,7 | 12 | 9.8 | 18 | 8.1 | 1.3 | 0.6, 2.7 | 0.49 | 1.6 | 0.7, 3.7 | 0.27 |
| 5 mGy | ||||||||||||
| X-ray of abdomen | 31 | 16.8 | 22 | 18.0 | 37 | 16.6 | 1.0 | 0.6, 1.8 | 0.92 | 1.0 | 0.5, 2.0 | 0.89 |
| 5 mGy | ||||||||||||
| CT of abdomen/pelvis | 2 | 1.1 | 2 | 1.6 | 3 | 1.3 | ||||||
| 10 mGy | ||||||||||||
| Lower GI series | 14 | 7.5 | 11 | 9.0 | 8 | 3.6 | 2.7 | 1.1, 6.8 | 0.03 | 3.6 | 1.2, 11.2 | 0.02 |
| 90 mGy | ||||||||||||
| Excluding 3 with undetermined timing in relation to conception | 12 | 6.5 | 9 | 7.4 | 7 | 3.1 | 2.6 | 1.0, 6.8 | 0.06 | 3.1 | 0.9, 10.8 | 0.08 |
| Interval before conception | ||||||||||||
| < 1 year | 1 | 0.5 | 1 | 0.8 | 1 | 0.4 | ||||||
| 1 – 9 years | 7 | 3.8 | 4 | 3.3 | 1 | 0.4 | ||||||
| 10 - 30 years | 4 | 2.2 | 4 | 3.3 | 5 | 2.2 | ||||||
No fathers reported a venogram of leg, MUGA scan, I131 thyroid treatment, or radiation treatment.
Results from logistic regression model with race (non-Hispanic white, other), educational level (less than college degree, college degree or greater), and child's birth year; odds ratios not calculated for procedures with fewer than 10 exposed participants.
Results from conditional logistic regression model with race (non-Hispanic white, other), educational level (less than college degree, college degree or greater), and child's birth year; odds ratios not calculated for procedures with fewer than 10 exposed participants.
For mothers, the ORs were close to 1.0 in the complete population and/or the case-control sets for the procedures with estimated ovarian doses from 1 - 5 mGy (hip/pelvic x-ray, IVP, lower back x-ray, x-ray of abdomen) (Table 3). A non-significantly higher proportion of case than control mothers reported upper GI series, a procedure with a moderate dose, with ORs of 1.7 (95% CI 0.9, 3.1, P=0.10) and 1.5 (95% CI 0.8, 2.9, P=0.25) in the complete population and case-control sets, respectively. For lower GI series (the highest dose procedure with sufficient numbers for analysis), the ORs were 7.6 – 7.7 in the two analyses and statistically significant based on 22 case and 5 control mothers with this procedure. For all time intervals before the child's conception, more case mothers had a lower GI series compared to control mothers, but the difference was smaller beyond 10 years.
Table 3.
Medical sources of gonadal radiation exposure of mothers of cases of retinoblastoma and controls prior to the index child's conception: frequencies and odds ratios
| Procedure† | All cases (n=204) | Cases (n=140) | Controls (n=260) | Complete population‡ |
Case-control sets§ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose to ovaries | # | % | # | % | # | % | OR | 95% CI | P | OR‡ | 95% CI§ | P |
| Hip/pelvic x-ray | 11 | 5.4 | 7 | 5.0 | 15 | 5.8 | 0.9 | 0.4, 2.2 | 0.90 | 0.9 | 0.3, 2.4 | 0.85 |
| 1 mGy | ||||||||||||
| IVP | 11 | 5.4 | 9 | 6.4 | 14 | 5.4 | 1.1 | 0.5, 2.4 | 0.89 | 1.1 | 0.4, 2.7 | 0.89 |
| 2 mGy | ||||||||||||
| CT of back | 2 | 1 | 2 | 1 | 6 | 2.3 | ||||||
