Abstract
In this study, we examined whether functional subunits of the ATP-dependent K+ channel (KATP) are expressed in trigeminal ganglia (TG), which contains sensory neurons that innervate oral and facial structures. We also investigated whether direct activation of the KATP effectively attenuates mechanical hypersensitivity in the context of an acute orofacial muscle pain condition. The KATP expression in TG and behavioral studies were conducted in age matched male and female Sprague Dawley rats. RT-PCR experiments showed that the mRNAs for the inwardly rectifying pore-forming subunits, Kir6.1 and Kir6.2, as well as the regulatory sulphonylurea subunits, SUR1 and SUR2, were reliably detected in TG. Subsequent western blot analysis confirmed that proteins for all 4 subunits are expressed in TG, and showed that Kir6.2 is expressed at a significantly higher level in male TG compared to that of female rats. This observation was confirmed by the immunohistochemical demonstration of higher percentages of Kir6 positive masseter afferents in female rats. Masseteric injection of capsaicin evokes a time dependent increase in masseter sensitivity to noxious mechanical stimulation. A specific KATP agonist, pinacidil, dose-dependently attenuated the capsaicin-induced mechanical hypersensitivity in male rats. The dose of pinacidil (20µg) that completely blocked the capsaicin responses in male rats was ineffective in female rats regardless of their estrus phases. Only at the highest dose (300µg) we used, pinacidil was partially effective in female rats. Similarly, another KATP agonist, diazoxide which targets different KATP subunits also showed sex specific responses in attenuating capsaicin-induced masseter hypersensitivity. These data suggested that sex differences in functional KATP expression in TG may underlie sex specific responses to KATP agonists. The present study provided novel information on sex differences in KATP expression in TG and its contribution under an orofacial muscle pain condition.
Keywords: trigeminal ganglia, muscle pain, peripheral, potassium channels, sex differences
1. Introduction
ATP-sensitive potassium channels (KATPs) are inwardly rectifying K+ channels (Kir6 family), and they consist of two structurally different subunits: a pore-forming subunit of the Kir6-family (Kir6.1 or Kir6.2) and a sulfonylurea receptor (SUR1 or SUR2) with regulatory activity (O’Rourke, 2000). Predominantly described in cardiac myocytes KATPs are believed to be cytoprotective, capable of preserving ionic homeostasis during metabolic stress, such as ischemic attacks (Chicco et al., 2007). In pancreatic cells, the KATP, which is regulated by intracellular ATP level, promotes the release of insulin during high glucose metabolism (Craig et al., 2008). KATP activation in neurons leads to membrane hyperpolarization, which results in reduced excitability and neurotransmitter release (Amoroso et al., 1990; Watts et al., 1995; Ye et al., 1997; Yamada et al., 2001).
At the spinal and supra-spinal levels KATPs mediate analgesia as a downstream target of opioid receptors through the activation of Gi/o proteins (North et al., 1987; Ocaña et al., 1990, 1993, 1995; Kang et al., 1998; Yang et al., 1998). Similarly, pharmacological manipulation of KATPs modulates the anti-hyperalgesic effects induced by peripheral opioid receptors in various pain models (Rodrigues and Duarte, 2000; Granados-Soto et al., 2002; Ortiz et al., 2002; Picolo et al., 2003; Amarante et al., 2004; Pacheco and Duarte, 2005), suggesting the involvement of KATPs in nociceptive processing at the level of primary afferent neurons.
Recent studies demonstrated that KATP subunits are expressed in sensory neurons in dorsal root ganglia (DRG), and that direct activation of KATPs regulates membrane excitability and reverses the inflammation-induced sensitization in dissociated DRG neurons (Chi et al., 2007; Kawano et al., 2009a). These data clearly suggest that KATPs in DRG neurons are functional and may potentially be an important therapeutic target for pain management. However, there is no information on whether KATP is also expressed in sensory neurons in trigeminal ganglia (TG), and whether direct activation of KATP can modulate pain arising from orofacial structures.
In the heart muscle, activation of KATP significantly reduces myocardiac infarct size and pharmacological blockade of KATP abolishes the infarct-sparing preconditioning effects of exercise, in a sex-dependent manner (Johnson et al., 2006; Chicco et al., 2007). A greater protective role of KATP in females can, in part, be explained by the greater expression levels of Kir6.2 and SUR2 in the myocardium from females than from males (Ranki et al., 2001; Brown et al., 2005). Treatment with 17β estradiol elicits a marked increase in the expression of both Kir6.2 and SUR2A (Ranki et al., 2002), further suggesting that sex differences in the mechanism of KATP are present at the expression level in the myocardium. Thus, it would be interesting to know whether there are sex differences in KATP expression in sensory neurons, and whether activation of KATP in sensory neurons leads to sex differences in anti-hyperalgesic responses.
In this study, we specifically investigated whether 1) all four subunits of KATP are expressed in TG, 2) there are sex differences in KATP expression in TG, and 3) direct activation of KATP results in attenuation of capsaicin-induced mechanical hyperalgesia in the orofacial muscle in a sex dependent manner.
2. Experimental Procedures
2.1 Animals
Adult male and female Sprague Dawley rats (250–350gm; Harlan, Indianapolis) were used in the present study. All animals were housed in a temperature-controlled room under a 12:12 light-dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and under a University of Maryland approved Institutional Animal Care and Use Committee protocol.
2.2 Determination of estrus phases
For females rats in which estrus cycle phase was determined, microscopic cytology of vaginal smears was examined daily for 2 weeks prior to behavioral experiments and tissue extraction. The estrus cycle phase of a female rat can be determined by observing the appearance of various cell types within the vaginal smear, which correlates with the status of the vaginal mucosa, uterus, and ovaries as well as the concentrations of the circulating sex steroids and gonadotropins (Goldman et al., 2007). Female rats in either the proestrus (Pro; predominantly nucleated epithelial cells, complete absence of leukocytes) or the diestrus (Di; predominantly leukocytes) phase were used for the experiments (Goldman et al., 2007, Marcondes et al., 2002).
