Abstract
Amyloidosis is a group of diverse disorders that fall into several major categories: primary, secondary, dialysis-associated, and hereditary forms. Clinically, amyloidosis may be categorized as localized or systemic. The gastrointestinal tract is among the most common places for deposition of amyloid, but large, localized amyloid deposits are an uncommon occurrence and rarely cause extraluminal bowel compression resulting in obstruction as was seen in the case presented in this clinical scenario.
Key Words: Amyloidosis, Bowel obstruction, Colon
Introduction
Amyloidosis is a group of diverse disorders characterized by extracellular deposits of abnormally folded protein that interferes with tissue function. These proteins form fibrillar, insoluble deposits with specific staining characteristics [1, 2]. Amyloidosis falls into several major categories: primary, secondary, dialysis-associated, and a variety of hereditary forms [2]. More than 25 different proteins are known to be involved in amyloidosis [1].
Primary amyloidosis is the most common form and is associated with the deposition of excess immunoglobulin light chains. This form is associated with multiple myeloma, lymphoma, and other lymphoproliferative disease. Secondary amyloidosis is associated with deposition of serum amyloid protein A in inflammatory and infectious conditions. Neoplastic conditions are less frequently associated with secondary amyloidosis. Patients on long-term hemodialysis form β2 microglobulin deposits [1, 2, 3, 4].
Amyloid deposits share distinct immunohistochemical characteristics and, at present, Congo Red staining of involved tissues remains the gold standard for detection [1]. These deposits appear pink under bright-field microscopy and have apple-green birefringence under polarized light. These two characteristics in combination are considered diagnostic of amyloid.
Clinically, amyloidosis may be categorized as localized or systemic [1]. Some forms are exclusively localized, including some cerebral amyloidoses, but most exist in both localized and systemic forms. In either case, the goals of treatment are to prevent organ dysfunction and to eliminate the source of the abnormal protein [1, 2].
The gastrointestinal tract is among the most common places for deposition of amyloid. The mouth is involved in 10–20% of primary amyloidosis patients, most commonly as macroglossia [2]. The esophagus, with symptoms including dysphagia, heartburn, and hematemesis, is involved in 13–22% of patients [2]. The stomach is involved in 8–12% of patients, but is usually asymptomatic [2, 5]. The small bowel is involved in up to 31%. Small bowel manifestations include diarrhea, constipation, obstruction, ischemia, and infarction [2, 3]. Large bowel involvement presents with symptoms mimicking ischemia, malignancy, or irritable bowel disease and can eventually result in obstruction, stricture, and perforation [2, 4].
Large, localized deposition of amyloid is an uncommon occurrence and gives rise to amyloid pseudo-tumor, or ‘amyloidoma’ [4]. Amyloidomas have been described in a variety of organs, including the heart, lungs, larynx, tongue, orbits, mediastinum, breast, bones, stomach, small bowel, and colon [1, 2, 3, 4, 5, 6, 7, 8].
We report a patient who presented with signs of bowel obstruction and was found to have a multitude of small amyloid implants throughout the abdomen and a solitary large amyloidoma encasing the ureters and rectosigmoid colon, resulting in obstruction.
Case Report
The patient was an 82-year-old male with a history of hypertension, osteoarthritis, and coronary artery disease who was in his usual state of fair health until approximately 3 years prior to the current hospital admission. At that time he noticed a slowly enlarging mass beneath his right mandible. A biopsy was performed that showed cells suggestive of a lymphoma. He then underwent resection of his submandibular gland for what was ultimately found to be an extranodal marginal zone B-cell MALT (mucosa-associated lymphoid tissue) lymphoma. He was treated at that time with cyclophosphamide, vincristine, and prednisone, and later with rituximab. Bone marrow biopsy showed no evidence of lymphoma. Laboratory studies showed that total serum protein, gamma chains, α1 protein, 1α2 protein, IgG, IgM, and β2 microglobulin were all elevated (table 1).
Table 1.