| 2 mGy | ||||||||||||
| X-ray of lumbar spine | 45 | 22 | 33 | 23.6 | 54 | 20.8 | 1.1 | 0.7, 1.8 | 0.61 | 1.2 | 0.7, 2.0 | 0.47 |
| 3 mGy | ||||||||||||
| Bone scan | 1 | 0.5 | 1 | 0.7 | 6 | 2.3 | ||||||
| 3 mGy | ||||||||||||
| Gall bladder scan | 3 | 1.5 | 3 | 2.1 | 2 | 0.8 | ||||||
| 3 mGy | ||||||||||||
| X-ray of abdomen | 36 | 17.6 | 20 | 14.3 | 33 | 12.7 | 1.5 | 0.9, 2.5 | 0.16 | 1.0 | 0.6, 1.9 | 0.90 |
| 5 mGY | ||||||||||||
| Nuclear stress test | 1 | 0.5 | 1 | 0.7 | 0 | 0 | ||||||
| 13 mGY | ||||||||||||
| Upper GI series | 26 | 12.7 | 18 | 12.9 | 23 | 8.8 | 1.7 | 0.9, 3.1 | 0.10 | 1.5 | 0.8, 2.9 | 0.25 |
| 13 mGy | ||||||||||||
| CT of abdomen/pelvis | 5 | 2.5 | 3 | 2.1 | 3 | 1.2 | ||||||
| 18 mGY | ||||||||||||
| Lower GI series | 22 | 11.8 | 19 | 13.6 | 5 | 1.9 | 7.7 | 2.8, 21.2 | <0.001 | 7.6 | 2.8, 20.7 | <0.001 |
| 36 mGy | ||||||||||||
| Interval before conception | ||||||||||||
| 1-4 years | 7 | 6 | 4.3 | 1 | 0.4 | |||||||
| 5-9 years | 8 | 7 | 5.0 | 1 | 0.4 | |||||||
| 10 - 26 years | 7 | 6 | 4.3 | 3 | 1.1 | |||||||
| I131 treatment for hyperthyroidism | 0 | 0 | 0 | 0 | 2 | 0.8 | ||||||
| 46 mGy | ||||||||||||
| Myelogram | 0 | 0 | 0 | 0 | 2 | 0.8 | ||||||
| 52 mGy | ||||||||||||
| Radiation treatment for breast cancer | 0 | 0 | 0 | 0 | 2 | 0.8 | ||||||
| 95 mGy | ||||||||||||
No mothers reported a PET scan or MUGA scan.
Results from logistic regression model with race (non-Hispanic white, other), educational level (less than college degree, college degree or greater), and child?s birth year; odds ratios not calculated for procedures with fewer than 10 exposed participants.
Results from conditional logistic regression model with race (non-Hispanic white, other), educational level (less than college degree, college
degree or greater), and child?s birth year; odds ratios not calculated for procedures with fewer than 10 exposed participants.
Radiation dose
The highest dose category (≥ 50 mGy, range 60 – 271, median 90 mGy for fathers; ≥ 25 mGy, range 27 – 248, median 50 mGy for mothers) was associated with significantly elevated ORs (Table 4). For paternal exposure, the ORs were 2.8 (95% CI 1.1, 6.7, P=0.03) in the complete population and 3.9 (95% CI 1.2, 14.4, P=0.02) in the case-control sets. For maternal exposure, the ORs were 2.6 (95% CI 1.3, 5.0, P =0.006) in the complete population and 3.0 (95% CI 1.4, 7.0, P =0.005) in the case-control sets. The one case father and one control father in the high dose group who did not have a lower GI series had multiple hip or abdominal x-rays. Four case mothers and 16 control mothers in the high dose category did not have lower GI series but had myelograms, I131 treatment for hyperthyroidism, breast cancer radiation, or combinations of, or multiple, upper GI series, abdominal/pelvic CTs, abdominal x-rays, lumbar spine x-rays, hip x-rays, and IVPs. For one control and three cases, both the mother and father had radiation exposure in the high dose category.
Table 4.