2.3 RT-PCR analyses
Total RNA was extracted from TG with Trizol (Sigma) and purified according to the RNeasy kit (Qiagen) that included a DNase treatment to remove genomic DNA. Reverse transcription was carried out using the Superscript First strand synthesis kit (Invitrogen). SuperScript II (Invitrogen) was used to generate cDNA from 1µg of RNA along with 2.5ng of random primer per reaction. The following were the primers for two structurally different subunits of KATP: a pore-forming subunit of the Kir6 family (Kir6.1 and Kir6.2) and a sulfonylurea receptor (SUR1 and SUR2) with regulatory activity: Kir6.1: sense 5’-AGCCACTGACCTTGTCAACC-3’, antisense 5’-GGAGTCATGAATTGCACCTT-3’; Kir6.2: sense 5’-CTGCCTTCCTTTTCTCCATC-3’, antisense 5’-TTACCACCCACACCGTTCTC-3 ’ ; S U R 1 : s e n s e 5 ’-TGGACCCAGAGAAGAAATGC-3’, antisense 5’-ACAAAGGAGGCAAAGACGC-3’; SUR2: sense 5’-CGAAAGAGCAGCATACTCATTA-3’, antisense 5’-CCTCTCTTCATCACAATGACC-3’. PCR products were separated by gel electrophoresis using a 2% agarose gel in TAE buffer containing ethidium bromide and visualized with an imaging system (Kodak ID 3.5).
2.4 Western blot experiment
TG were homogenized in a lysis buffer including a protease inhibitor cocktail. Denatured proteins were fractionated on NuPAGE gels with a running buffer containing SDS (Invitrogen). Fractionated proteins were transferred onto PVDF membranes. After blocking with 5% milk in 1x phosphate buffer solution (PBS) for one hour at room temperature, the membranes were incubated in primary antibodies at 4°C overnight. The bound primary antibodies were detected with appropriate secondary antibodies conjugated to HRP. The immunocomplex was visualized with ECL reagents. Four KATP subunit antibodies were used. (1) A polyclonal rabbit antibody corresponding to amino acid residues 382–396 of rat Kir6.1 was raised against the peptide C-KRNSMRRNNSMRRSN (1:500, Alomone Labs). (2) A polyclonal rabbit antibody corresponding to amino acid residues 372–385 of rat Kir6.2 was raised against the peptide C-SVAVAKAKPKFSIS (1:500, Alomone Labs). (3) A polyclonal goat antibody was raised against a peptide mapping the c-terminus of human SUR1 (1:200, Santa Cruz Biotechnology). (4) A polyclonal goat antibody was raised against amino acids 921–1000 mapping an internal region of human SUR2 (1:200, Santa Cruz Biotechnology). The membranes were re-probed with anti-GAPDH antibody and the protein levels for each KATP subunit were normalized to that of GADPH in the same sample. For peptide control experiments, samples were incubated with control antigen provided by the respective companies in combination with the primary antibody. Antibody:antigen concentrations were as follows: Kir6.1 1:5, Kir6.2 1:3, SUR1 1:3, SUR2 1:3.
2.5 Immunohistochemistry for KATP subunits in masseter afferents
Fast Blue (FB) (2%; 10µl) was injected into multiple sites in the masseter muscle, using aseptic techniques, in order to retrogradely label muscle afferents in the TG of three male and three female rats. To avoid any leakage of the tracer the injection needle was left in place for 1–2 min before it was slowly retracted. After 7 days, animals were perfused transcardially with PBS followed by 4% paraformaldehyde in PBS (250 ml; pH 7.2). The TG from each rat were extracted and post-fixed at 4°C overnight, placed in 30% sucrose solution at 4°C overnight and sectioned coronally at 12µm. Every eighth section was collected and mounted on gelatin-coated slides for double-labeling immunohistochemistry. The sections were incubated overnight with the primary antisera for Kir6.1 (1:1000) or Kir6.2 (1:1500), the same antibodies described in the Western blot experiment. For immunofluorescence the sections were incubated with Alexa 488 conjugated goat anti-rabbit antiserum (West Grove, PA; 1:250) at room temperature for one hour. The primary antibody for each KATP subunit was omitted from the processing of selected sections to control for non-specific background staining.
Kir6 subunits and FB positive cells were counted from 8 representative sections per ganglion from three male and three female TG. Trigeminal and facial motor nuclei were also evaluated as positive and negative controls for FB labeling, respectively. Only the labeled neurons that showed a clear nucleolus were included in the counting. Percentages of masseter afferents double labeled with Kir6.1 or Kir6.2 were calculated and presented as mean ± standard error of the mean (SE). The estrus cycle phase of female rats were not determined in these experiments.
2.6 Behavioral studies
It is well established that noxious chemical or mechanical stimulation of the masseter muscle evokes characteristic shaking of the ipsilateral hindpaw in lightly anesthetized rats (Han et al., 2008; Ro et al., 2003; Sánchez et al., 2010). We have previously described the use of this behavior for testing mechanical sensitivity of the masseter muscle in rats (Ro et al., 2007, 2009). Briefly, rats were initially anesthetized with an intraperitoneal injection of sodium pentobarbital (40mg/kg for male, 35mg/kg for female). A level of ‘light’ anesthesia was determined by providing a noxious pinch to the tail or the hindpaw with a serrated forceps. Male rats typically responded to the noxious pinch on the tail with an abdominal contraction and with a withdrawal reflex to the noxious pinch of a hindpaw within 30 min after the initial anesthesia. It typically took 45 to 60 min for female rats to show similar responses. Once the animal reached this level a metal clip calibrated to produce 600 gm of force was applied 5 consecutive times, and experiments were continued only after the animals showed reliable reflex responses to every clip application. A tail vein was connected to an infusion pump (Harvard Apparatus, Pump11) for continuous infusion of pentobarbital. The rate of infusion was adjusted to maintain a relatively light level of anesthesia throughout the duration of the experiment (3–5 mg/h).
A baseline mechanical threshold for evoking the hindpaw responses was determined 15 min prior to drug injections using the electronic von Frey (VF) anesthesiometer (IITC Life Science, Inc, Woodland Hills, CA). A rigid tip (2–3mm diameter) attached to the VF meter was applied to the masseter muscle until the animals responded with hindpaw shaking. The animal’s head was rested flat against the surface of the table when pressing the anesthesiometer on the masseter in order to provide a stable set-up. The threshold was defined as the lowest force necessary to evoke the hindpaw response.
Changes in masseter sensitivity were then assessed at 15, 30, 45, 60 and 90 min following drug treatments. We calculated percent changes in VF thresholds following drug treatments with respect to the baseline threshold and plotted against time. In order to assess the overall magnitude of drug-induced changes in masseter sensitivity over time, the area under the curve (AUC) was calculated for the normalized data for each rat using the trapezoid rule. All behavioral observations were made by one experimenter who was blinded to the experimental conditions in order to maintain the consistency of assessing behavioral responses. All animals were kept warm throughout the experiments with thermal blankets.
