Abstract
Background: T‐wave alternans (TWA), a harbinger of sudden cardiac death, associates to a broad variety of pathologies. In a previous study, we observed the presence of unstable and low‐amplitude TWA also in healthy subjects, and considered it as “physiological TWA.” The possible existence of different TWA characteristics between males and female is investigated in the present work.
Methods: Resting ECG recordings from 142 control healthy subjects, 77 males and 65 females, were submitted to our adaptive match filter (AMF) based method for TWA detection and characterization in terms of duration, amplitude, and their product. The 99.5th percentile of these parameters distributions over the entire control population and over the male and female subgroups, were used to define thresholds which delimit a gender‐independent and male‐ and female‐related TWA normality regions, respectively, out of which abnormal TWA cases (TWA+) are expected to fall. Clinical usefulness of these regions was tested using a population of 151 coronary artery disease (CAD) patients, divided into 128 males and 23 females.
Results: In our control‐female population, TWA duration was significantly longer than in control‐male population (65 ± 13 beat vs 52 ± 14 beat; P < 10−6). Our gender‐related normality regions allowed identification of 36 (23.8%) TWA+ cases among the CAD patients, 17 more than those obtained from a gender‐independent region. All these 17 patients were CAD males with over‐threshold TWA duration.
Conclusions: TWA is a gender‐related phenomenon. Definition of gender‐related TWA normality regions improves identification of patients at increased TWA stability (i.e., prolonged TWA duration) and, thus, at increased risk of arrhythmic events.
Ann Noninvasive Electrocardiol 2010;15(4):328‐336
Keywords: repolarization variability, sudden cardiac death, ECG signal processing
T‐wave alternans (TWA), an electrophysiologic phenomenon consisting in an alternation of the electrocardiographic (ECG) T‐wave morphology, has known a growing interest in the last decades because of its association with malignant ventricular arrhythmias and sudden cardiac death. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 After Adam et al. 9 reported the existence of microvolt TWA, too small in amplitude to be visually detected at standard display scales, several methods have been proposed for its automatic detection and quantification. 10 , 11 , 12 , 13 , 14 , 15 Some of these techniques, such as the spectral method 8 , 10 and the Laplacian likelihood ratio method, 15 ascribe to noise the low‐TWA levels, not associated with cardiac instability, which are even detected under physiological conditions. This methodological approach aroused from the early hypothesis that TWA could be an on–off phenomenon, usually not present in health. 16 After the recent experimental study by Pruvot et al., 17 who demonstrated the possibility of inducing various levels of TWA, some of which not necessarily associated to cardiac instability, the hypothesis that TWA is a phenomenon characterized by a continuously changing amplitude from physiological to pathological condition has gained increasing consideration. The existence of physiological levels of TWA is supported by our more recent study, 18 where ECG recordings from a control group of healthy subjects, in resting condition and with normal heart rate (68 ± 10 bpm), were submitted to our adaptive match filter (AMF) method 14 , 19 for TWA identification. This technique, which is based on the TWA continuity hypothesis, allowed the identification of a TWA normality region useful to discriminate normal TWA levels from abnormal ones, most likely associated to arrhythmic events. These findings raise the question as to whether the observed physiological levels of TWA show different characteristics in healthy males and females, thus implying the possible existence of two different gender‐related normality regions. The present study was designed to address this issue in the perspective of improving reliability of nonphysiological TWA levels discrimination in males and females.
METHODS
Clinical Data
All participants in this study pertain to the Intercity Digital Electrocardiology Alliance (IDEAL) Study, whose protocol was approved by Research Subject Review Board of the University of Rochester. The IDEAL Study was conducted following the required rules for human subjects’ research principles, according to the Declaration of Helsinki, as well as to Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects, Revised November 13, 2001, effective December 13, 2001.