Estimated total dose of gonadal radiation exposure from medical sources of parents of cases of retinoblastoma and controls: frequencies and odds ratios
| Complete population† |
Case-control sets§ |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All cases # (%) | Cases in case-control sets* # (%) | Controls* # (%) | OR | 95% CI‡ | P | OR | 95% CI | P | |
| Fathers | 183 (100) | 122 (100) | 223 (100) | ||||||
| 0 mGy | 124 (68) | 78 (64) | 166 (74) | 1.0 | 1.0 | ||||
| 1-49 mGy | 442 (23) | 32 (26) | 48 (22) | 1.3 | 0.8, 2.2 | 0.26 | 1.6 | 0.8, 3.0 | 0.16 |
| 50–271 mGy | 17 (9) | 12 (10) | 9 (4) | 2.8 | 1.1, 6.7 | 0.03 | 3.9 | 1.2, 14.4 | 0.02 |
| Mothers | 203 (100) | 140 (100) | 260 (100) | ||||||
| 0 mGy | 113 (56) | 74 (54) | 160 (62) | 1.0 | 1.0 | ||||
| 1-24 mGy | 61 (30) | 43 (31) | 79 (30) | 1.1 | 0.7, 1.8 | 0.59 | 1.0 | 0.6, 1.8 | 1.00 |
| 25 - 248 mGy | 29 (14) | 23 (16) | 21 (8) | 2.6 | 1.3, 5.0 | 0.006 | 3.0 | 1.4, 7.0 | 0.005 |
Results from logistic regression model with mother's radiation exposure, father's radiation exposure, father's race (non-Hispanic white, other), father's educational level (less than college degree, college degree or greater), and child's birth year; the analysis included the 182 cases and 218 controls with radiation data for both mothers and fathers.
Results from conditional logistic regression model with mother's radiation exposure, father's radiation exposure, father's race (non-Hispanic white, other), father's educational level (less than college degree, college degree or greater), and child's birth year; the analysis included the 119 case-control sets (119 cases, 218 controls) with radiation data for both mothers and fathers.
Confidence interval
To assess the robustness of the findings, we tried using other cutpoints in analyzing the complete population (data not shown). With cutpoints at 40, 60, and 80 mGy, the ORs for paternal exposure were 2.4 to 2.8 with P values of 0.03 to 0.07. Only 6 fathers had doses of 100 mGy or greater so cutpoints above 80 mGy were not explored. For mother's exposure, cutpoints of 20, 30, 40, and 50 mGy resulted in ORs of 2.1 to 3.2 with P values < 0.05. With higher cutpoints of 60 – 100 mGy, ORs were in the same range but with 15 or fewer exposed mothers and non-significant P values. When dose was analyzed as a continuous variable, the ORs for maternal dose and paternal dose were both 1.01 per 1 mGy increase, each with a P value of 0.05 (data not shown).
We analyzed the complete population to assess whether the results for radiation were confounded by parental medical conditions and symptoms. Among the father's medical conditions and symptoms, ORs of at least 2.0 and/or ORs that were statistically significantly greater than 1.0 were observed for irritable bowel syndrome, blood in vomit or stool, kidney or bladder infections, and lung problems. When each condition was included in the analysis along with race/ethnicity, birth year, and educational level, the OR for high radiation dose ranged from 2.6 to 3.0 with P < 0.05 (Supplemental Table 1). When all of these conditions were included in one analysis along with the three demographic covariates, the OR for high radiation exposure was 2.1 (95% CI 0.8, 5.5, P=0.11).
ORs of at least 2.0 and/or ORs significantly greater than 1.0 were observed for mother's injury to the upper leg, hips, back, pelvis or internal organs; back problem other than scoliosis, slipped disc, arthritis, pain or surgery; arthritis of knees or hips; hip pain; constipation; blood in urine; bladder or kidney problem other than infection or blood in urine; surgery of the digestive system or internal organs; and injury to the head, neck or face (Supplemental Table 2). Another indication for lower GI series, irritable bowel syndrome, had an OR of 1.9 and P value of 0.07. When individual medical conditions were added to the model with radiation dose group and the three demographic covariates, the OR for high radiation dose was 1.9 to 2.5, with P values of 0.005 to 0.07. When all the above mentioned medical conditions were included in the model, the OR for high radiation exposure was 1.7 (95% CI 1.1, 2.6, P=0.02).
We assessed whether the time interval between the case's diagnosis and the parent's interview affected the results (Supplemental Table 3). When the interval was dichotomized (≤ 22 months, ≥23 months), the association with paternal lower GI series and high dose and with maternal lower GI series occurred in both strata. The association with maternal radiation dose was restricted to the shorter time interval.
Mutation spectrum
The RB1 mutation spectrum of cases whose parents had high radiation exposure did not differ significantly from that of cases whose parents had less exposure (Table 5).