2.7 Experimental and control groups for behavioral studies
To examine whether activation of KATP blocks capsaicin-induced mechanical hypersensitivity, the masseter muscle was pretreated with a specific KATP opener, pinacidil (2, 20, 100, 300µg/10µl) or the vehicle 5 min prior to capsaicin (0.1%, 100µl) injection in both male and female rats. There is a possibility that pinacidil injected into the masseter can mediate its effects by activating centrally located KATPs. Therefore, in order to evaluate possible systemic effects, the highest dose of pinacidil (300µg) was administered into the masseter muscle contralateral to the capsaicin injection in a separate group of animals. In order to examine potential hormonal influences on the KATP effect, a dose of pinacidil (20µg), which showed significant effects in male rats, was tested in female rats in Pro and Di phases. Finally, another KATP opener, diazoxide (100, 300µg/10µl) was tested in the same manner to confirm the functional role of the KATP.
2.8 Drug preparation and administration
Capsaicin (0.1%; Sigma) was dissolved in ethanol (23%), Tween 80 (7%) and PBS (70%). DPDPE (Tocris Cookson) was dissolved in PBS. Pinacidil and diazoxide (Tocris Cookson) were dissolved in DMSO. In order to make sure that the drugs and vehicles were administered in the same target region of the muscle the injection site was determined by palpating the masseter muscle between the zygomatic bone and the angle of the mandible. Injections were made with a 27-gauge needle. Upon contacting the mandible the needle was slowly withdrawn into the mid-region of the masseter and injections were made for 5–10 seconds.
2.9 Data Analysis
The Western blot data were analyzed with a one-way ANOVA and the immunohistochemistry data with a t-test. For behavioral studies, the time-dependent mean percent changes in mechanical thresholds were normalized to the baseline threshold and analyzed with a two-way ANOVA with repeated measures. In addition, either the student t-test or one-way ANOVA was used to evaluate the overall magnitude of mechanical hypersensitivity assessed as the area under the curve (AUC), which was calculated from the normalized data for each rat. All multiple group comparisons were followed by a post hoc test (Dunnett’s). The significance of all statistical analyses was set at p<0.05.
3. Results
3.1 Sex differences in KATP subunit expression in TG
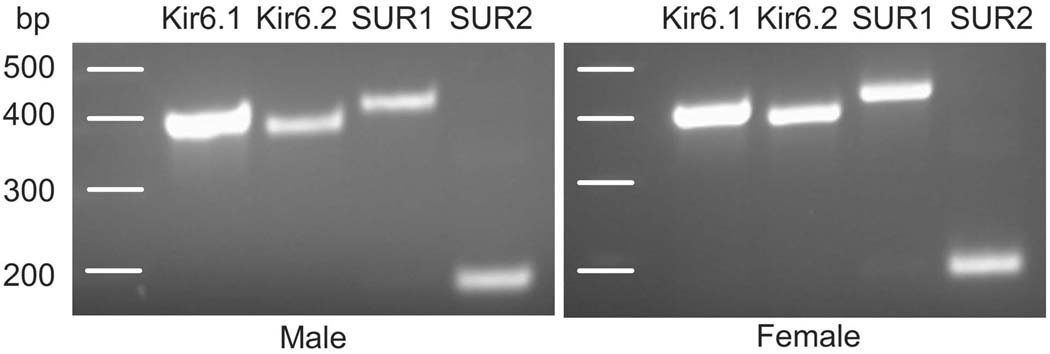
The genes for the four KATP subunits were amplified from TG cDNA through RT-PCR reactions and resulted in a single band for each subunit: two pore forming subunits, Kir6.1 (386 bp) and Kir6.2 (394 bp), and two regulatory subunits, Sulfonylurea Receptor SUR1 (402 bp) and SUR2 (173 bp) (Fig 1). The primers for SUR2 were designed to target the SUR2B splicing variant. Similar results were obtained from male and female rats, indicating that mRNAs of all the subunits necessary for forming functional KATPs are expressed in the trigeminal ganglia of both male and female rats.
Figure 1.
Conventional RT-PCR analysis demonstrated the presence of mRNAs for Kir6.1, Kir6.2, SUR1, and SUR2 subunits in the TG of both male and female rats.
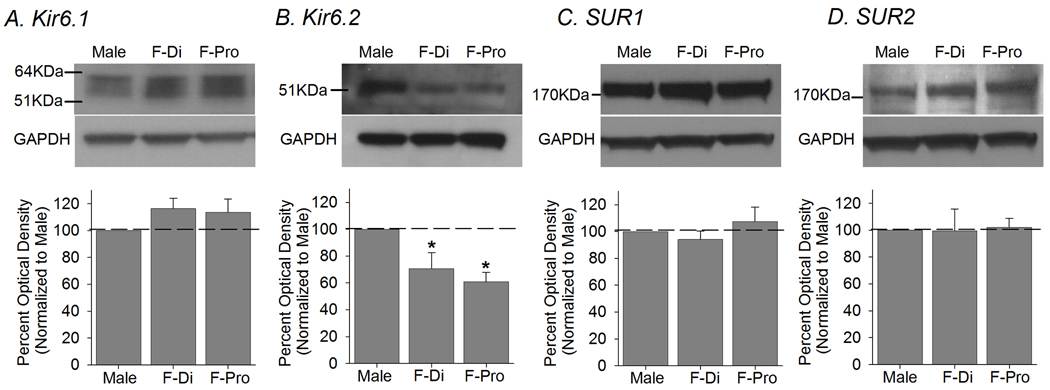
Subsequent Western blot experiments confirmed the RT-PCR data that protein for each KATP subunit is expressed in TG (Fig 2). A single band corresponding to Kir6.1, Kir6.2, SUR1 or SUR2 was reliably detected in the TG of male as well as female rats, regardless of the estrus phase. The antibody specificity was confirmed with peptide control experiments (data not shown), and the omission of primary antibody failed to produce any protein bands.
Figure 2.