Physiological levels of TWA were determined using a population of 142 control subjects, divided into a group of 77 males and a group of 65 females. All they had no history of diabetes, hypertension, and cardiovascular diseases. Control‐male and control‐female groups were selected to be matched for age, body mass index (BMI), systolic (SAP), and diastolic (DAP) arterial pressure (Table 1). Usefulness of determining TWA physiological levels was tested by involving in this study one more population of 151 coronary artery disease (CAD) patients, who are known to be at increased risk to develop TWA. 1 , 2 , 5 , 19 The CAD population was divided into a group of 128 males and a group of 23 females, also matched for age, BMI, SAP, and DAP (Table 2). All these CAD patients were characterized by positive angiogram and either exercise induced ischemia or evidence of previous myocardial infarction.
Table 1.
Clinical and TWA Parameters (mean ± SD) for the 142 Control Healthy Subjects (77 Males and 65 Females)
| Control Population (142) | Control Males (77) | Control Females (65) | P‐Value | |
|---|---|---|---|---|
| SAP (mmHg) | 108 ± 35 | 112 ± 32 | 104 ± 40 | NS |
| DAP (mmHg) | 68 ± 23 | 72 ± 20 | 66 ± 25 | NS |
| BMI (kg/m2) | 24 ± 5 | 25 ± 3 | 24 ± 7 | NS |
| Age (year) | 39 ± 15 | 37 ± 13 | 40 ± 17 | NS |
| MNN (ms) | 886 ± 139 | 927 ± 144 | 837 ± 116 | <10−4 |
| SDNN (ms) | 24 ± 17 | 27 ± 19 | 22 ± 15 | NS |
| Mean TWA duration (beat) | 58 ± 15 | 52 ± 14 | 65 ± 13 | <10−6 |
| Mean TWA amplitude (μV) | 35 ± 12 | 35 ± 12 | 34 ± 11 | NS |
| Mean TWA product (beat·μV) | 2085 ± 899 | 1913 ± 772 | 2290 ± 998 | NS |
| TWA duration SD (beat) | 9 ± 3 | 10 ± 4 | 9 ± 2 | NS |
| TWA amplitude SD (μV) | 6 ± 4 | 7 ± 4 | 5±3 | NS |
| TWA product SD (beat·μV) | 523 ± 289 | 539 ± 327 | 505 ± 238 | NS |
P‐value refers to statistical comparison between control‐male and control‐female groups. P < 10−4 and P < 10−6, statistical significance (unpaired Student's t‐test with Bonferroni's method).
NS: no significant difference (P ≥ 0.006).
Table 2.
Clinical and TWA Parameters (mean ± SD) Values for the 151 Coronary Artery Disease (CAD) Patient (128 Males and 23 Females)
| CAD Population (151) | CAD Males (128) | CAD Females (23) | P‐Value | |
|---|---|---|---|---|
| SAP (mmHg) | 128 ± 17* | 127 ± 16* | 131 ± 23* | NS |
| DAP (mmHg) | 78 ± 10* | 78 ± 10* | 78 ± 11 | NS |
| BMI (kg/m2) | 27 ± 4* | 30 ± 4* | 27 ± 5 | NS |
| AGE (year) | 56 ± 15* | 56 ± 16* | 60 ± 9* | NS |
| MNN (ms) | 929 ± 141 | 928 ± 143 | 932 ± 127* | NS |
| SDNN (ms) | 19 ± 14* | 19 ± 15* | 15 ± 5 | NS |
| Mean TWA duration (beat) | 64 ± 16* | 64 ± 15* | 65 ± 18 | NS |
| Mean TWA amplitude (μV) | 40 ± 22 | 40 ± 23 | 40 ± 17 | NS |
| Mean TWA product (beat·μV) | 2558 ± 1510* | 2537 ± 1478* | 2678 ± 1707 | NS |
| TWA duration SD (beat) | 10 ± 4 | 10 ± 4 | 11 ± 5* | NS |
| TWA amplitude SD (μV) | 8 ± 7 | 8 ± 8 | 8 ± 6* | NS |
| TWA product SD (beat·μV) | 698 ± 677 | 667 ± 636 | 871 ± 867* | NS |
NS: statistically not significant (P ≥ 0.006) when comparing CAD males versus CAD females.
P‐value refers to statistical comparison between CAD‐male and CAD‐female groups.
*P < 0.004 identifying significant differences (unpaired Student's t‐test with Bonferroni's method) when comparing CAD patients versus control subjects, CAD males versus control males, and CAD females versus control females.