Table 5.
RB1 mutation spectrum for retinoblastoma cases by parental radiation exposure†
| Father's dose |
Mother's dose |
|||
|---|---|---|---|---|
| Mutation type | < 50 mGy # (%) | ≥50 mGy # (%) | < 25 mGy # (%) | ≥ 25 mGy # (%) |
| Transition at CpG site | 25 (16) | 3 (19) | 25 (15) | 5 (20) |
| Other transition | 24 (15) | 1 (6) | 23 (14) | 5 (20) |
| Transversion | 16 (10) | 1 (6) | 17 (10) | 3 (12) |
| Small insertion or deletion | 46 (30) | 6 (37) | 49 (30) | 6 (24) |
| Large deletion or rearrangement | 26 (17) | 2 (12) | 28 (17) | 3 (12) |
| Mutation not found | 18 (12) | 3 (19) | 20 (12) | 3 (12) |
| Total | 155 (100) | 16 (100) | 162 (100) | 25 (100) |
All case parents who provided medical radiation information and DNA for mutation testing are included in this table regardless of whether they had a control.
Discussion
We observed an association between imaging procedures with relatively high gonadal radiation exposure in both men and women, mainly from lower GI series, and the risk of retinoblastoma occurring in their children from a new germline RB1 mutation. These results replicate findings from a small previous study in which ORs of about 2.0 that were not statistically significant were observed for paternal and maternal exposure5.
The effect of paternal exposure on risk is consistent with the fact that 85% of new germline RB1 mutations occur on the father's allele 6, 7. The exposure of nearly all the fathers with the highest doses reportedly occurred longer before the index child's conception than the approximately three months it takes for sperm to differentiate from stem cells 17. Therefore, the exposure must have occurred to stem cell spermatogonia, cells that persist throughout reproductive life. Radiation to stem cell spermatogonia in animals is well known to induce new heritable mutations 1. It would seem that an effect on a stem cell might result in multiple affected children within a single family. However, as there are an estimated 6,000 stem cell spermatogonia in mice 18, the number of these cells in humans is also assumed to be large. With a large number of stem cells, the probability would be small that an acquired mutation would result in multiple children with retinoblastoma within a family.
In contrast to the animal data, an increase in germline mutation or conditions caused by germline mutation has not been observed in survivors of the atomic bombs or of cancer. Recently, researchers have assessed germline mutation in mini- and micro-satellite loci, the most unstable loci in the human genome, in radiation-exposed groups. Significantly increased mutation rates occurred in families exposed to contamination from the Chernobyl accident, Soviet nuclear weapon tests, and a radiation accident in Brazil, but not in families of Chernobyl cleanup workers, atomic bomb survivors, or a small sample of irradiated cancer patients 2, 3, 19. Possible explanations for germline radiation effects being detected in some but not all exposed populations include few or no mutations occurred, only some types and timings of radiation exposure cause mutations, the effect was missed in some studies because of small sample sizes, and the long period between exposure and conception (e.g. in the atomic bomb survivors) allowed successful repair of DNA damage 3. Although the lack of observed effect in other exposed populations is not understood, the positive Eastern European and Brazilian studies provide the first evidence that ionizing radiation may cause detectable germline mutation in humans. However, whether the results for micro- and minisatellites apply to single-copy gene loci is not known. Our results add to the evidence of a possible effect on germline mutation.
In mice, the radiation-induced germline mutations that result in congenital anomalies or growth retardation are mostly large deletions 2. Based on these data, it has been assumed that germline mutations that affect human health will also be large deletions. In our study, only 12% of the parents with high exposure had a child with a large RB1 deletion. Although not seen in radiation-induced mouse abnormalities, ionizing radiation can induce other types of mutations including point mutations and very small insertions and deletions 20. Thus, the dearth of large deletions differs from mouse mutations in anomalies and growth retardation, but not from other known mutagenic effects of radiation.