Western blot experiments confirmed that protein for each KATP subunit is expressed in TG. (Top) Examples of immunoblots for Kir and SUR subunits along with GAPDH from TG of males and females in Pro and Di phases are shown. (Bottom) The group data showed that Kir6.2 protein level in female TG was substantially less compared to that of male. Other KATP subunits were expressed at comparable levels between the two sexes (n=6 for each group). p<0.05
In order to normalize KATP expression to GAPDH, a standard curve for GAPDH was generated to determine the amount of protein that falls within the linear range of the optical density. The western blot analysis showed that there were no significant differences in the levels of Kir6.1, SUR1 or SUR2 protein expression between the TG of male and that of female rats in either Pro or Di estrus phase (Fig 2A,C,D). However, the level of Kir6.2 protein in TG of female rats was significantly lower than that of male TG (F=6.48, p=0.009; Fig 2B). A post-hoc analysis showed that the level of Kir6.2 protein in females in either Di or Pro phase was significantly less than that of male TG.
3.2 Sex differences in KATP subunit expression in masseter afferents in TG
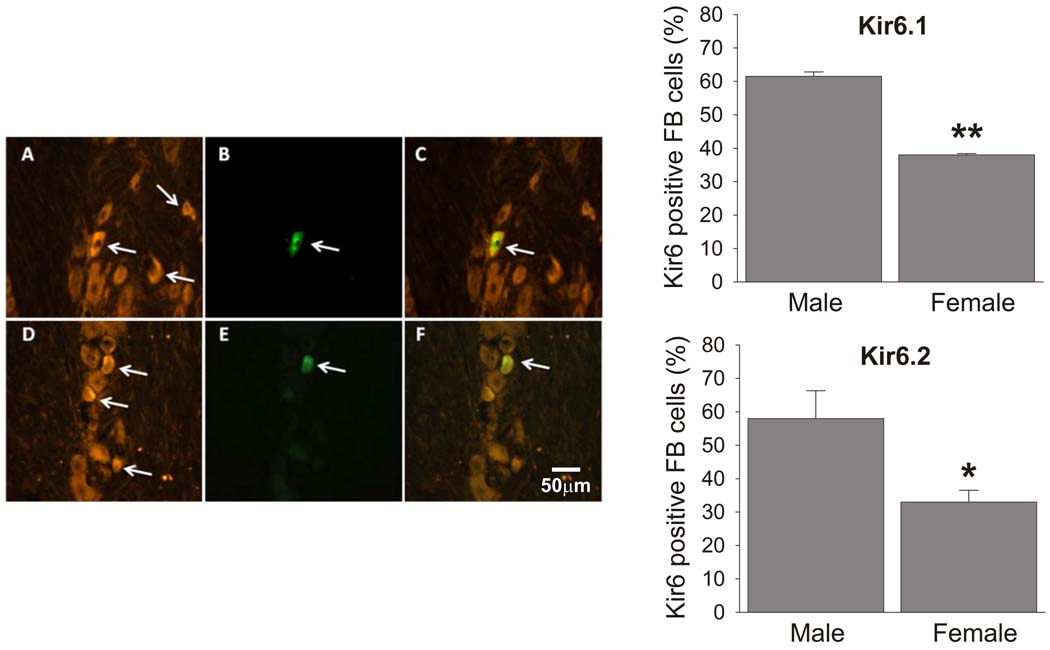
The western blot analysis detected bulk expression levels of the KATP subunits in entire TG. In order to confirm the sex differences in Kir6.2 expression at a more functionally relevant level, we compared the percentages of masseter afferents expressing Kir6.1 or Kir6.2 between male and female rats. Both Kir6.1 and Kir6.2 positive neurons were widely distributed in cells with all soma size in all three divisions of the TG. There were no obvious differences in morphological features between Kir6.1 and Kir6.2 positive neurons. The retrograde labeling by FB was limited to the mandibular division of TG and appeared to label small to medium size TG neurons (Ro et al., 2009). FB labeled neurons could also be found in the trigeminal motor nucleus while there were no labeled cells in the facial motor nucleus, suggesting that the tracer did not leak out to overlying cutaneous tissue. Both Kir6 subunits were localized in FB positive muscle afferents in the TG of both male and female rats (Fig 3A–F). The percentage of Kir6.1 positive muscle afferents in male TG (61.5%±2.6 from 183 neurons) was significantly higher than that in female rats (38%±0.96 from 136 neurons) (Fig 3). The percentage of Kir6.2 positive muscle afferents in male TG (58%±16.6 from 196 neurons) was also significantly higher than those in female rats (33%±7 from 178 neurons, respectively) (Fig 3).
Figure 3.
Kir6.1 and Kir6.2 are expressed in trigeminal ganglion neurons (A and D, respectively). The somata of masseter afferents labeled by retrograde transport of Fast Blue (FB; B and E) expressed Kir6.1 (C) or Kir6.2 (F). Bar graphs represent sex differences in the percentages of Kir6.1 (top) and Kir6.2 (bottom) positive masseter afferents in TG. * p<0.05, **p<0.01 (n= 4 in each group). The estrus cycle phase of the female rats were not determined for these experiments.
