Abstract
We examined whether absence or blocking of transient receptor potential vanilloid subtype 1 (TRPV1) affects the level of inflammation and fibrosis/scarring during healing of injured tissue using an alkali burn model of cornea in mice. A cornea burn was produced with 1 N NaOH instilled into one eye of TRPV1−/− (KO) (n = 88) or TRPV1+/+ (n = 94) mice. Examinations of the corneal surface and eye globe size suggested that the loss of TRPV1 suppressed inflammation and fibrosis/scarring after alkali burn, and this was confirmed by histology, IHC, and gene expression analysis. The loss of TRPV1 inhibited inflammatory cell invasion and myofibroblast generation in association with reduction of expression of proinflammatory and profibrogenic components. Experiments of bone marrow transplantation between either genotype of mice showed that KO corneal tissue resident cells, but not KO bone marrow–derived cells, are responsible for KO-type wound healing with reduced inflammation and fibrosis. The absence of TRPV1 attenuated expression of transforming growth factor β 1 (TGFβ1) and other proinflammatory gene expression in cultured ocular fibroblasts, but did not affect TGFβ1 expression in macrophages. Loss of TRPV1 inhibited myofibroblast transdifferentiation in cultured fibroblasts. Systemic TRPV1 antagonists reproduced the KO type of healing. In conclusion, absence or blocking of TRPV1 suppressed inflammation and fibrosis/scarring during healing of alkali-burned mouse cornea. TRPV1 is a potential drug target for improving the outcome of inflammatory/fibrogenic wound healing.
The cornea is an avascular transparent tissue located at the outermost part of the eye. It must remain transparent to properly refract light for normal vision. Ocular trauma resulting from a corneal alkali burn is a serious clinical problem and may cause severe and permanent visual impairment by inducing tissue inflammation, fibrosis, and scarring, leading to subsequent corneal opacification.1 The acute corneal injury sequence after alkali burn includes inflammation and degradation of the matrix of the epithelial basement membrane and stroma.2–4 Influx of inflammatory cells [ie, macrophages and/or polymorphonuclear leukocytes (PMNs)], activation of corneal fibroblasts (keratocytes), formation of myofibroblasts, and subsequent tissue scarring are all involved in the wound healing response in an alkali-burned cornea.2,3 Keratocyte activation results in myofibroblast transdifferentiation and tissue contraction with increased extracellular matrix expression.5 Despite aggressive treatment of severe injury with anti-inflammatory drugs and surgery, vision restoration often fails.1,6,7 This limitation is the basis for efforts to develop new and more effective prevention/treatment strategies.
Transient receptor potential (TRP) channels are polymodal receptors that are activated by a host of stimuli to mediate sensory transduction. The TRP superfamily is composed of 28 different genes that are subdivided into seven different subfamilies (TRPA, TRPC, TRPM, TRPML, TRPN, TRPP, and TRPV).8 Each of them possesses variable cation permeability. They are activated by multiple endogenous and external stimuli.9,10 They could be activated by the following: i) direct ligand binding, ii) depletion of intracellular Ca2+ store and Ca2+/calmodulin-dependent activation, and iii) indirect activation by osmotic stress, temperature variation, pheromones, taste, and mechanical as well as other stimuli.
The capsaicin receptor, TRPV1, is a nocioceptor and one of the isoforms belonging to the seven-member TRPV subfamily. It elicits responses to a variety of diverse noxious stimuli that include various ligand-like agents and a plethora of seemingly unrelated stimuli such as chemical irritants, inflammatory mediators, tissue-damaging stimuli, a decline in pH (<6.0), moderate heat (≥43°C), and hypertonic challenges. All of them lead to nocioceptions and evoke pain in human beings and pain-related behaviors in animals.11–14 TRPV1 is a cationic nonselective channel whose activation leads to increases in Ca2+ influx through a highly permeable cation channel, and has an outward-rectifying current–voltage relationship.15 TRPV1 activation causes release of tachykinin neuropeptides [eg, substance P (SP), neurokinin A, and calcitonin gene-related peptide] from sensory nerves, eliciting neurogenic inflammation in the surrounding area. Studies using mice lacking TRPV1 have shown that TRPV1 is essential for the development of heat hyperalgesia in response to tissue inflammation.16,17
The present study was undertaken to elucidate the role of corneal alkali burn–induced TRPV1 activation in eliciting inflammation and scarring during wound healing. The results show that loss of TRPV1 expression or blockage of its activation suppressed severe and persistent corneal inflammation and fibrosis/scarring, resulting in marked improvement in the restoration of tissue transparency.
Materials and Methods
Experimental protocols and the use of experimental mice were approved by the DNA Recombination Experiment Committee and the Animal Care and Use Committee of Wakayama Medical University and conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
IHC for TRPV1 in Wild-Type Mouse Eyes
Intact or alkali-burned mouse corneas were fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) for 24 hours, embedded in paraffin, and then processed for histology. Paraffin sections (5-μm thick) were deparaffinized, rehydrated, and subjected to immunohistochemistry (IHC) for TRPV1. The rabbit polyclonal anti-TRPV1 antibody (1:500; Neuromics, Edina, MN) was diluted in PBS.
Alkali Burn in Mouse Eyes
A total of 3 μL of 1 N NaOH solution was applied to the right eye of 6- to 8-week-old TRPV1-null (KO) (n = 88) mice or wild-type (WT) (n = 94) mice under general anesthesia to produce an ocular surface alkali burn.18,19 Ofloxacin ointment was administered topically twice a week to reduce the risk of bacterial infection. The eyes with obvious bacterial infection were excluded from the study. Eye globe diameters were measured from photographs obtained under a microscope. The corneal tissue then was processed for histology, IHC, Western blotting, or quantitative RT-PCR (qRT-PCR) on days 1, 2, 5, 10, and 20 after alkali burn.
Bone Marrow Transplantation and Ocular Alkali Burn
Reciprocal bone marrow transplantation (BMT) was performed. Briefly, BM cells were obtained by flushing the tibia and femur of experimental TRPV1 KO and WT mice with PBS. A total of 2 × 106 WT BM cells were transplanted via tail vein infusion into recipient mice that had received whole-body irradiation of 12 Gy before BMT (from WT mice to KO mice or vice versa). The mice were subjected to alkali burn on the right eyes 3 weeks after BMT, as described earlier. Ten days later, the experimental mice were sacrificed and excised corneas were subjected to histology and IHC examination. Repopulation of transplanted BM was confirmed by RT-PCR detection of TRPV1 mRNA in the spleens of transplanted mice (not shown).
To assess the percentage of macrophages derived from the transplanted BM in total macrophages in an alkali-burned, healing, corneal stroma with inflammation, we used a transgenic mouse with green fluorescent protein (GFP) expression (Riken, Tokyo, Japan). TRPV1−/−/GFP+/+ and TRPV1+/+/GFP+/+ mice were used as BM donors, and the recipient was a WT or a KO mouse. Three weeks after the BMT procedure, the cornea was affected by an alkali exposure as described earlier. Cryosections were cut and processed for F4/80 IHC (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) 10 days after the alkali treatment. After binding of tetramethyl rhodamine isothiocyanate (TRITC)-labeled secondary antibodies (1:200; Dako Cytomation, Carpinteria, CA), the specimens were observed under a microscope followed by mounting with VectaShield (Vector Laboratories, Burlingame, CA) for nuclear DAPI staining.