The observation of a strong association with maternal radiation exposure prior to the child's conception is surprising given that only an estimated 15% of new germline mutations in RB1 are of maternal origin and that animal studies show that oocytes are much less sensitive to mutagens compared to sperm. In a previous study, we observed an OR of 2 for gonadal radiation exposure that was not statistically significant 5 and interpreted it as a chance finding. In the current study that assessed exposure more rigorously, we observed a strong and significant association and, thus, have to consider explanations other than chance alone. One possibility is that radiation plays an etiologic role in a substantial proportion of the maternal mutations. Consistent with this possibility, researchers observed maternally derived germline mutations in microsatellite DNA after a radiation accident in Brazil19. Mutations in human single-gene loci can also arise in oocytes but these are thought to be mostly large deletions 21, which occurred rarely in the children of exposed mothers. In mice, radiation induces mutations in mature and maturing oocytes, but an effect has not been demonstrated in arrested oocytes 22, the cells that were exposed given the timing of the procedure in relation to the child's conception. However, at least one chemical, ethylnitrosourea, can induce mutation in arrested oocytes 23. The finding in the current study for mothers was unexpected and differs from data in irradiated mice and thus, our finding may be spurious. However, the fact that there are now two studies with similar results indicates the need for further investigation.
Information on whether the parental origin of the child's RB1 mutation matched the parent with the radiation exposure would enhance the study. However, the determination of parental origin usually requires a tumor sample, but fewer than half of children with bilateral retinoblastoma have their tumors removed surgically. We did not collect tumor samples and, even if we had, we would be able to determine parental origin in only a minority of patients.
The effect of maternal radiation exposure appears to be greater than that of paternal exposure as judged by the ORs and the doses associated with those ORs. However, the CIs for maternal and paternal ORs were wide and overlapping, indicating that the differences were likely due to chance. In addition, dose estimation was, by necessity, crude and the 2.5 fold difference between testicular and ovarian doses for lower GI series may not be meaningful.
The findings for both paternal and maternal exposure were consistent in analyses of the complete population and the matched sets, analyses using different cutpoints for the high dose category and using dose as a continuous variable, and analyses adjusted for confounders. The results presented here were adjusted for race and education. We also considered parental age and medical conditions/symptoms as possible confounders. Parental age could confound the results as it may be associated with the risk of retinoblastoma and the likelihood of having a high dose procedure. However, adjustment for parental age did not change the results. A number of medical conditions and symptoms were associated with risk, some of which were indications for diagnostic procedures with high gonadal radiation exposure (e.g. irritable bowel syndrome and lower GI series) and some of which were not (e.g. injury to the head, neck, or face). The results in analyses that adjusted for the medical conditions/symptoms individually or together are consistent with some degree of confounding that reduced but did not eliminate the observed effect of radiation. The meaning of the elevated ORs for medical conditions is not clear, as they have not been studied in either animals or humans as possible risk factors for new germline mutation. One could speculate that the number of conditions with elevated ORs, especially for mothers, is high enough to suggest recall bias. While the meaning of the associations with medical conditions is not clear, the elevated ORs associated with radiation exposure remained, although somewhat attenuated, with adjustment for medical conditions. Although the results for radiation were robust to different analytic methods, cutpoints, and confounders, we could not, of course, exclude confounding by other characteristics that we did not measure.
The small numbers of parents with high exposure, the reliance on self-reporting of procedures and treatments, and the crude dose estimates were the main limitations of our study. The observed case-control differences may have occurred by chance, given the small numbers of highly exposed parents. However, the consistency with a previous study suggests explanations other than chance. The assessment of past radiation exposure relied completely on self-reporting by parents. We attempted to increase the accuracy and completeness of parental recall by first asking about common indications for the diagnostic procedures and then asking about the procedures. Even if this approach improved recall, reporting undoubtedly remained imperfect. If parents of children with cancer recalled their procedures differently than control parents, recall bias could have resulted in misleading observations. In this study, the associations were not seen for low dose procedures and were strongest for the highest dose procedures. This pattern argues against recall bias as the explanation, unless case parents knew both the hypothesis and the procedures with the highest doses, which seems unlikely. The dose estimates were necessarily crude as we did not have information on the characteristics of x-ray machines, lengths of procedures, numbers of films taken, or sizes of individuals, all of which would affect the dose. However, our goal was modest - to distinguish between procedures with low and high doses. One procedure had estimated doses much higher than other common procedures and it appeared to increase risk. The pattern of higher risk with higher dose and the fact that crude dose estimates are generally expected to attenuate an association support the validity of the findings.