3.3 Sex differences in anti-hyperalgesic effects of the peripheral KATP
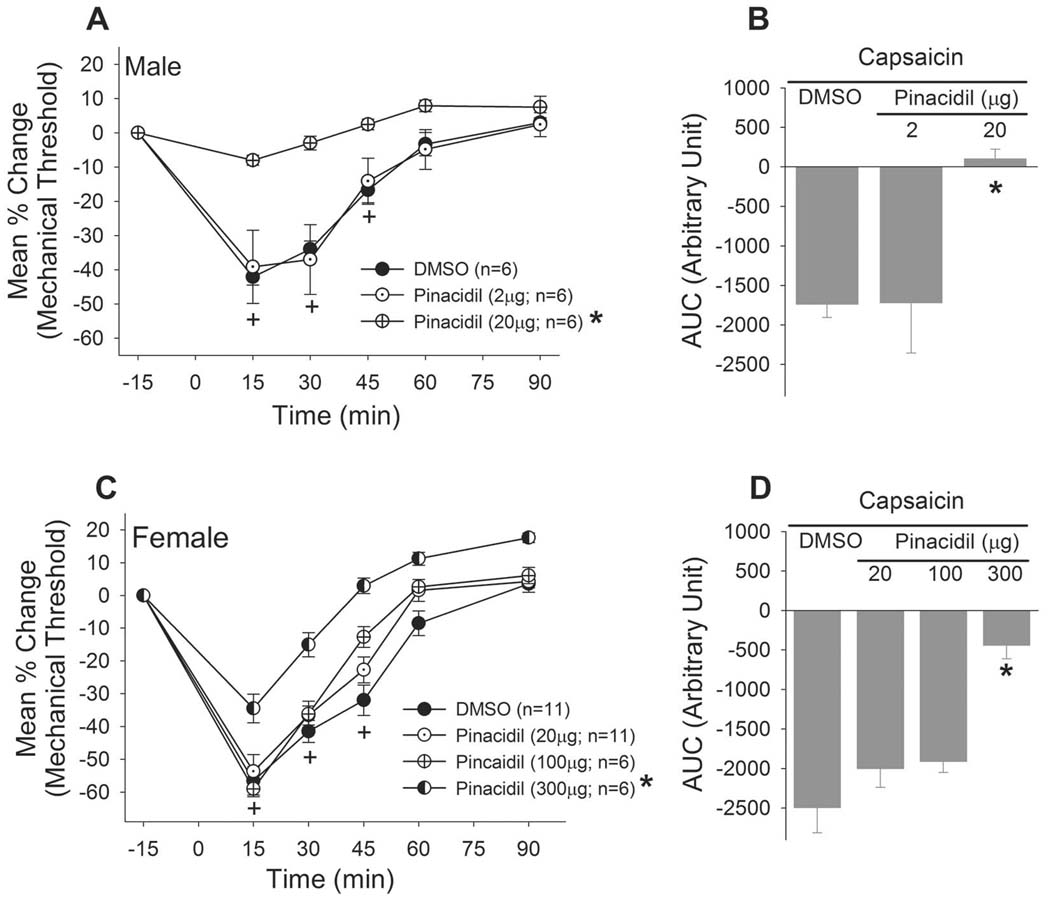
We then examined whether direct activation of KATP modulates pain responses arising from the masseter, and whether there are sex differences in the function of the KATP. The baseline mechanical threshold that evoked the responses ranged approximately 500–700 g, which did not differ between the sexes. This range of baseline was consistent across various treatment conditions and also with our previous studies (Lee and Ro, 2007). Both male and female rats developed acute mechanical hypersensitivity in the masseter muscle following capsaicin administration. Female rats tended to show a greater level of hypersensitivity compare to males, but the difference in AUC at baseline was not statistically significant (t=1.839, p=0.096). Pretreatment of them asseter with a specific KATP opener, pinacidil, dose-dependently and significantly attenuated the capsaicin-induced masseter hypersensitivity in male rats (Fig 4A). Pinacidil at 20µg completely blocked the overall hypersensitivity responses (Fig 4B). The same dose of pinacidil was ineffective when given in female rats (Fig 4C). A 15 fold higher dose of pinacidil (300µg) partially, but significantly, attenuated the capsaicin-induced masseter hypersensitivity in female rats (Fig 4C,D). Pinacidil (300µg) was without effect when injected in the contralateral masseter muscle suggesting that the drug effect is not due to systemic responses.
Figure 4.
(Left) Line graphs show the time course of the effects of a specific KATP opener, pinacidil, on the capsaicin-induced masseter hypersensitivity. The dose of pinacidil (20µg) that effectively attenuated the masseter hypersensitivity in male rats did not produce significant changes in female rats. Pinacidil at 300µg significantly attenuated the masseter hypersensitivity in female rats (Right). Bar graphs, which represent the overall magnitude of the response, show similar results. p<0.05
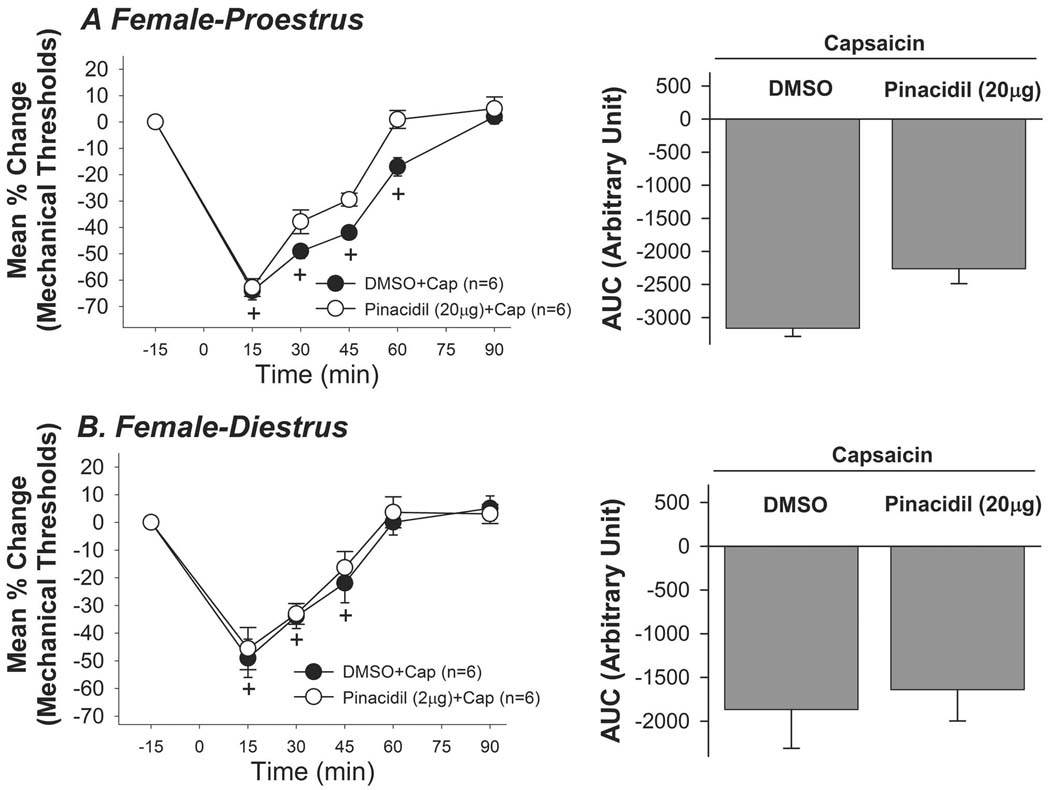
In order to investigate whether the different estrus phases influence the pinacidil effect, we repeated parts of the behavioral experiments in female rats in both the proestrus (Pro) and diestrus (Di) phases of the estrus cycle. We chose to test pinacidil at 20µg since this dose completely attenuated the masseter hypersensitivity in male rats. Pinacidil failed to attenuate the capsaicin-induced mechanical hypersensitivity in female rats in either the Pro or Di phase (Fig 5A,B). These data confirmed that peripheral application of pinacidil is less potent in female rats regardless of the estrus phase.