Treatment of Corneal Alkali Burn in WT Mice with TRPV1 Antagonists
We determined if the KO phenotype is reproduced by intraperitoneal injection into WT mice (n = 36) after a corneal alkali burn of one of two different TRPV1 antagonists. These antagonists [SB366791 (0.5 mg/kg)20 and JYL1421 (2.0 mg/kg)21] or their vehicle were administered daily until euthanasia. Ofloxacin ointment was administered topically twice a week to reduce the risk of bacterial infection. Infected eyes were excluded from the study. Eyes then were processed for histology or IHC at days 5, 10, and 20 after alkali burn.
Histology and IHC
Paraffin sections (5 μm) were processed for H&E staining and IHC as previously reported.19 The following antibodies were diluted in PBS: rabbit polyclonal anti-TRPV1 antibody (1:500; Neuromics), and mouse monoclonal anti–α smooth muscle actin (α-SMA) antibody (1:200; Neomarker, Fremont, CA). The presence of monocytes/macrophages was examined by using rat monoclonal F4/80 antimacrophage antigen antibody. Neutrophil presence was examined by using rabbit polyclonal myeloperoxidase (MPO) antibody (1:200; Neomarker). IHC for transforming growth factor β 1 (TGFβ1) was performed as previously reported.18,22 The antibody used here detects only the active form of TGFβ1, but does not react with the latent form. Negative control staining was performed by omission of each primary antibody and did not yield specific staining (not shown).
Western Blotting
To semiquantify the expression level of F4/80, α-SMA, and fibronectin we also conducted Western blotting as previously reported.23,24 In brief, the corneas were harvested in Sigma Mammalian Tissue Lysis buffer (Sigma-Aldrich, St. Louis, MO) (50 μL/4 corneas) or the cells were harvested in Sigma-Aldrich Mammalian Cell Lysis buffer (100 μL/dish) and processed for SDS-PAGE and Western blotting for F4/80 (clone A3-1, 1:1000; BMA Biomedicals, August, Switzerland), α-SMA (1:2000; Neomarker), and fibronectin (1:500; Santa Cruz Biotechnology) as previously reported.23,24 The membrane then was stripped and restained for β-actin.
qRT-PCR
Total RNA was extracted from corneal tissue excised from 4 burned mouse eyes using a Sigma RNA extraction kit (St. Louis, MO) according to the manufacturer's protocol and processed for qRT-PCR. The corneas were processed for total RNA extraction and qRT-PCR for collagen Ia1, α-SMA, F4/80, MPO, TGFβ1, vascular endothelial growth factor, monocyte/macrophage-chemoattractant protein-1 (MCP-1), IL-6, and SP.23 qRT-PCR using the TaqMan one-step RT-PCR master mix reagents kit and the Applied Biosystems Prism 7300 (Applied Biosystems, Foster City, CA) were used. Primers and oligonucleotide probes used are listed in Table 1 and were designed according to the cDNA sequences in the GenBank database, using Primers Express software (Applied Biosystems, Foster City, CA). Data at each time point were analyzed for significance by using the Mann–Whitney U test.
Table 1.
Primers and Oligonucleotide Probes Used
| Primer | Oligonucleotide Probe |
|---|---|
| α-SMA | Mm01204962_gh |
| F4/80 | Mm00802524_ml |
| MPO | Mm01298422_gl |
| Collagen 1a1 | Mm00801666_gl |
| MCP-1 | Mm9999056_ml |
| VEGF | Mm01281447_ml |
| SP | Mm01166996_ml |
| IL-6 | Mm01210732_gl |
| GAPDH | Mm03302249_gl |
α-SMA, α-smooth muscle actin; MPO, myeloperoxidase; MCP-1, monocyte chemoattractant protein-1; VEGF, vascular endothelial growth factor; SP, substance P; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
mRNA Expression of TGFβ1 by Macrophages
Mouse macrophages were obtained from the peritoneal cavity using a glycogen stimulation method.19 In brief, 1 mL of 5% sterilized oyster glycogen (Sigma-Aldrich) was injected into the peritoneal cavity of either a WT or KO mouse. After 4 days, the peritoneal cavity was irrigated with culture medium to harvest macrophages. Approximately 90% of the cells obtained by this method were positive for F4/80. The cells in medium were allowed to adhere to 60-mm culture dishes for 6 hours, and the nonadherent cells were washed out with PBS. The RNA extracted from the adherent cells (macrophages) was analyzed by qRT-PCR for TGFβ1 mRNA. Five dishes were prepared for each condition. Data were analyzed statistically by using the nonpaired Student's t-test.
Cell Culture Experiments of Ocular Fibroblasts
The eye shells (including cornea and sclera) of WT and KO mice were minced and explanted in a 60-mm culture dish (Falcon; Fisher Scientific, Waltham, MA) on postnatal day 1 for eliciting outgrowth of ocular fibroblasts. The primary cultured cells were used directly without passage. Cells were grown to confluence and then treated with recombinant human TGFβ1 (1.0 ng/mL; R&D Systems, Minneapolis, MN) or vehicle control in the medium. Total RNA prepared from the cells was subjected to qRT-PCR to determine the expression levels of collagen Ia1, SP, IL-6, TGFβ1, vascular endothelial growth factor, MCP-1, and α-SMA expression. Five dishes were prepared for each condition. Data were analyzed statistically by analysis of variance. Another set of cultures was incubated for 24 or 48 hours with or without exogenous TGFβ1 at 1.0 ng/mL and was processed for Western blotting for fibronectin protein as previously reported.23,24
Co-Culture of Ocular Fibroblasts and Macrophages
We used a co-culture model to determine whether fibrosis after an alkali burn corneal injury is caused by TRPV1 activation on corneal fibroblasts rather than infiltrating macrophages. Co-culture experiments were performed using these two cell types obtained from WT and KO mice as previously reported.19 A suspension of WT or KO macrophages (2.4 × 106 cells) in culture medium supplemented with 3% fetal calf serum was added to confluent WT/KO fibroblast cultures in 60-mm dishes and further incubated for 24 hours, thereafter total RNA obtained from the cells was subjected to qRT-PCR for expression of collagen Ia1 mRNA. Four dishes were prepared as described.
Results
IHC for TRPV1 in WT Mouse Eyes
TRPV1 expression in the intact mouse cornea is restricted to the basal epithelial cell layer. On the other hand, TRPV1 also was detected in stromal cells of alkali-burned corneas healed for 10 days (Figure 1), suggesting that alkali burn activates stromal cell TRPV1 expression.
Figure 1.