As in many case-control studies, interviewer and selection bias might also have affected our results. The interviewers were not blinded to whether subjects were cases or controls and this knowledge may have affected how they administered the questionnaire. To produce the observed results, the interviewers would have had to elicit the reporting of more procedures by cases compared to controls. This bias would presumably have affected the reporting of procedures regardless of dose, as the interviewers did not know which procedures had high and low doses. Interviewer bias seems unlikely to explain the observed results, as associations occurred only at high doses. Selection bias resulting in a non-representative case or control group may also have affected our study. For example, as some cases were recruited several years after diagnosis, some of those no longer followed at these institutions were missed. Differences between the cases who returned for follow-up and those who did not might have biased the results if, for example, those who returned had parents with higher utilization of health services such as diagnostic imaging procedures. We have little information on non-participating cases and cannot assess this possibility. However, when we restricted the analysis to the two institutions that attempted to recruit all cases regardless of whether they were still being followed, the findings remained unchanged. Additionally, even if the cases were non-representative in their health services utilization, their controls had similar socioeconomic status and likely similar access to and utilization of health services. Thus, the differences between cases and their matched controls seem unlikely to be due to the cases’ non-representative utilization of health services since the differences were limited to high dose procedures and higher utilization would presumably not be restricted to high dose procedures. Another potential selection bias might be an over-representation of cases who survived longer. However, this cannot be a major bias for retinoblastoma for which the survival rate is over 95%.
It is important to note that although the relative risk associated with radiation exposure appeared substantial, the resulting absolute risk would be very small. Sporadic heritable retinoblastoma occurs in about 1 in 50,000 births 24. If the observed results are real, children born to parents with high gonadal radiation doses prior to the child's conception would have an estimated risk of 1 in 13,000 – 18,000. The low absolute risk should not deter individuals from procedures needed for diagnosis and treatment.
We have estimated the population attributable risk percent, i.e., the percentage of cases of sporadic heritable retinoblastoma in the population that would be attributable to high radiation exposure, if these results are real. The percents are 6% (95% CI 0%, 13%) for paternal exposure and 9% (1%, 17%) for maternal exposure 25. These attributable risks are fairly small and quite imprecise because of the small numbers of highly exposed parents. The percentages apply to all cases rather than only to cases with a mutation on the RB1 allele inherited from that parent. The attributable risk for paternal exposure suggests that a small proportion of the 85% of cases of paternal origin might be due to men's radiation exposure. The 9% for maternal exposure, given that only an estimated 15% of cases are of maternal origin, suggests that a majority of such cases might occur as a result of women's radiation exposure. Whether these attributable risks are accurate requires further study of radiation exposure and perhaps of the parental origin of RB1 mutations in patients with sporadic bilateral retinoblastoma, now that nearly all mutations can be detected and characterized.
Our results for both men and women were associated with low doses of radiation, roughly estimated at 50 – 100 mGy in the highest dose group. These are much lower than 1 Gy, the current estimate for the doubling dose 26, the estimated dose that would double the background rate of spontaneous germline mutation in humans. Medical radiation exposure is generally acute rather than chronic and with acute exposure, some estimates of the doubling dose have been as low as 160 – 500 mGy 2, doses that are closer to doses in this study. However, the effect we observed is still large for the estimated dose and we cannot exclude that chance and/or bias produced our findings. Nevertheless, our findings suggest the possibility that there may be genetic effects at lower doses.
In summary, we observed that individuals exposed to medical radiation might experience an increased risk of new germline mutation that results in retinoblastoma in their children. Along with recent results on minisatellite loci, our study adds to the evidence that men exposed to radiation may experience increased germline mutation. However, the low dose at which the increased risk occurred and the lack of large deletions differs from current knowledge of germline mutation induced by radiation. Our observation that mothers exposed to medical radiation may experience an increased risk of new germline mutation is surprising given that only a small proportion of new RB1 mutations are of maternal origin and that radiation has not been shown to induce mutation in arrested oocytes in animals. Although the results for both mothers and fathers are based on self-reported exposure and differ from some aspects of current knowledge of radiation mutagenesis, the strength of these associations, their occurrence at only high estimated doses, their persistence after adjustment for possible confounders, and their corroboration of similar findings from a previous study indicate that they are worthy of further attention.