Figure 5.
20µg of pinacidil failed to attenuate the capsaicin-induced mechanical hypersensitivity in female rats in either the Pro (A) or Di (B) phase.
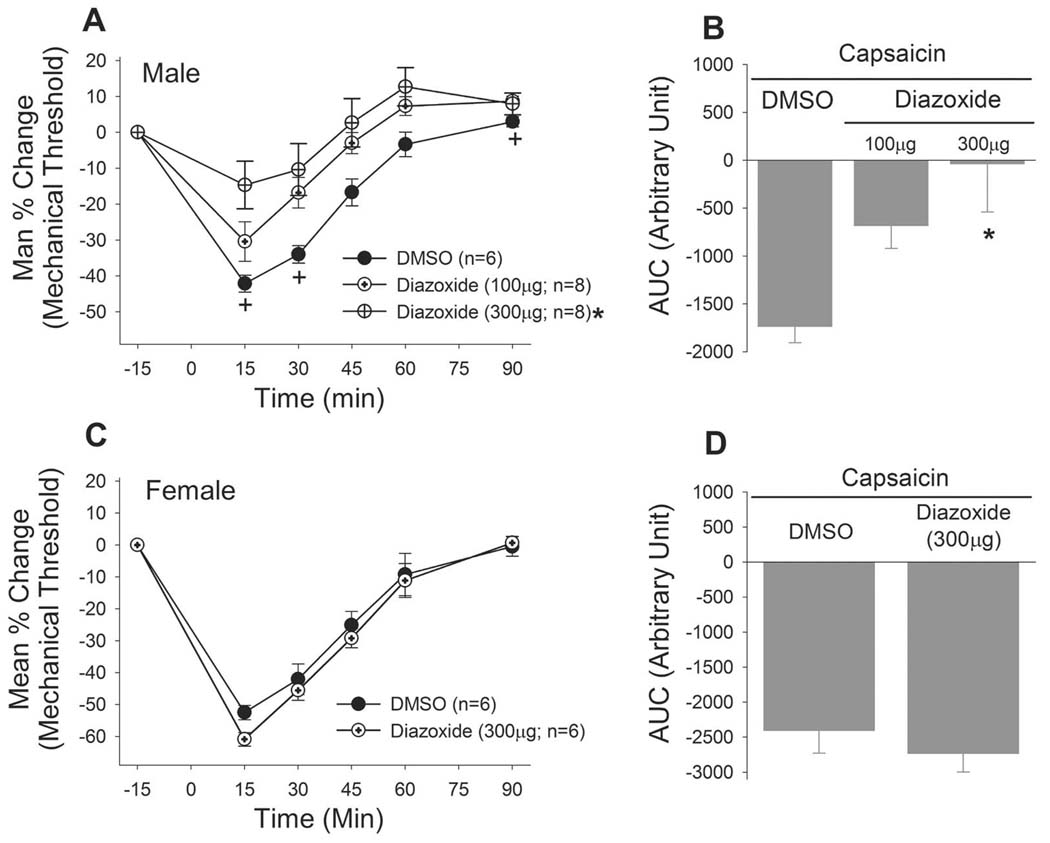
We confirmed the sex differences in the effects of KATP activation with another KATP opener, diazoxide. Diazoxide also significantly attenuated the mechanical hypersensitivity in male rats (Fig 6A). The high dose of diazoxide (300µg), administered in the same manner, failed to block the capsaicin-induced mechanical hypersensitivity in female rats (Fig 6B). Since the pinacidil effect was not influenced by estrus stage in female rats we did not test the diazoxide effect in females in different estrus phases.
Figure 6.
The sex differences in the anti-hyperalgesic effects of KATP were confirmed with another KATP opener, diazoxide.
4. Discussion
4.1 Expression of KATP subunits in TG
Functional KATPs require both Kir6 and SUR subunits (Ocaña et al., 2004). In the present study, we demonstrated that mRNA and protein for all four subunits of the KATP, i.e. Kir6.1, Kir6.2, SUR1 and SUR2, are expressed in TG. The Kir6 subunit expression in TG was further confirmed by the immunohistochemistry data. Two recent studies report the presence of the KATP in the rat DRG, but in one case SUR2 was not detected and in another Kir6.1 (Chi et al., 2007; Kawano et al., 2009a), suggesting that the subunit compositions of KATP in DRG may be different from those in TG. However, neither of the two studies employed both mRNA and protein analyses in the same study. In an earlier study, mRNAs for all 4 subunits were indeed detected in DRG (Kawabata et al., 2006). Also, functional KATPs composed of SUR1 with Kir6.1 and Kir6.2 as well as SUR2 with Kir6.1 and Kir6.2 have been documented at the single cell level in DRG (Chi et al., 2007; Kawano et al., 2009a). Thus, it is possible that since all subunits are also present in TG, the KATP composition is comparable between DRG and TG.
4.2 Sex differences in KATP expression in TG
Sex differences in KATP expression have been shown in cardiac muscle tissue. Higher levels of both Kir6.2 and SUR2 proteins in cardiac membrane fractions in female tissue compared with male tissue have been proposed as the cellular basis for sex differences in the susceptibility of the heart to infarction and exercise-induced cardioprotection (Ranki et al., 2001; Brown et al., 2005). Sex differences in myocardiac infarct size and exercise-induced cardioprotection are abolished when KATP is pharmacologically blocked, further suggesting that KATP plays a critical role in sex differences in cardiac tissue (Johnson et al., 2006; Chicco et al., 2007). There has been a lack of data on sex differences in the expression of KATP in other cell types including neurons. Thus, we provide the first evidence that there are sex differences in KATP expression in trigeminal sensory neurons.
Specifically, we showed that the relative expression level of Kir6.2 in TG was significantly lower in female rats regardless of the estrus stage. The immunohistochemistry data confirmed that Kir6.2 was expressed at a relatively lower level in masseter afferents in female rats. The discrepant results between western blot and immunohistochemistry data for Kir6.1 were surprising. A possible explanation could be that the sex difference in Kir6.2 is widespread in all sensory neurons whereas the sex difference in Kir6.1 is selective for certain afferent types, such as muscle afferents. A lower level of Kir6 subunits in female compared to male rats may contribute to higher nociceptive responses in female rats.