ECG tracings were taken from the three pseudo‐orthogonal (X, Y, and Z) leads available at the Telemetric and Holter ECG Warehouse (http://www.thew-project.org), acquired in resting supine conditions using the SpaceLab‐Burdick digital Holter recorder (SpaceLab‐Burdick, Inc., Deerfield, WI, USA; sampling frequency 200 Hz; amplitude resolution 10 μV). In the effort to rule out noise effects on our assessment of a “physiologic” TWA, ECG tracings of selected participants were required to satisfy a noise control criterion based on a correlative approach. 12 , 18 Cases characterized by the presence of repeated ventricular beats were also discarded. 12 , 18
TWA Identification by Adaptive Match Filter
TWA analysis was performed in 20‐minute ECG recordings by means of a procedure that removes artifacts and nonsinus beats during preprocessing and, subsequently, submits 128 consecutive beats ECG data to a recursive (every 10 seconds) AMF processing. 18 After parameterization of heart rate (HR) in terms of mean interval between two sinus beats over each 128 beat sequence (MNN128) over each 128 beat sequence, the AMF technique detects TWA by filtering out every ECG component but the TWA, which, by definition, is characterized by a frequency, fTWA, equal to 1/(2·MNN128). TWA is then quantified by three parameters, that is, TWA duration (beat; defined as the total number of beats with alternating T waves), TWA amplitude (μV; defined as the mean amplitude over all alternating T waves), and the product of these two, named TWA product (μV·beat; defined as the product of TWA amplitude times duration). 14 , 18 , 19 To control for distributed noise, our AMF algorithm incorporates a specific test to reduce the effect of distributed noise. In particular, TWA amplitude is required to be greater than 5 μV for at least seven consecutive beats for TWA to be detected. 14 Mean and standard deviation (SD) values of TWA duration, amplitude, and product over 20 minutes are used to characterize TWA and TWA variability in each subject. 18
Our AMF‐based TWA identification procedure was applied to all our control subjects and CAD patients.
Definition of TWA Normality Regions
To investigate whether physiological levels of TWA show different characteristics in healthy males and females, three TWA normality regions were defined, respectively, in relation to the entire control population and its male and female subgroups.
According to our previous studies, 18 , 19 a TWA normality region for the control population was identified by defining a set of three thresholds as the 99.5th percentiles of mean TWA duration, mean TWA amplitude, and mean TWA product distributions. This implies that the cases characterized by at least one TWA parameter exceeding the corresponding threshold identify abnormal levels of TWA (TWA+). Accordingly, separate normality regions of healthy males and females were delimited by thresholds on TWA duration, amplitude and product defined as the 99.5th percentiles of mean TWA duration, mean TWA amplitude, and mean TWA product distributions over our control‐male group and the control‐female group, respectively.
Mean TWA duration, mean TWA amplitude, and mean TWA product determined in individual control and CAD participants were analyzed in relation to their location with respect to the above defined normality regions.
Statistics
Lilliefors test 20 was used to evaluate the hypothesis that each parameter vector had a normal distribution (significance was set at 5% level) and could be expressed as mean ± SD. Comparisons between two groups of normally distributed samples were performed with 2‐tailed, nonpaired Student's t‐test (statistically significant difference was assumed at P < 0.05). According to Bonferroni's method, 21 statistical significance at 5% of this multiple‐comparison problem required P < 0.006 (i.e., P < 0.05/8, where 8 counts for mean and SD values of NN intervals over 20‐min recording, denominated MNN and SDNN, respectively, mean and SD of TWA duration, mean and SD of TWA amplitude, and mean and SD of TWA product) when comparing males against females within a population (being age, BMI, SAP, and DAP matched by definition), and P < 0.004 (i.e., P < 0.05/12, where 12 counts for age, BMI, SAP, DAP, MNN, SDNN, mean and SD of TWA duration, mean and SD of TWA amplitude, and mean and SD of TWA product) when comparing corresponding quantities between populations.