Selected laboratory values
| Test | Value | Normal range |
|---|---|---|
| Total serum protein | 8.2 | 5.8–8.0 |
| Gamma chains | 2.1 | 0.48–1.34 |
| αl protein | 0.4 | 0.1–0.3 |
| α2 protein | 1.1 | 0.48–1.34 |
| IgG | 2,108 | 666–1,494 |
| IgM | 418 | 25–231 |
| β2 microglobulin | 3.17 | <1.85 |
The patient again presented to medical attention approximately 12 months ago due to symptoms of constipation and partial bowel obstruction. Physical examination at that time showed a chronically ill-appearing elderly man with a distended abdomen and mild tenderness to deep palpation in the region of the umbilicus; there was no rebound tenderness and no guarding. Computed tomography of the abdomen showed a pelvic mass that measured 7.0 cm in greatest dimension and was localized to the left pelvic wall in the region of the rectosigmoid junction. Over the intervening 12 months he underwent additional rounds of chemotherapy and radiation for what was presumed to be a part of his lymphoproliferative disease, but was never proven on biopsy.
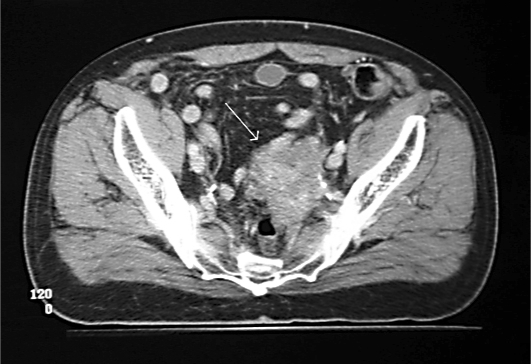
At the time of his final hospitalization, again for symptoms of bowel obstruction, he underwent a barium enema that showed a significant stricture at the rectosigmoid junction. The pelvic mass had progressively grown in size and now caused near-complete colonic obstruction. A contrast enema just prior to surgery revealed a string sign consistent with a long-segment, non-transient stricture of the colon at the rectosigmoid junction. Computed tomography confirmed the location of the enlarged mass in the pelvis (fig. 1).
Fig. 1.
Computed tomography of the abdomen demonstrates a pelvic mass with encasement of the left ureter and near-complete obstruction of the sigmoid colonic lumen (arrow).
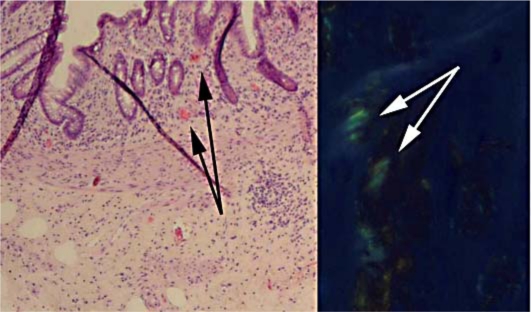
The patient was taken to the operating room for exploratory laparotomy with the intention of resecting the pelvic mass to relieve his obstruction. At laparotomy, he was found to have multiple small nodules in the abdomen and pelvis not visualized on CT scan. The dominant mass was found in the pelvis and completely encased his ureters, the iliac vessels, and the rectosigmoid colon. Given the extent of disease, it was felt that the mass could not be safely resected and to relieve his obstruction an end-colostomy was created. Microscopic examination of the multiple nodules that were excised showed extensive amyloid deposition. This histological finding was confirmed by typical findings on Congo Red staining (fig. 2). Special stains to determine the specific protein responsible for forming the amyloidoma were not performed.
Fig. 2.
Histopathology demonstrates typical amorphous pink staining under bright-field microscopy (black arrows) and apple-green birefringence on Congo Red staining under polarized light (white arrows). Magnification 120×.
The patient survived for approximately 2 months after surgery and died in hospice care.