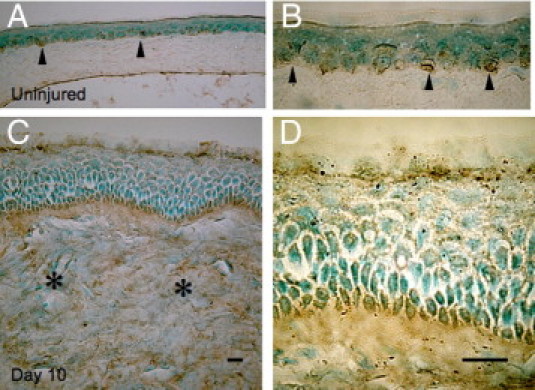
TRPV1-immunostaining in corneas of WT mice. The localization of TRPV1 in the intact mouse cornea is restricted to the basal cell layers of the epithelial cells (A, arrowheads). B: Higher magnification of the epithelium (arrowheads). On the other hand, TRPV1 is detected in stromal cells (asterisks) in a healing burned cornea besides basal epithelial cells (C). D: Higher magnification of the epithelium. Scale bar = 10 μm.
Alkali Burn in Mouse Eyes
To examine the role of TRPV1 in modulating wound healing of alkali-burned corneas, we first compared corneal haze development in the injured corneas of TRPV1 KO and WT mice. At each time point, the incidence and degree of epithelial defect/ulceration and opacification in the burned cornea were more severe in WT mice than those in TRPV1 KO mice (Figure 2A). The healing stroma was thicker in WT corneas as compared with KO corneas throughout the interval examined, suggesting the presence of more severe tissue swelling or edema in the presence of TRPV1. The eye globe diameters of alkali-burned eyes were determined after various periods of healing. WT globes have a smaller diameter at 20 days than those of KO mice (Figure 2B). This finding suggested that myofibroblast transdifferentiation is greater in the WT cornea as compared with KO corneas. To further characterize the tissue reaction to an alkali burn, we next conducted IHC and qRT-PCR to assess the variations in inflammation between WT and TRPV1 KO mice.
Figure 2.
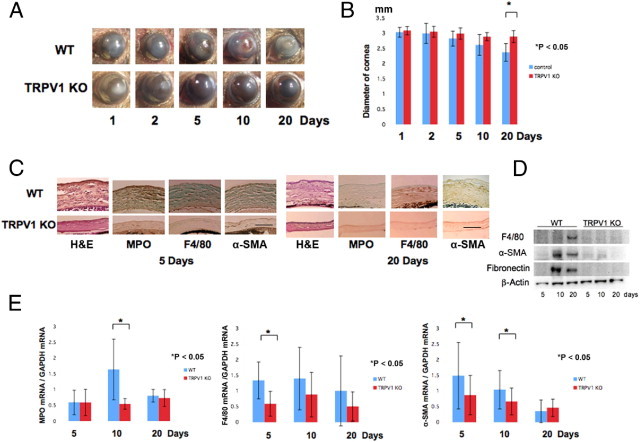
Healing of alkali-burned corneas in TPV1 KO mice. A: The corneas of WT and KO mice at 1, 2, 5, 10, or 20 days. On each of these days, the incidence and degree of opacification in the burned cornea was more prominent in WT mice than TRPV1 KO mice. At day 20, the iris is observed through the transparent cornea in a KO mouse, although not in a WT mouse. B: Evaluation of the alteration of the diameter of the eyeball during wound healing after alkali burn shows that WT globes have a smaller diameter at 20 days than the KO globes. C: Histology of burned corneas stained with H&E and IHC findings at days 5 and 20. H&E staining shows that the burned cornea shows a higher cell population and more severely disorganized tissue in WT corneas as compared with KO tissues at both days 5 and 20. The stroma was thicker in WT corneas as compared with KO corneas throughout the healing interval examined. IHC suggests that the density of the MPO-labeled cells at day 5 and F4/80-labeled cells at day 20 is greater in a WT cornea as compared with a KO cornea. Burned corneas that are healing seem to contain many α-SMA–positive myofibroblasts in WT mice at days 5 and 20. However, the majority of corneal fibroblasts were not labeled with anti–α-SMA antibody in TRPV1 KO mice. Scale bar = 100 μm. D: Western blotting shows expression of F4/80, α-SMA, and fibronectin were higher in WT tissue at 10 and 20 days after alkali burn. E: qRT-PCR also shows that the loss of TRPV1 suppresses mRNA expression levels of MPO, F4/80, and α-SMA during wound healing after alkali burn. Data represent mean ± SEM from five specimens in each condition. *P < 0.05.
Histology and IHC
The persistent and severe inflammation induced by alkali burn very markedly worsened the wound healing outcome in WT mice. For example, in WT mice there was a higher level of MPO (PMN marker) and F4/80 (macrophage marker) staining than that in KO mice (Figure 2C). Immunostaining with anti–α-SMA, a marker of myofibroblasts,24 revealed that there was an immense increase in stromal myofibroblasts of alkali-burned corneas of WT mice at 5 to 20 days, in contrast, the majority of stromal cells of alkali-burned corneas of TRPV1 KO mice were negative for α-SMA (Figure 2C), suggesting that a much higher number of fibroblasts underwent transdifferentiation into myofibroblasts in the WT mice. We performed Western blotting for F4/80, α-SMA, and fibronectin. Expression of F4/80, α-SMA, and fibronectin was higher in WT tissue at 10 and 20 days after alkali burn (Figure 2D). Thus, we then performed qRT-PCR for mRNA expression of MPO, F4/80, and α-SMA to verify the immunostaining observations. The data confirmed that there were significantly fewer inflammatory cells in KO tissue than WT tissue at most time points, except for the presence of PMN at day 5 after alkali burn (Figure 2E). Therefore, the improved wound healing outcome in the KO mice is associated with less inflammation and myofibroblast transdifferentiation.
Inflammatory and Fibrogenic Gene Expression
qRT-PCR showed that lacking TRPV1 significantly suppressed the mRNA levels of IL-6, MCP-1, SP, and collagen Ia1 in the healing alkali-burned corneas at certain time point(s) throughout the wound closure interval (Figure 3). We immunostained the active form of TGFβ1 in tissue. Expression of active TGFβ1 was much more marked in a WT stroma as compared with a KO stroma at day 10 (Figure 4).
Figure 3.
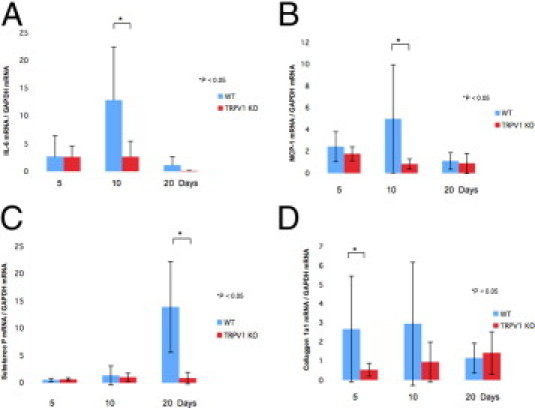
Expression pattern of wound healing–related genes in an alkali-burned cornea. Expression of IL-6 (A) and MCP-1 (B) was transiently increased at 10 days in the healing alkali-burned WT corneas and then decreases. Lacking TRPV1 abolished the IL-6 (A) and MCP-1 (B) mRNA expression peaks. C: Expression of SP increased markedly at 20 days in the healing alkali-burned WT corneas, and was suppressed by lacking TRPV1 in the healing alkali-burned mouse corneas at this time point. D: Expression of collagen Ia1 mRNA increased at 5 to 10 days in the healing alkali-burned WT corneas, and was reduced by the loss of TRPV1. Data represent mean ± SEM from five specimens in each condition. *P < 0.05. Scale bar = mean ± SEM.