RESULTS
TWA was detected in all 142 control subjects and 151 CAD patients. TWA characteristic parameters (mean and SD of TWA duration, mean and SD of TWA amplitude, and mean and SD of TWA product) and clinical data (SAP, DAP, BMI, age, MNN, and SDNN) are reported in Table 1 for our control population and related control‐male and control‐female subgroups, and in Table 2 for our CAD population and related male and female subgroups.
Overall, the control population showed mean TWA duration (58 ± 15 beat) and mean TWA product (2085 ± 899 beat·μV) significantly lower than the CAD population (mean TWA duration = 64 ± 16 beat; mean TWA product = 2558 ± 1510 beat·μV). No significant difference was found between mean TWA amplitude levels of the two groups (control: mean TWA amplitude = 35 ± 12 μV; CAD: mean TWA amplitude = 40 ± 22 μV).
Within the control population, significant differences between genders were only observed in the MNN and mean TWA duration values. In particular, compared to the control‐female group, the control‐male group was characterized by 11% (P < 10−4) higher MNN and 20% (P < 10−6) lower mean TWA duration. Within the CAD population, no significant differences were observed in clinical and TWA parameters, when comparing the male and female groups.
Gender‐related comparison between control and CAD populations showed significantly higher values of mean TWA duration in the CAD‐male group compared to the control‐male group (2537 ± 1478 beat·μV vs 1913 ± 772 beat·μV, P < 0.004; respectively), while the CAD‐female group showed significantly higher levels of all TWA variability (SD of TWA duration, amplitude, and product) than the control‐female group (11 ± 5 beat vs 9 ± 2 beat, P < 0.004; 8 ± 6 μV vs 5 ± 3 μV, P < 0.004; and 871 ± 867 beat·μV vs 505 ± 238 beat·μV, P < 0.004; respectively).
Values of the thresholds delimiting the normality regions for the entire control population and, separately, for the control‐male group and the control‐female group, are reported in Table 3. The assumption of threshold values for the control population to identify abnormal levels of TWA (TWA+) yielded 19 TWA+ cases among the CAD patients (15 CAD males and 4 CAD females) and 2 TWA+ cases among the control subjects (1 male and 1 female; Fig. 1A). After assumption of gender‐related threshold values, no control subject (either male or female) fell in the TWA+ region, while the total number of TWA+ CAD cases increased to 36, divided into 32 CAD males (the 15 CAD‐male patients previously identified as TWA+ without gender separation, plus other 17 now characterized by a mean TWA duration over threshold) and 4 CAD females (the same 4 identified as TWA+ without gender separation; Fig. 1B and C, respectively).
Table 3.
Normality Threshold Values Identified over the Entire Control Healthy Population and, Separately, in the Control Healthy Males and Females
| Control Population (142) | Control Males (77) | Control Females (65) | |
|---|---|---|---|
| TWA duration threshold (beat) | 92 | 78 | 93 |
| TWA amplitude threshold (μV) | 67 | 68 | 61 |
| TWA product threshold (beat·μV) | 5026 | 3928 | 5057 |
Figure 1.
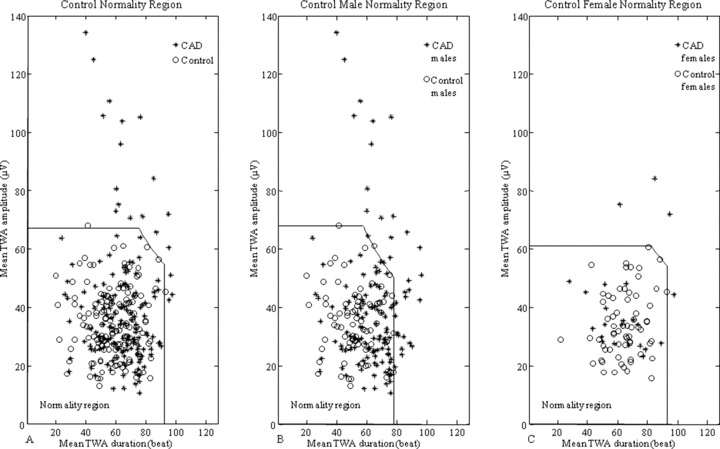
TWA normality regions defined over the entire control population (panel A) or over the two genders separately (panels B and C), together with TWA levels measured in the control and CAD populations.