Supplementary Material
Statements on novelty and impact.
We present evidence suggesting that gonadal radiation exposure of fathers from medical sources prior to a child's conception may increase the risk of retinoblastoma in the child by causing a new germline mutation, although other explanations of the finding such as bias, confounding, or chance cannot be ruled out. The imaging procedure with the highest testicular dose and the total estimated testicular dose were significantly associated with increased risk. Our approach was novel since we studied a disease known to result from a new germline mutation rather than studying radiation-exposed groups such as atomic bomb and cancer survivors.
We observed an effect of maternal as well as paternal gonadal radiation exposure from medical sources, a surprising finding given that only a minority of RB1 germline mutations occurs on the mother's allele and that radiation exposure has not been observed to induce new germline mutations in arrested oocytes, the cell population most relevant to our results. However, our finding corroborates that of a previous study and does not appear to be a result of recall bias as the finding only occurred at high radiation doses. The study of germline mutation in human microsatellite DNA following a radiation accident provides additional data consistent with our findings since maternal as well as paternal exposure appeared to contribute to increased germline mutation in the children.
Acknowledgments
This work was supported by a grant from the US National Institutes of Health (R01CA081012). We thank the families of the patients and their relatives and friends who participated as controls. We also thank the clinical research staff at the participating centers and study staff at Children's Hospital of Philadelphia for their diligent efforts particularly Bethany Barone, Jaclyn Bosco, Greta Anschuetz, Sheila Kearney, and the late Jean Rodwell. We are grateful for the insights of Alfred G. Knudson that improved the paper and for the assistance with data analysis from Hong Zhou and Yun Teng.
Abbreviations
- CI
confidence interval
- CT
computerized axial tomography
- GI
gastrointestinal
- IVP
intravenous pyelogram
- OR
odds ratio
References
- 1.Russell LB. Role of mouse germ-cell mutagenesis in understanding genetic risk and in generating mutations that are prime tools for studies in modern biology. Environ Mol Mutagen. 1994;23:S24, 23–9. doi: 10.1002/em.2850230608. [DOI] [PubMed] [Google Scholar]
- 2.Board on Radiation Effects Research, National Research Council, editor. Heritable genetic effects of radiation in human populations Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. The National Academies Press; Washington, DC: 2006. pp. 91–131. [Google Scholar]
- 3.Bouffler SD, Bridges BA, Cooper DN, Dubrova Y, McMillan TJ, Thacker J, Wright EG, Waters R. Assessing radiation-associated mutational risk to the germline: repetitive DNA sequences as mutational targets and biomarkers. Radiat Res. 2006;165:249–68. doi: 10.1667/rr3506.1. [DOI] [PubMed] [Google Scholar]
- 4.Wyrobek AJ, Mulvihill JJ, Wassom JS, Malling HV, Shelby MD, Lewis SE, Witt KL, Preston RJ, Perreault SD, Allen JW, Demarini DM, Woychik RP, et al. Assessing human germ-cell mutagenesis in the Postgenome Era: a celebration of the legacy of William Lawson (Bill) Russell. Environ Mol Mutagen. 2007;48:71–95. doi: 10.1002/em.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunin GR, Meadows AT, Emanuel BS, Buckley JD, Woods WG, Hammond GD. Pre- and post-conception factors associated with heritable and non-heritable retinoblastoma. Cancer Res. 1989;49:5730–5. [PubMed] [Google Scholar]
- 6.Dryja TP, Morrow JF, Rapaport JM. Quantification of the paternal allele bias for new germline mutations in the retinoblastoma gene. Hum Genet. 1997;100:446–9. doi: 10.1007/s004390050531. [DOI] [PubMed] [Google Scholar]
- 7.Zhu XP, Dunn J, Phillips R, Goddard A, Paton K, Becker A, Gallie B. Preferential germline mutation of the paternal allele in retinoblastoma. Nature. 1989;340:312–3. doi: 10.1038/340312a0. [DOI] [PubMed] [Google Scholar]