Figure 4.
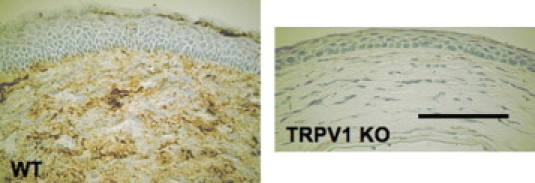
IHC of the active form of TGFβ1 in alkali-burned cornea. Expression of active TGFβ1 was much more marked in a WT stroma as compared with a KO stroma at day 10. Scale bar = 100 μm.
mRNA Expression of TGFβ1 by Cultured Macrophages
There was no difference in the expression level of TGFβ1 mRNA between cultured WT and KO macrophages (Figure 5).
Figure 5.
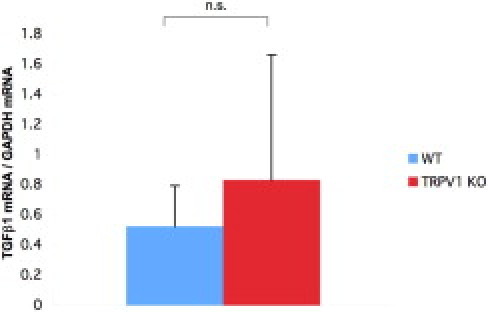
mRNA expression of TGFb1 by cultured macrophages. There was no difference in the expression level of TGFβ1 mRNA between cultured WT and KO macrophages. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., not significant.
Fibrogenic Gene Expression in Cultured Ocular Fibroblasts
Adding exogenous TGFβ1 up-regulated TRPV1 mRNA expression in WT ocular fibroblasts (Figure 6A). Any increase in mRNA expression levels induced by TGFβ1 was validated by showing that in KO ocular fibroblasts such effects were ablated (Figure 6, B–H). Loss of TRPV1 receptor reduced the mRNA expression level of TGFβ1 in ocular fibroblasts (Figure 6B). Expression of IL-6 mRNA was markedly up-regulated by adding exogenous TGFβ1, but such up-regulation was abolished by the loss of TRPV1 gene in the fibroblasts (Figure 6C). Expression of MCP-1 and vascular endothelial growth factor also was suppressed in ocular fibroblasts lacking TRPV1, but the expression pattern was not affected by exogenous TGFβ1 (Figure 6, D and E). There was no difference in the expression level of SP mRNA between cultured WT and KO ocular fibroblasts, and the expression pattern also was not affected by exogenous TGFβ1 (Figure 6F). Expression of the major fibrogenic markers, mRNAs of collagen Iα1 and α-SMA, was up-regulated by adding exogenous TGFβ1, but such up-regulation was abolished by the loss of TRPV1 gene in the fibroblasts (Figure 6, G and H).
Figure 6.
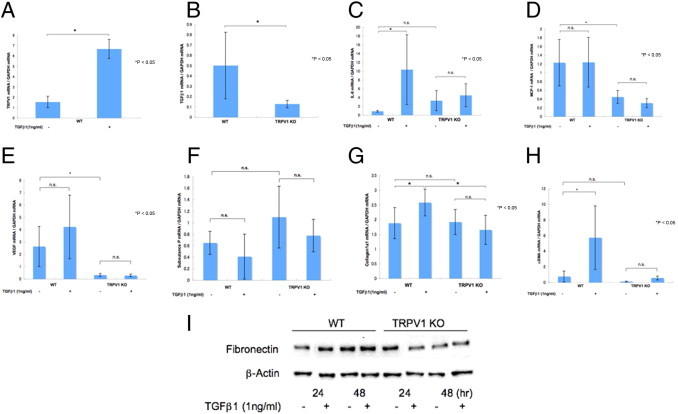
Fibrogenic gene expression in cultured ocular fibroblasts. A: Adding exogenous TGFβ1 up-regulated TRPV1 mRNA expression in WT ocular fibroblasts. B: Loss of TRPV1 reduced the mRNA expression level of TGFβ1 in ocular fibroblasts. C: Expression of mRNAs of IL-6 was markedly up-regulated by adding exogenous TGFβ1, but such up-regulation was abolished by the loss of TRPV1 gene in the fibroblasts. Expression of MCP-1 (D) and vascular endothelial growth factor (VEGF) (E) also was suppressed in ocular fibroblasts by lacking the TRPV1 gene, and the expression pattern was not affected by exogenous TGFβ1. F: There was no difference in the expression level of SP mRNA between cultured WT and KO ocular fibroblasts, and the expression pattern was not affected by exogenous TGFβ1. G: Expression of mRNAs of collagen Iα1, the major fibrogenic marker, was up-regulated by adding exogenous TGFβ1, but such up-regulation was abolished by the loss of TRPV1 gene in the fibroblasts. H: Expression of mRNAs of α-SMA, a myofibroblast marker, was markedly up-regulated by adding exogenous TGFβ1, but such up-regulation was completely abolished by the loss of TRPV1 gene in the fibroblasts. I: Fibronectin was suppressed in ocular fibroblasts by lacking TRPV1. Adding exogenous TGFβ1 up-regulated fibronectin in WT ocular fibroblasts, but such up-regulation was abolished by the loss of TRPV1 gene in the fibroblasts. Data represent mean ± SEM from five specimens in each condition. *P < 0.05. Scale bar = mean ± SEM. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blotting also showed that fibronectin also was suppressed in ocular fibroblasts lacking TRPV1. Adding exogenous TGFβ1 up-regulated fibronectin in WT ocular fibroblasts, but such up-regulation was abolished by the loss of TRPV1 gene in the fibroblasts (Figure 6I).
Co-Culture of Fibroblasts and Macrophages
The in vitro data described earlier suggested that the resident tissue cell (ie, the fibroblast), but not the inflammatory cells such as macrophages, is responsible for the better outcome of alkali-burned corneas seen in TRPV1 KO mice. To test this hypothesis, we measured the expression levels of fibrogenic genes (ie, collagen Ia1) by fibroblasts in reciprocal co-cultures of ocular fibroblasts and macrophages from WT and KO mice. Both WT and KO macrophages promoted collagen Ia1 mRNA expression more prominently in WT fibroblasts, however, the KO fibroblasts did not up-regulate collagen Ia1 expression regardless of whether the macrophages were obtained from WT or KO mice (Figure 7). These observations are consistent with the notion that the presence of TRPV1 gene in fibroblasts is responsible for mediating inflammatory responses during the healing of corneal alkali burn.
Figure 7.