DISCUSSION
The assumption of TWA as a physiological phenomenon characterized by a continuously changing amplitude and duration, which may show a transition to an abnormal condition, 17 requires the set up of methods that allow identification of a TWA normality region to improve reliability of nonphysiological TWA levels discrimination. The first attempt to quantify amplitude and duration of TWA in a population of control healthy subjects, in order to assess a threshold‐based borderline of TWA normality region, was performed by ourselves in a previous work, 18 without discriminating male from female gender. The hypothesis of TWA being gender‐dependent was tested in the present study.
It is worth to notice that the definition of a TWA normality region 18 is expected to be affected by the ECG recording conditions, ECG length, beyond the TWA detection technique. Therefore, there is a need to define standardized conditions under which ECG tracings are to be recorded. The supine resting condition has been considered preferable in the present study, to limit the amount of noise affecting the recordings. Moreover, a 20‐minute ECG, sufficiently short for recording in supine position, was selected as representing a good compromise between computational efforts and TWA identification reliability. 18 , 22 The AMF‐based method, applied here for TWA identification, underwent several validation tests in previous reports, 14 , 18 , 19 , 22 , 23 and is to be considered particularly suitable because the TWA continuity hypothesis underplays its algorithm. Other well known methods for TWA identification, such as the spectral method, 8 the complex demodulation method, 11 and the modified moving average method, 13 allow measurement of TWA amplitude as an index of cardiac electrical instability. 24 , 25 , 26 , 27 Compared to them, our AMF‐based method has the further and peculiar advantage to provide TWA duration and product, beyond TWA amplitude, thus allowing better characterization of stationary as well as transient TWA. 18 , 19 , 23
Once TWA parameterization is accomplished, the main point is how threshold levels of these parameters can be defined to discriminate physiological TWA from abnormal TWA (which is referred to as TWA+). In the absence of outcome monitoring, these thresholds have been statistically defined here over our control population (control‐male and control‐female populations for gender‐related investigation), on basis of the consideration that the number of TWA+ cases is expected to be extremely low, if not zero, in such a population. Considering 0.5% a suitable choice for each threshold, 99.5th percentiles of mean TWA duration, mean TWA amplitude, and mean TWA product distributions were assumed to define thresholds on TWA duration, amplitude, and product, respectively. Use of percentiles, rather than mean and SD, makes the procedure independent of the assumption of normal distribution for parameter estimates. After definition of TWA normality regions (Fig. 1), the cases falling outside are classified as TWA+ to indicate that they are characterized by increased levels of TWA in relation to the defined physiological levels. Belonging to TWA+ class can be considered a necessary, but not sufficient, condition for increased risk of arrhythmic events and sudden cardiac death. The definition of the exact TWA level over which TWA is closely linked to the occurrence of ventricular arrhythmias requires follow‐up studies and is beyond the scope the present study.
Any TWA detection algorithm is required to control for the noise level on the ECG, to discard the hypothesis that “physiological” TWA might, rather, pertain to noise. To this aim, our AMF‐based procedure includes a preprocessing stage designed to identify R peaks and to remove the beats recognized as noisy or nonsinus. Removed beats are replaced with a mean beat (over 128); however, an ECG tracing is considered eligible for TWA analysis only if the number of replaced beat is less than 8%. 12 , 18 In addition, our AMF removes any kind of noise (baseline, respiration modulation, etc.) characterized by frequency components incompatible with its narrow (0.12 Hz) passing band centered at the TWA frequency. 14 , 23 To reduce the effect of compatible noise, our AMF algorithm incorporates a further specific test involving the phase of the TWA‐signal (i.e., the output signal of the AMF). More specifically, to make sure that a detected TWA‐signal is due to the alternation of the T‐waves and not to other kinds of alternation (noise, and even QRS alternation, for instance), the maxima and minima of the TWA‐signal are required to fall in correspondence of the ST segment. 19 Eventually, to avoid that local noise‐induced alternation is detected as TWA, the TWA‐signal amplitude is required to be greater than 5 μV for at least seven consecutive beats (see Methods). As an example, Figure 2 shows a segment of 3‐lead ECG tracings, from one of the two control subjects falling outside the gender‐independent TWA normality region. Some T‐wave variability is visible, more properly than TWA, because, as it is generally the case, noise, baseline wanderings, and respiration modulation, beyond physiological oscillations, superimpose. TWA is unmasked by our AMF method, with relatively high levels of mean TWA duration = 41 beat; mean TWA amplitude = 68 μV, and mean TWA product = 2955 beat·μV. Figure 3 shows superimposition of 128 consecutive T‐waves from a control subjects (the same as used in Fig. 2), and a CAD patient (mean TWA duration = 76 beat, mean TWA amplitude = 105 μV, and mean TWA product = 8151 beat·μV) falling in the TWA+ region. Some T‐wave variability is evident in both cases (especially in channel Z), more in the CAD patient than in the control subject, though.