- 8.Nichols K, Houseknecht M, Godmilow L, Bunin G, Shields C, Meadows A, Ganguly A. Sensitive multi-step clinical molecular screening of 180 unrelated individuals with retinoblastoma detects 36 novel mutations in the RB1 gene. Hum Mutat. 2005;25:566–74. doi: 10.1002/humu.20184. [DOI] [PubMed] [Google Scholar]
- 9.Richter S, Vandezande K, Chen N, Zhang K, Sutherland J, Anderson J, Han L, Panton R, Branco P, Gallie B. Sensitive and efficient detection of RB1 gene mutations enhances care for families with retinoblastoma. Am J Hum Genet. 2003;72:253–69. doi: 10.1086/345651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PCXMC, version 1.0. Finnish Centre for Radiation and Nuclear Safety; Helsinki: 1997. [Google Scholar]
- 11.ImPACT CT Patient Dosimetry Calculator, version 0.99x. National Radiation Protection Board; London: 2006. [Google Scholar]
- 12.Radiation dose to patients from radiopharmaceuticals, International Commission on Radiological Protection, Publication 53ed. Pergamon Press; New York: 1988. [Google Scholar]
- 13.Radiation dose to patients from radiopharmaceuticals, International Commission on Radiological Protection, Publication 80 ed. Pergamon Press; New York: 1998. [Google Scholar]
- 14.Stabin MG, Stubbs JB, Toohey RE. Radiation dose estimates for radiopharmaceuticals, NuReg/CR 6345ed., vol. NuReg/CR 6345. Oak Ridge Institute for Science and Education; Oak Ridge: 1996. [Google Scholar]
- 15.Ramakrishnan C, V P. Radiation doses to patients from X-ray examinations involving fluoroscopy. Indian J Radiol Imaging. 2001;11:181–4. [Google Scholar]
- 16.Mazonakis M, Damilakis J, Varveris H, Gourtsoyiannis N. Therapeutic external irradiation in women of reproductive age: risk estimation of hereditary effects. Br J Radiol. 2004;77:847–50. doi: 10.1259/bjr/88840344. [DOI] [PubMed] [Google Scholar]
- 17.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 18.Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod. 2003;69:701–7. doi: 10.1095/biolreprod.103.016352. [DOI] [PubMed] [Google Scholar]
- 19.da Cruz AD, de Melo e Silva D, da Silva CC, Nelson RJ, Ribeiro LM, Pedrosa ER, Jayme JC, Curado MP. Microsatellite mutations in the offspring of irradiated parents 19 years after the Cesium-137 accident. Mutat Res. 2008;652:175–9. doi: 10.1016/j.mrgentox.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz JL, Jordan R, Sun J, Ma H, Hsieb AW. Dose-dependent changes in the spectrum of mutations induced by ionizing radiation. Radiat Res. 2000;153:312–7. doi: 10.1667/0033-7587(2000)153[0312:ddcits]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Crow JF. The origins, patterns and implications of human spontaneous mutation. 2000;1:40–6. doi: 10.1038/35049558. www.nature.com/reviews/genetics. [DOI] [PubMed]
- 22.Eichenlaub-Ritter U, Adler ID, Carere A, Pacchierotti F. Gender differences in germ-cell mutagenesis and genetic risk. Environ Res. 2007;104:22–36. doi: 10.1016/j.envres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Russell LB, Russell WL. Frequency and nature of specific-locus mutations induced in female mice by radiations and chemicals: a review. Mutat Res. 1992;296:107–27. doi: 10.1016/0165-1110(92)90035-8. [DOI] [PubMed] [Google Scholar]
- 24.Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995, NIH Pub. No. 99-4649 ed. National Cancer Institute; Bethesda, Maryland: 1999. [Google Scholar]
- 25.Natarajan S, Lipsitz SR, Rimm E. A simple method of determining confidence intervals for population attributable risk from complex surveys. Stat Med. 2007;26:3229–39. doi: 10.1002/sim.2779. [DOI] [PubMed] [Google Scholar]
- 26.Sankaranarayanan K, Chakraborty R. Ionizing radiation and genetic risks. XI. The doubling dose estimates from the mid-1950s to the present and the conceptual change to the use of human data on spontaneous mutation rates and mouse data on induced mutation rates for doubling dose calculations. Mutat Res. 2000;453:107–27. doi: 10.1016/s0027-5107(00)00108-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.