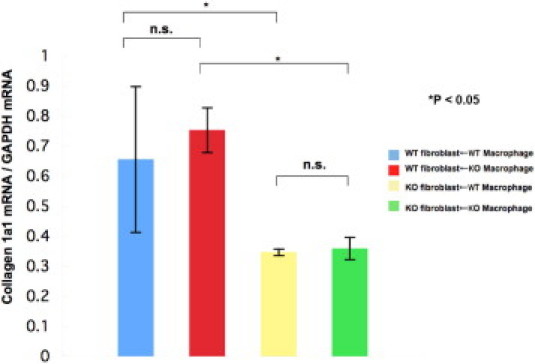
mRNA expression of collagen Ia1 in ocular fibroblasts co-cultured with macrophages. Either WT or KO macrophages promoted more collagen Ia1 mRNA expression in WT fibroblasts, but not in KO fibroblast culture. Data represent mean ± SEM from five specimens in each condition. Scale bar = mean ± SEM. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
BMT and Ocular Alkali Burn
The results of in vitro experiments suggest that resident corneal cells (ie, ocular fibroblasts, epithelial cells, and endothelial cells), but not inflammatory cells (ie, macrophages and neutrophils), might be responsible for the wound healing phenotype of the KO mice, which shows less inflammation and tissue fibrosis/scarring. To further test this hypothesis, we then used in vivo chimera mice generated by reciprocal BMT of WT and KO mice to determine the roles of infiltrating inflammatory cells in eliciting the aforementioned KO healing phenotype in response to corneal alkali burn.
We compared the corneal healing response of chimera mice that had received reciprocal BM from WT with KO mice and vice versa (KO-to-WT and WT-to-KO group) 5, 10, and 20 days after an alkali burn. The chimera mice of WT mice receiving WT BM (WT-to-WT) and KO BM (KO-to-WT) showed no difference in the alkali-burned cornea healing pattern (data not shown). By using RT-PCR, we detected TRPV1 mRNA in the spleen of mice of the WT-to-KO group, indicating that WT BM had reconstituted successfully in KO mice (data not shown). In contrast, 10 days after alkali burn, the chimera mice of KO mice receiving WT BM (WT-to-KO) still displayed much less opacification (scarring) similar to what was seen in KO mice as compared with that of chimera mice of WT mice receiving KO BM (KO-to-WT) and of WT mice (compare Figure 8A with Figure 2A). H&E histology in corneas of KO-to-WT chimeras showed more stromal cellularity and swelling than those of WT-to-KO chimeras (Figure 8A). IHC revealed that the cornea of a WT-to-KO chimera mouse had less stromal α-SMA staining as well as lower levels of MPO, F4/80, and active TGFβ1 immunoreactivity as compared with that of the KO-to-WT chimeras (Figure 8B). These findings are consistent with the notion that the expression of TRPV1 by corneal cells of WT genetic background is needed to elicit severe inflammation in alkali-burned corneas (ie, inflammatory cell infiltration, myofibroblast transdifferentiation, and activation of TGFβ1 activity).
Figure 8.
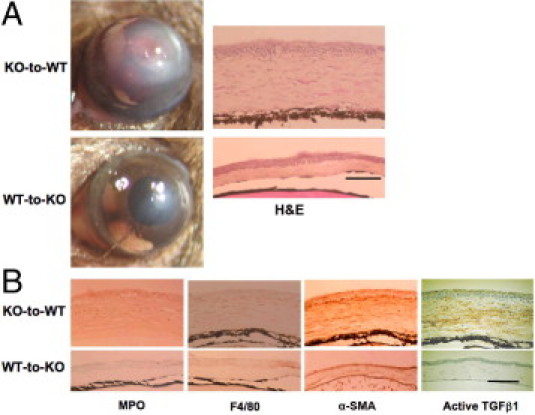
Healing an alkali-burned cornea in a mouse that has received a BMT at day 10 of healing. A: Ten days after alkali burning, a WT mouse that had received BM from a KO mouse (KO-to-WT group) showed much more opacification and neovascularization as compared with a KO mouse that had received BM from a WT mouse (WT-to-KO group). H&E histology shows larger cell populations in the swollen stroma of a KO-to-WT cornea as compared with WT-to-KO tissue. B: IHC shows that the cornea of a WT-to-KO mouse has less stromal α-SMA (the myofibroblast marker) staining as well as lower levels of immunoreactivity of MPO (a neutrophil marker), F4/80 (a macrophage marker), and active TGFβ1 as compared with the KO-to-WT tissue. Scale bar = 100 μm.
TRPV1−/−/GFP+/+ and TRPV1+/+/GFP+/+ mice were used to examine the chimeric status of the local inflammation in the healing, burned, corneal stromal tissue. The results showed that the ratio of GFP-positive cells in total F4/80-labeled macrophages was 80.06% ± 4.64% or 85.52% ± 8.59% in the cornea of a WT mouse with TRPV1+/+/GFP+/+ BM (TRPV1+/+/GFP+/+-to-WT) or with TRPV1−/−/GFP+/+ BM (Figure 9). The ratio was not obtained in KO mice that had received BMT from TRPV1+/+/GFP+/+ mice because of much less severe inflammation in the burned tissue.
Figure 9.
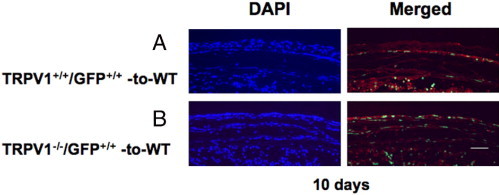
Healing an alkali-burned cornea in a mouse that has received GFP-positive BMT at day 10 of healing. A: Ten days after alkali burning, a WT mouse that had received BM from a TRPV1+/+/GFP+/+ mouse (TRPV1+/+/GFP+/+-to-WT group). B: WT mouse that had received BM from a TRPV1−/GFP+ mouse (TRPV1−/−/GFP+/+-to-WT group). Green staining shows GFP-positive cells. Red staining shows F4/80 localization and blue DAPI staining shows cell nuclei. Scale bar = 100 μm.
Treatment of Corneal Alkali Burn in WT Mice with TRPV1 Antagonists
We finally planned to reproduce the favorable phenotype of a KO mouse with less inflammation and scarring/fibrosis in the alkali-burned cornea by using chemical antagonists of the TRPV1 receptor. Healing of the corneal surface was evaluated by observing the degree of corneal stromal opacification (scarring/fibrosis). Corneal transparency restoration is improved markedly in mice treated with both TRPV1 antagonists [ie, SB366791 (Figure 10A) and JYL1421 (data not shown)]. Similar to a KO mouse, the globe diameter did not change in mice whereas in the untreated mice the globe diameter shrank at 20 days (Figure 10B), suggesting that tissue contraction caused by wound healing was more marked in the untreated control group as compared with the TRPV1 antagonist group. The stromal organization is poorer in untreated mice than in antagonist-treated mice as judged by H&E histology. The antagonist-treated mice have lower levels of infiltration of MPO-labeled macrophages and F4/80-positive PMNs as well as more marked α-SMA staining (Figure 10C). Expression of active TGFβ1 protein was much more marked in untreated mouse stroma as compared with an antagonist-treated mouse stroma at day 10 (Figure 10C). IHC results indicate less inflammatory cell infiltration and myofibroblast transdifferentiation in the antagonists group than in the untreated mice. The wound healing outcome obtained with either of these two antagonists mimics the result seen in the KO mice.
Figure 10.