4.3 KATP in TG exerts anti-hyperalgesic effects in a sex-dependent manner
KATP mediates approximately 10–25% of total K+ currents in DRG neurons, which is significantly attenuated following peripheral nerve injury (Sarantopoulos et al., 2003). The loss of KATP currents after painful nerve injury occurs selectively in large diameter primary afferents, which has been proposed as an underlying basis for phenotypic changes in large A-type afferents that contribute to the generation of neuropathic pain (Kawano et al., 2009a). Direct activation of KATP in small and medium size DRG neurons suppresses the neuronal hyperexcitability induced by prostaglandin, an inflammatory agent (Chi et al., 2007). These studies with dissociated preparations suggest that KATP in somatic sensory neurons modulate neuronal excitability under inflammatory or nerve injury conditions.
In this report, we provided additional evidence that KATP plays a functionally important role in modulating nociceptive processing in sensory neurons under acute an inflammatory condition. Using the established model of an acute orofacial myositis condition (Ro et al., 2003, 2007, 2009), we demonstrated that direct activation of KATP significantly attenuated the capsaicin-induced masseter hypersensitivity in a dose dependent manner. A specific agonist for KATP, pinacidil, which targets the SUR2 containing KATPs, and diazoxide, which is the only KATP opener that can activate SUR1 containing KATPs (Moreau et al., 2005), effectively attenuated the mechanical hypersensitivity at doses that do not produce systemic effects in male rats. These findings indicate that Kir6 subunits form functional KATPs with both SUR1 and SUR2 in TG neurons.
Peripherally localized KATPs function as a downstream effector of µ and δ, but not κ, opioid receptors, (Rodrigues and Duarte, 2000; Aramante et al., 2004; Pacheco and Duarte, 2005), and this effect is likely mediated by the nitric oxide and cGMP pathway (Sachs et al., 2004; Kawano et al., 2009b). Consistent with these observations we have recently shown that capsaicin-induced masseter hypersensitivity is attenuated by direct activation of δ opioid receptors (DOR) in TG and that the DOR-mediated anti-hyperalgesic responses are reversed when KATP is blocked by a specific KATP antagonist, glibenclamide (Saloman et al., 2010). Interestingly, the DOR-mediated responses were significantly more potent in male compared to female rats.
In this study, we showed that there are also sex differences in KATP-mediated anti-hyperalgesic responses in that KATP agonists were more potent in male compared to female rats. Since neither pinacidil nor diazoxide discriminates Kir6 subunits, these behavioral responses suggest that the sex differences, at least in part, result from the reduced level of functional Kir6.2 in female rats. Thus, it is possible that sex differences in peripheral DOR-mediated responses are expressed as a result of sex differences in the expression level of downstream targets such as KATP. Sex differences in nociceptive responses are eliminated in mice lacking a specific subunit of the G-protein coupled inwardly rectifying K+ channel (GIRK), which also serves as a downstream effector of opioid receptor activation in the spinal cord (Mitrovic et al., 2003). The impact of the deletion of the GIRK subunit on morphine is lower in female mice, suggesting that inwardly rectifying K+ channels confer sex differences in analgesia (Blednov et al., 2003). These data, along with the data on KATP expression and function in cardiac muscles, suggest K+ channels are a potentially important source of sex specific responses in the nociceptive system.
As the importance of sex differences in the management of acute and chronic types of pain is increasingly recognized, an understanding of the underlying mechanisms becomes critical for the development of sex specific therapeutic interventions. An important new finding in this study is that sex differences exist in KATP responses, which may result from the differential expression of Kir6 subunits in TG. Therefore, behavioral or cellular responses implicating K+ channels observed in one sex should not be generalized to both sexes. These findings have significant clinical implications since potassium channels themselves are considered as new targets for alleviating pain and hyperalgesia in acute and chronic pain conditions (Ocaña et al., 2004). The novel findings from this study should offer important new insights for the development of sex specific pharmacological treatments that can be directed at the peripheral trigeminal system to ameliorate persistent muscle pain conditions, such as temporomandibular disorders.
Acknowledgements
The authors thank Drs. Misono Hiroaki and Jongseok Lee for technical assistance in biochemical experiments. This study was supported by NIH grant RO1 DE19448 (JYR).
Abbreviations
- AUC
Area under curve
- DRG
Dorsal root ganglia
- FB
Fast blue
- KATP
ATP sensitive potassium channels
- SUR
Sulfonylurea receptors
- TG
Trigeminal ganglia
- VF
Von Frey
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: There are no conflicts of interest associated with the present study.