Discussion
Amyloidoma is an uncommon manifestation of amyloidosis. Abnormal glycoprotein, usually light chain immunoglobulin, or less commonly serum amyloid A protein, is deposited locally, forming a solitary mass. Amyloidomas have been reported in a variety of anatomical sites including the gastrointestinal tract, central and peripheral nervous systems, soft tissue, bone, and the lung [7]. The distribution of amyloid usually displays an organ preference determined by its type [6]. The gastrointestinal tract is involved in 70–100% of patients. MALT lymphomas of the stomach are frequently associated with gastric Helicobacter pylori colonization, which this patient did not have. When MALT tumors are found at other sites they are frequently associated with chronic autoimmune diseases. MALT lymphomas have been associated with amyloidomas on a number of occasions and there are reported cases in whom intraluminal amyloidoma was the cause of intestinal obstruction [3, 4]. This is the first report we are aware of in which an extraluminal amyloidoma caused bowel obstruction by external compression.
The lack of specific radiological characteristics makes a preoperative diagnosis of amyloidoma extremely difficult. It is often mistaken for a malignant neoplasm such as metastatic carcinoma or lymphoma, and while it is not a true neoplastic process, amyloidosis can have devastating consequences for the patient. Accurate diagnosis is only possible with histological examination of the involved tissues. Staining with Congo Red and observation of apple-green birefringence under polarized light is essential to detect amyloid deposits [1].
Management of this patient's disease was complicated due to extensive spread throughout the peritoneal cavity and its proximity to vital structures. Surgical debulking has been described in treatment of limited disease by relieving the space-occupying effects of the amyloidoma [7]. Radiotherapy can be used to decrease the size of bone-occupying lesions. Aggressive chemotherapy has been used in cases where underlying lymphoproliferative disorders were known, sometimes in combination with marrow ablation and stem cell rescue [1, 3]. This patient's underlying extranodal marginal zone B-cell MALT lymphoma complicated the picture as amyloidosis is very rare in this subtype of malignancy. This erroneous assumption led to treatment of peritoneal disease for over a year with chemotherapy and radiation targeted at the lymphoproliferative malignancy without knowledge of the amyloidosis component. This case highlights the difficulty in diagnosis and management of this uncommon condition in combination with an underlying lymphoma, and the overall poor prognosis of disseminated amyloidosis.
References
- 1.Picken MM. Amyloidosis – where are we now and where are we heading? Arch Pathol Lab Med. 2010;134:545–551. doi: 10.5858/134.4.545. [DOI] [PubMed] [Google Scholar]
- 2.Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol. 2008;103:776–787. doi: 10.1111/j.1572-0241.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Cho HY, Ha SY, Chung DH, Kim NR, An JS. Marginal zone B-cell lymphoma of MALT in small intestine associated with amyloidosis: a rare condition. J Korean Med Sci. 2011;26:686–689. doi: 10.3346/jkms.2011.26.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raccanelli V, D'Amore FP. Localized AL amyloidosis of the colon and clinical features of intestinal obstruction. A case report. Ann Ital Med Int. 1999;14:58–60. [PubMed] [Google Scholar]
- 5.Dastur KJ, Ward JF. Amyloidoma of the stomach. Gastrointest Radiol. 1980;5:17–20. doi: 10.1007/BF01888593. [DOI] [PubMed] [Google Scholar]
- 6.Wilk W, Papla B. Amyloidoma and marginal zone malignant lymphoma (MALT type) in the gastric antrum. A case report. Pol J Pathol. 1998;49:183–186. [PubMed] [Google Scholar]
- 7.Podbielski FJ, Nelson DG, Pearsall GF, Marquez GD, Connolly MM. Nodular pulmonary amyloidoma. J Thorac Cardiovasc Surg. 1997;114:289–291. doi: 10.1016/S0022-5223(97)70161-2. [DOI] [PubMed] [Google Scholar]
- 8.Shiels SA, Hansan SI, Darowski A. Collapse in a 79-year-old: a rare case of amyloid tumour of the pelvis. Age Ageing. 2005;34:648–649. doi: 10.1093/ageing/afi161. [DOI] [PubMed] [Google Scholar]