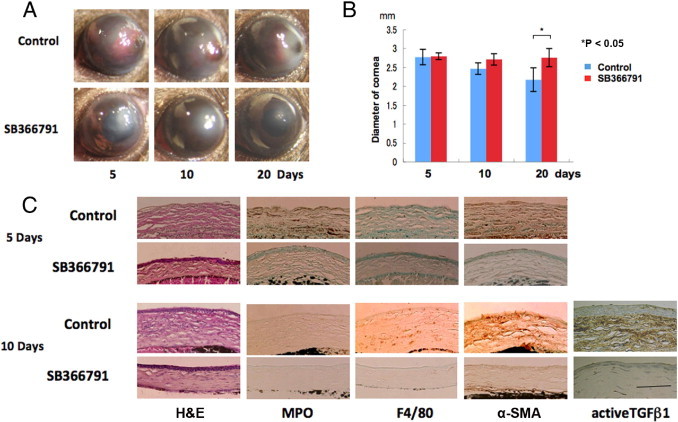
Treatment of corneal alkali burn in WT mice with a TRPV1 antagonist of SB366791. A: The corneas of WT mice treated with or without SB366791 at 5, 10, or 20 days, respectively. At each time point, corneal transparency restoration is markedly improved in the mice treated with the TRPV1 antagonist SB366791. At day 20 the iris is seen through the transparent cornea in an antagonist-treated mouse, although not in an untreated mouse. B: Evaluation of the alteration of the diameter of the eyeball during wound healing after alkali burn shows that untreated globes have a smaller diameter at 20 days than antagonist-treated globes. C: Histology of burned corneas stained with H&E and IHC findings at days 5 and 10. The stromal organization is poorer in untreated mice than in antagonist-treated mice as judged by H&E histology. The antagonist-treated mice have lower levels of infiltration of MPO-labeled neutrophils at day 5 and F4/80-positive macrophages at day 10 as well as less marked α-SMA and active TGFβ1 staining at both time points. Scale bar = 100 μm.
Discussion
An alkali corneal burn induces severe inflammation and subsequent tissue fibrosis resulting in scarring that causes opacification of the stroma. In the present study, we show for the first time that lacking TRPV1 signaling was beneficial in the restoration of corneal transparency after an alkali burn to mouse corneas. A more prominent pathogenic tissue response, that is, inflammation and subsequent tissue swelling (edema) and fibrogenic reaction as indicated by greater myofibroblast transdifferentiation and matrix elaboration, was observed in an alkali-burned WT mouse cornea as compared with that of a TRPV1 KO cornea. This difference in responses also was evident based on the evaluation of ocular globe contraction, histology, IHC, and gene expression analysis.
In a healing alkali-burned cornea, enormous numbers of PMNs first invade into the injured stroma followed by macrophage infiltration and up-regulation of proinflammatory growth factor/cytokines. In the present study, IHC and qRT-PCR showed that lacking TRPV1 decreased the PMN and macrophage infiltration into alkali-burned mouse corneas. Various growth factors/cytokines might be involved in the pathogenic inflammatory response of an alkali-burned cornea. Stromal swelling (edema) in the WT mice might be attributable to severe inflammation and failure of the epithelial barrier function to be adequately restored owing to delayed epithelial wound closure. Incomplete healing provides a leak pathway for the stroma to imbibe fluid and become edematous. However, our previous reports showed that TGFβ1 is one of the most critical growth factors in establishing the pathologic (inflammatory and fibrogenic) lesion after a corneal alkali burn.25 Its importance in corneal wound healing was substantiated by showing that in mouse corneas either lacking Smad3 expression, the main TGFβ signal transmitter, or overexpressing Smad7, the inhibitory Smad, both almost completely abrogated the development of a severe lesion caused by alkali burn.25 Expression levels of the active form of TGFβ1 and other proinflammatory factors (ie, MCP-1 and IL-6), were reduced in the alkali-burned cornea of TRPV1 KO mice as compared with those of WT mice. MCP-1 and IL-6 are known to hasten and augment inflammation by serving as chemoattractants to inflammatory cells.26–28 Suppression of inflammatory cell infiltration (ie, PMNs and macrophages) might lead to a further reduction in tissue levels of inflammatory or fibrogenic cytokines.
Myofibroblast transdifferentiation of fibroblasts is a reflection of increases in α-SMA expression levels and increases in collagen type I expression, which are the hallmarks of tissue fibrosis.29–32 This phenomenon is enhanced by various growth factors, especially by TGFβ. This is also the case in a healing corneal stroma and also was suppressed by gene ablation of TRPV1 as revealed by IHC and qRT-PCR. Lessened fibrosis is also in agreement with the lack of eye globe contraction after alkali burn healing seen in KO mice in contrast to that of WT mice. Myofibroblasts that highly express collagen type I are reportedly dependent on the activation of latent TGFβ1 in situ.31 A decline in TGFβ activation in alkali-burned corneas of KO mice may result in fewer contractile α-SMA–positive myofibroblasts than that in the WT mice, which may in part explain the smaller eye globe diameters seen in WT mice.
We determined in vivo whether loss of TRPV1 activation by injury on inflammatory cells (macrophages and/or PMNs) or resident stromal fibroblasts or keratocytes accounts for suppression of inflammation or the fibrogenic process (ie, myofibroblast generation and collagen expression) in a healing KO cornea. Namely, we asked the following: Was suppression of tissue inflammation resulting in reduced expression levels of fibrogenic cytokines/growth factors (ie, TGFβ1) a cause of less fibrogenic fibroblast reaction to injury in the KO tissue, or did the loss of injury-induced TRPV1 signaling directly suppress myofibroblast transdifferentiation?
IHC clearly detected up-regulation of TRPV1 protein in corneal stromal fibroblasts or keratocytes in a healing, alkali-burned cornea, suggesting that TGFβ1 up-regulates TRPV1 expression in corneal stromal cells. This notion was supported by results obtained with cultured ocular fibroblasts. Exogenous TGFβ1 up-regulated fibronectin and mRNA expression of TRPV1, TGFβ1, MCP-1, IL-6, and vascular endothelial growth factor in WT fibroblasts. All of these factors either induce or are chemoattractants for inflammatory cell types.33–38 On the other hand, these increases of cytokines were suppressed in TRPV1 lacking ocular fibroblasts. Nevertheless, expression of TGFβ1 mRNA was unaltered by the loss of TRPV1 in cultured macrophages. These findings support the notion that TRPV1 signal activation in stromal cells (ie, corneal fibroblasts or keratocytes after exposure to alkali) is involved in the activation of latent forms of proinflammatory cytokines/growth factors (eg, TGFβ1). Although inflammatory cytokines/growth factors expressed by inflammatory cells are believed to play important roles in the pathogenic process of stromal inflammation, cytokines/growth factors secreted by resident stromal cells (corneal fibroblasts or keratocytes) or nerve fibers in an injured tissue also are involved in the initiation of inflammatory cell infiltration on tissue damage. SP is known to be a proinflammatory neuropeptide that is expressed by neuronal cells and other cell types.39,40 However, we found that SP expression in ocular fibroblasts was not affected by exogenous TGFβ1 or TRPV1 gene ablation even though in nerve fibers TRPV1 activation induces SP release. Our in vivo data showed that SP was not up-regulated at day 10 after burn, although its level of expression was not the same in the WT and KO genotypes. It remains to be determined if such a difference in SP expression levels might be correlated with nerve fiber regeneration. Nevertheless, the unique phenotype in the KO mice seen after healing appears not to be attributable to a difference in SP expression level between KO and WT alkali-burned corneas because they were not different from one another.