References
- Amarante LH, Alves DP, Duarte ID. Study of the involvement of K+ channels in the peripheral antinociception of the kappa-opioid receptor agonist bremazocine. Eur J Pharmacol. 2004;494:155–160. doi: 10.1016/j.ejphar.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: Activation of GIRK2 channels. Proc Natl Acad Sci U S A. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol (Lond) 2005;569:913–924. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi XX, Jiang X, Nicol GD. ATP-sensitive potassium currents reduce the PGE2-mediated enhancement of excitability in adult rat sensory neurons. Brain Res. 2007;1145:28–40. doi: 10.1016/j.brainres.2007.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicco AJ, Johnson MS, Armstrong CJ, Lynch JM, Gardner RT, Fasen GS, Gillenwater CP, Moore RL. Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am J Physiol Heart Circ Physiol. 2007;292:H2432–H2437. doi: 10.1152/ajpheart.01301.2006. [DOI] [PubMed] [Google Scholar]
- Craig TJ, Ashcroft FM, Proks P. How ATP inhibits the open K(ATP) channel. J Gen Physiol. 2008;132(1):131–144. doi: 10.1085/jgp.200709874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth defects research. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Granados-Soto V, Arguelles CF, Ortiz MI. The peripheral antinociceptive effect of resveratrol is associated with activation of potassium channels. Neuropharmacology. 2002;43:917–923. doi: 10.1016/s0028-3908(02)00130-2. [DOI] [PubMed] [Google Scholar]
- Han SR, Lee MK, Lim KH, Yang GY, Jeon HJ, Ju JS, Yoon YW, Kim SK, Ahn DK. Intramuscular administration of morphine reduces mustard-oil-induced craniofacial-muscle pain behavior in lightly anesthetized rats. Eur. J. Pain. 2007;12(3):361–370. doi: 10.1016/j.ejpain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Moore RL, Brown DA. Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol. 2006;290:H2644–H2647. doi: 10.1152/ajpheart.01291.2005. [DOI] [PubMed] [Google Scholar]
- Kang YM, Zhang ZH, Yang SW, Qiao JT, Dafny N. ATP-sensitive K+ channels are involved in the mediation of intrathecal norepinephrine- or morphine-induced antinociception at the spinal level: a study using EMG planimetry of flexor reflex in rats. Brain Res Bull. 1998;45(3):269–273. doi: 10.1016/s0361-9230(97)00345-6. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kawao N, Hironaka Y, Ishiki T, Matsunami M, Sekiguchi F. Antiallodynic effect of etidronate, a bisphosphonate, in rats with adjuvant-induced arthritis: involvement of ATP-sensitive K+ channels. Neuropharmacology. 2006;51(2):182–190. doi: 10.1016/j.neuropharm.2006.03.015. 2006. [DOI] [PubMed] [Google Scholar]
- Kawano T, Zoga V, McCallum JB, Wu HE, Gemes G, Liang MY, Abram S, Kwok WM, Hogan QH, Sarantopoulos CD. ATP-sensitive potassium currents in rat primary afferent neurons: Biophysical, pharmacological properties, and alterations by painful nerve injury. Neuroscience. 2009a;162:431–443. doi: 10.1016/j.neuroscience.2009.04.076. [DOI] [PubMed] [Google Scholar]
- Kawano T, Zoga V, Kimura M, Liang MY, Wu HE, Gemes G, McCallum JB, Kwok WM, Hogan QH, Sarantopoulos CD. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation. Mol Pain. 2009b;14(5):12. doi: 10.1186/1744-8069-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz J Biol. 2002;62(4A):609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci U S A. 2003;100:271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Prost AL, Derand R, Vivaudou M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol. 2005;38:951–963. doi: 10.1016/j.yjmcc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaña M, Del Pozo E, Barrios M, Robles LI, Baeyens JM. An ATP-dependent potassium channel blocker antagonizes morphine analgesia. Eur J Pharmacol. 1990;186(2–3):377–378. doi: 10.1016/0014-2999(90)90466-j. [DOI] [PubMed] [Google Scholar]
- Ocaña M, Del Pozo E, Baeyens JM. ATP-dependent K+ channel blockers antagonize morphine- but not U-504,88H-induced antinociception. Eur J Pharmacol. 1993;230(2):203–207. doi: 10.1016/0014-2999(93)90803-p. [DOI] [PubMed] [Google Scholar]
- Ocaña M, Del Pozo E, Barrios M, Baeyens JM. Subgroups among mu-opioid receptor agonists distinguished by ATP-sensitive K+ channel-acting drugs. Br J Pharmacol. 1995;114(6):1296–1302. doi: 10.1111/j.1476-5381.1995.tb13346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaña M, Cendan CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: Present realities and future opportunities. Eur J Pharmacol. 2004;500:203–219. doi: 10.1016/j.ejphar.2004.07.026. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J Physiol. 2000;529(Pt 1):23–36. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz MI, Torres-López JE, Castañeda-Hernández G, Rosas R, Vidal-Cantú GC, Granados-Soto V. Pharmacological evidence for the activation of K(+) channels by diclofenac. Eur J Pharmacol. 2002;438(1–2):85–91. doi: 10.1016/s0014-2999(02)01288-8. [DOI] [PubMed] [Google Scholar]
- Pacheco DF, Duarte ID. Delta-opioid receptor agonist SNC80 induces peripheral antinociception via activation of ATP-sensitive K+ channels. Eur J Pharmacol. 2005;512:23–28. doi: 10.1016/j.ejphar.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Picolo G, Cassola AC, Cury Y. Activation of peripheral ATP-sensitive K+ channels mediates the antinociceptive effect of crotalus durissus terrificus snake venom. Eur J Pharmacol. 2003;469:57–64. doi: 10.1016/s0014-2999(03)01676-5. [DOI] [PubMed] [Google Scholar]
- Ranki HJ, Budas GR, Crawford RM, Jovanovic A. Gender-specific difference in cardiac ATP-sensitive K(+) channels. J Am Coll Cardiol. 2001;38:906–915. doi: 10.1016/s0735-1097(01)01428-0. [DOI] [PubMed] [Google Scholar]
- Ranki HJ, Budas GR, Crawford RM, Davies AM, Jovanovic A. 17Beta-estradiol regulates expression of K(ATP) channels in heart-derived H9c2 cells. J Am Coll Cardiol. 2002;40:367–374. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Ro JY, Capra N, Masri R. Development of a behavioral assessment of craniofacial muscle pain in lightly anesthetized rats. Pain. 2003;104:179–185. doi: 10.1016/s0304-3959(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain Res. 2007;1184:141–148. doi: 10.1016/j.brainres.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AR, Duarte ID. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K(+) channels. Br J Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci U S A. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloman JL, Niu K, Ro JY. Activation of peripheral δ-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. 2010 doi: 10.1016/j.neuroscience.2011.05.062. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez EM, Bagües A, Martín MI. Contributions of peripheral and central opioid receptors to antinociception in rat muscle pain models. Pharmacol. Biochem. Behav. 2010;96(4):488–495. doi: 10.1016/j.pbb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos C, McCallum B, Sapunar D, Kwok WM, Hogan Q. ATP-sensitive potassium channels in rat primary afferent neurons: the effect of neuropathic injury and gabapentin. Neurosci Lett. 2003;343(3):185–189. doi: 10.1016/s0304-3940(03)00383-5. [DOI] [PubMed] [Google Scholar]
- Watts AE, Hicks GA, Henderson G. Putative pre- and postsynaptic ATP-sensitive potassium channels in the rat substantia nigra in vitro. J Neurosci. 1995;15(4):3065–3074. doi: 10.1523/JNEUROSCI.15-04-03065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SW, Kang YM, Guo YQ, Qiao JT, Dafny N. ATP-sensitive potassium channels mediate norepinephrine- and morphine-induced antinociception at the spinal cord level. Int J Neurosci. 1998;93(3–4):217–223. doi: 10.3109/00207459808986427. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292(5521):1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- Ye GL, Leung CK, Yung WH. Pre-synaptic effect of the ATP-sensitive potassium channel opener diazoxide on rat substantia nigra pars reticulata neurons. Brain Res. 1997;753(1):1–7. doi: 10.1016/s0006-8993(96)01473-4. [DOI] [PubMed] [Google Scholar]