Once inflammatory cells populate an injured tissue, factors secreted by these cells are considered to further augment the tissue inflammation. The present findings obtained from in vivo and in vitro experiments strongly suggest that the phenotype of the healing response of an alkali-burned mouse cornea, as evaluated by the level of inflammation, depends on the genotype of resident corneal cells [ie, corneal stromal cells (keratocytes or corneal fibroblasts)] instead of inflammatory cells (ie, macrophages and neutrophils). Suppression of expression of inflammatory cytokines/growth factors in KO resident corneal cells appears to interrupt the inflammatory cycle augmentation by infiltrating inflammatory cells in the healing of alkali-burned corneas. Furthermore, KO ocular fibroblast exposure to TGFβ1 did not elicit myofibroblast transdifferentiation as determined by the lack of α-SMA expression. Although the exact mechanism for this blockage requires additional clarification, loss of this response along with declines in cytokines/growth factors also may contribute to lessened fibrosis observed in a KO healing cornea.
The notion that the KO healing phenotype (less inflammation and fibrosis) is attributable to the absence of TRPV1 expression in tissue resident cells is supported further by the results from experiments using chimera mice of reciprocal BMT transplantation and co-culture of ocular fibroblasts and macrophages, and treatments with TRPV1 antagonists. The co-culture experiment also indicated that WT ocular fibroblasts expressed a high level of collagen Ia1 mRNA as compared with KO cells regardless of the source of macrophages (from either KO or WT mice). The experiments with chimeras from BMT showed that TRPV1 KO mice receiving WT BM still had a better wound healing outcome (less inflammation and less fibrosis) than their WT counterpart chimeras constituting BM of KO mice. Indeed, more than 80% of the macrophages were derived from transplanted BM in WT mice that had received BMT from either a WT or a KO mouse with labeling of the GFP expression. These results further indicate that injury-induced TRPV1 activation on resident stromal cells rather than on infiltrating inflammatory cells determines the outcome of the wound healing response.
Similar findings of suppression of tissue inflammation in a TRPV1 KO mouse were reported, endotoxin-induced airway inflammation41 or inflammation in the knee joint induced by capsaicin was attenuated by TRPV1 gene loss.42 Either sulfate induced colitis in mice or TRPV1 activation by dextran-enhanced neutrophil accumulation and histopathologic changes.43,44 Also, in a human study, TRPV1 mRNA and protein expression levels along with nerve growth factor expression were significantly greater in patients with erosive esophagitis than in healthy controls.45
The present study clearly showed that the loss of TRPV1 signal blocks inflammatory/fibrogenic reaction after chemical injury in an alkali-burned cornea in mice. The results suggest that chemical blocking of the TRPV1 channel could be beneficial in treating inflammation-based corneal diseases. To test this possibility, we examined the individual effects of systemic i.p. administration of two different TRPV1 antagonists on the wound healing outcome in an alkali-burned cornea in mice. Both of the two TRPV1 receptor antagonists (ie, SB366791 and JYL1421), reproduced the results seen in the KO tissue; namely, suppression of inflammation and tissue fibrosis. All of the findings in this series of experiments suggest that a novel strategy to treat a chemical corneal burn could be obtained by blocking TRPV1-induced signaling. Such an approach is expected to lessen or even prevent declines in visual acuity by suppressing TRPV1-mediated inflammatory/fibrogenic reactions. This approach also could be applicable for suppression of inflammation and subsequent undesirable loss of function in various other tissues.
Acknowledgments
We thank Dr. Jee Woo Lee (Seoul National University) for providing the JYL1421 compound.
Footnotes
Supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (C21592241 to Y.O.; C19592036 to S.S.); the Mitsui Life Social Welfare Foundation, Mochida Memorial Foundation, Takeda Science Foundation, and Uehara Foundation (S.S.); EY04795 (P.S.R.); NIH grants EY011845 and EY013755, and the Research to Prevent Blindness and Ohio Lions Eye Research Foundation (W.W.Y.K.).
References
- 1.Brodovsky S.C., McCarty C.A., Snibson G., Loughan M., Sullivan L., Daniell M., Taylar H.R. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835. doi: 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 2.Ishizaki M., Zhu G., Hasebe T., Shafer S.S., Kao W.W.-Y. Expression of collagen I, smooth muscle a-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993;34:3320–3328. [PubMed] [Google Scholar]
- 3.Saika S., Kobata S., Hashizume N., Okada Y., Yamanaka O. Epithelial basement membrane in alkali-burned corneas in rats: Immunohistochemical study. Cornea. 1993;12:383–390. doi: 10.1097/00003226-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Saika S., Uenoyama K., Hiroi K., Tanioka H., Takase K., Hikita M. Ascorbic acid phosphate ester and wound healing in rabbit corneal alkali burns: epithelial basement membrane and stroma. Graefes Arch Clin Exp Ophthalmol. 1993;231:221–227. doi: 10.1007/BF00918845. [DOI] [PubMed] [Google Scholar]
- 5.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2008;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 6.Sridhar M.S., Bansal A.K., Sangwan V.S., Rao G.N. Amniotic membrane transplantation in acute chemical and thermal injury. Am J Ophthalmol. 2000;130:124–137. doi: 10.1016/s0002-9394(00)00500-6. [DOI] [PubMed] [Google Scholar]
- 7.Meller D., Pires R.T., Mack R.J., Figueiredo F., Heiligenhaus, Park W.C., Prabhasawat P., John T., McLeod S.D., Steuhl K.P., Tseng S.C. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen S.F., Owsianik G., Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey I.S., Delling M., Clapham D.E. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 10.Owsianik G., Talavera K., Voets T., Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 11.Montell C., Birnbaumer L., Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 12.Ciura S., Bourque C.W. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steen K.H., Reeh P.W., Anton F., Handwerker H.O. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Hirschfeld J., López-Briones L.G., Belmonte C., Valdeolmillos M. Intracellular free calcium responses to protons and capsaicin in cultured trigeminal neurons. Neuroscience. 1995;67:235–243. doi: 10.1016/0306-4522(95)00055-n. [DOI] [PubMed] [Google Scholar]
- 15.Caterina M.J., Schumacher M., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 16.Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Petersen-Zeitz K.R., Kolzenburg M., Basbaum A.I., Julius D. Impaired nocioception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288 doi: 10.1126/science.288.5464.306. 306–303. [DOI] [PubMed] [Google Scholar]
- 17.Davis J.B., Gray J., Gunthorpe M.J., Hatcher J.P., Davey P.T., Overend P., Harries M.H., Latcham J., Clapham C., Atkinson K., Hughes S.A., Rance K., Grau E., Harper A.J., Pugh P.L., Roger D.C., Blingham S., Randall A., Sheardown S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 18.Saika S., Miyamoto T., Yamanaka O., Kato T., Ohnishi Y., Flanders K.C., Ikeda K., Nakajima Y., Kao WW- Y., Sato M., Muragaki Y., Ooshima A. Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor-B, in treatment of corneal alkali burns in mice. Am J Pathol. 2005;166:1393–1403. doi: 10.1016/s0002-9440(10)62357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saika S., Ikeda K., Yamanaka O., Flanders K.C., Okada Y., Miyamoto T., Kitano A., Ooshima A., Nakajima Y., Ohnishi Y., Kao W.W. Loss of tumor necrosis factor a potentiates transforming growth factor b-mediated pathogenic tissue response during wound healing. Am J Pathol. 2006;168:1848–1860. doi: 10.2353/ajpath.2006.050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga A., Németh J., Szabó A., McDougall J.J., Zhang C., Elekes K., Pintér E., Szolcsányi J., Helyes Z. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in rat. Neurosci Lett. 2005;385:137–142. doi: 10.1016/j.neulet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Jakab B., Helyes Z., Varga A., Bölcskei K., Szabó A., Sándor K., Elekes K., Börzsei R., Keszthelyi D., Pintér E., Petho G., Németh J., Szolcsányi J. Pharmacological characterization of the TRPV1 receptor antagonist JYL1421 (SC0030) in vitro and in vivo in the rat. Eur J Pharmacol. 2005;517:35–44. doi: 10.1016/j.ejphar.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Flanders K.C., Ludecke G., Engels S., Cissel D.S., Roberts A.B., Kondaiah P., Lafyatis R., Sporn M.B., Unsicker K. Localization and actions of transforming growth factor-bs in the embryonic nervous system. Development. 1991;113:183–191. doi: 10.1242/dev.113.1.183. [DOI] [PubMed] [Google Scholar]
- 23.Saika S., Ikeda K., Yamanaka O., Flanders K.C., Ohnishi Y., Nakajima Y., Muragaki Y., Ooshima A. Adenoviral gene transfer of BMP-7: Id2 or Id3 suppresses injury-induced epithelial-mesenchymal transition of lens epithelium in mice. Am J Physiol Cell Physiol. 2006;290:C282–C289. doi: 10.1152/ajpcell.00306.2005. [DOI] [PubMed] [Google Scholar]
- 24.Saika S., Yamanaka O., Okada Y., Tanaka S., Miyamoto T., Sumioka T., Kitano A., Shirai K., Ikeda K. TGF beta in fibroproliferative diseases in the eye. Front Biosci (Schol Ed) 2009;1:376–390. doi: 10.2741/S32. [DOI] [PubMed] [Google Scholar]
- 25.Saika S., Ikeda K., Yamanaka O., Miyamoto T., Ohnishi Y., Sato M., Muragaki Y., Ooshima A., Nakajima Y., Kao W.W., Flanders K.C., Roberts A.B. Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am J Pathol. 2005;166:1405–1418. doi: 10.1016/S0002-9440(10)62358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukacs N.W., Chensue S.W., Smith R.E., Strieter R.M., Warmington K., Wilke C., Kunkel S.L. Production of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 alpha by inflammatory granuloma fibroblasts. Am J Pathol. 1994;44:711–718. [PMC free article] [PubMed] [Google Scholar]
- 27.Helme R.D., Andrews P.V., Watson B.A. Neurogenic inflammation caused by wool fabric in the rat; possible mediation by substance P. Neurosci Lett. 1986;66:333–337. doi: 10.1016/0304-3940(86)90041-8. [DOI] [PubMed] [Google Scholar]
- 28.Sehgal P.B. Interleukin-6: molecular pathophysiology. J Invest Dermatol. 1990;94:2S–6S. doi: 10.1111/1523-1747.ep12874963. [DOI] [PubMed] [Google Scholar]
- 29.Serini G., Bochaton-Piallat M.L., Ropraz P., Geinoz A., Borsi L., Zardi L., Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta 1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasek J.J., Gabbiani G., Hinz B., Chponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 31.Hester J.V., Huang J., Petroll W.M., Chavanagh H.D. TGFβ induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFβ: PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- 32.Desmouliere A., Darby I.A., Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- 33.Brodovsky S.C., McCarty C.A., Snibson G. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835. doi: 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 34.Meller D., Pires R.T., Mack R.J., Figueiredo F., Heiligenhaus A., Park W.C., Prabhasawat P., John T., McLeod S.D., Steuhl K.P., Tseng S.C. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 35.Saika S., Yamanaka O., Sumioka T., Miyamoto T., Miyazaki K., Okada Y., Kitano A., Shirai K., Tanaka S., Ikeda K. Fibrotic disorders in the eye: targets of gene therapy. Prog Retin Eye Res. 2008;27:177–196. doi: 10.1016/j.preteyeres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Azar D.T. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 37.Papathanassiou M., Theodossiadis P.G., Liarakos V.S., Rouvas A., Giamarellos-Bourboulis E.J., Vergados I.A. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am J Ophthalmol. 2008;145:424–431. doi: 10.1016/j.ajo.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Saika S. TGFb pathobiology in the eye. Lab Invest. 2006;86:106–115. doi: 10.1038/labinvest.3700375. [DOI] [PubMed] [Google Scholar]
- 39.Nassini R., De Siena G., DeCesaris F., Geppetti P. Transient receptor potential channels as novel drug targets in respiratory diseases. Curr Opin Investig Drugs. 2010;11:535–542. [PubMed] [Google Scholar]
- 40.Szolcsanyi J. Hot target on nociceptors: perspectives, caveats and unique features. Br J Pharmacol. 2008;155:1142–1144. doi: 10.1038/bjp.2008.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helyes Z., Elekes K., Németh J., Pozsgai G., Sándor K., Kereskai L., Börzsei R., Pintér E., Szabó A., Szolcsányi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1173–L1181. doi: 10.1152/ajplung.00406.2006. [DOI] [PubMed] [Google Scholar]
- 42.Keeble J.E., Brain S.D. Capsaicin-induced vasoconstriction in the mouse knee joint: a study using TRPV1 knockout mice. Neurosci Lett. 2006;401:55–58. doi: 10.1016/j.neulet.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 43.Pan X.Q., Gonzalez J.A., Chang S., Chacko S., Wein A.J., Malykhina A.P. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Exp Neurol. 2010;225:262–273. doi: 10.1016/j.expneurol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szitter I., Pozsgai G., Sandor K., Elekes K., Kemeny A., Perkecz A., Szolcsanyi J., Helyes Z., Pinter E. The role of transient receptor potential vanilloid 1 (Trpv1) receptors in dextran sulfate-induced colitis in mice. J Mol Neurosci. 2010;42:80–88. doi: 10.1007/s12031-010-9366-5. [DOI] [PubMed] [Google Scholar]
- 45.Shieh K.R., Yi C.H., Liu T.T., Tseng H.L., Ho H.C., Hsieh H.T., Chen C.L. Evidence for neurotrophic factors associating with TRPV1 gene expression in the inflamed human esophagus. Neurogastroenterol Motil. 2010;22:971–977. doi: 10.1111/j.1365-2982.2010.01530.x. [DOI] [PubMed] [Google Scholar]