Figure 2.
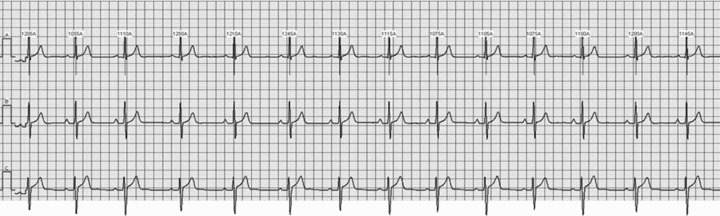
ECG tracing (X, Y, and Z leads from top to bottom panels) from a control subject.
Figure 3.
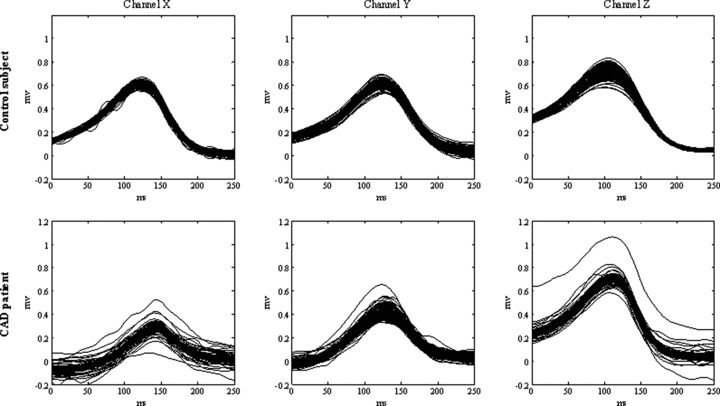
Superimposition of 128 consecutive T‐waves (X, Y, and Z leads) from ECG tracings of the control subject of Figure 2 (TWA duration = 41 beat; TWA amplitude = 68 μV, TWA product = 2955 beat·μV), and a CAD patient (TWA duration = 76 beat; TWA amplitude = 105 μV, and TWA product = 8151 beat·μV). Both cases fall in the TWA+ region.
Significantly lower mean TWA duration in our control‐male subgroup, compared with the control‐female (Table 1) makes the difference in the definition of gender‐related normality regions, and enhances the need of referring to them for a more reliable identification of abnormal TWA cases (TWA+). Indeed, after gender‐related definition of normality regions, TWA+ cases among the CAD patients increased from 12.6% (19 out of 151) to 23.8% (36 out of 151). To understand the origin and the implications of this increase in TWA+ cases, it is worth to compare in one figure (Fig. 4) the threshold lines delimiting the normality regions as defined over the entire control population (solid line), the control‐male subgroup (dashed line), and the control‐female subgroup (dotted line). Compared to the thresholds identifying the control normality region, the control‐female normality region shows a reduction of TWA amplitude threshold (from 67 to 61 μV; Table 3), which, however, does not contribute to identify new TWA+ cases among the CAD females. By contrast, the control‐male normality region is characterized by a significant reduction in the TWA duration threshold (from 92 to 78 beat; Table 3), which gives rise to the shaded zone where 17 extra TWA+ CAD‐male patients fall.
Figure 4.
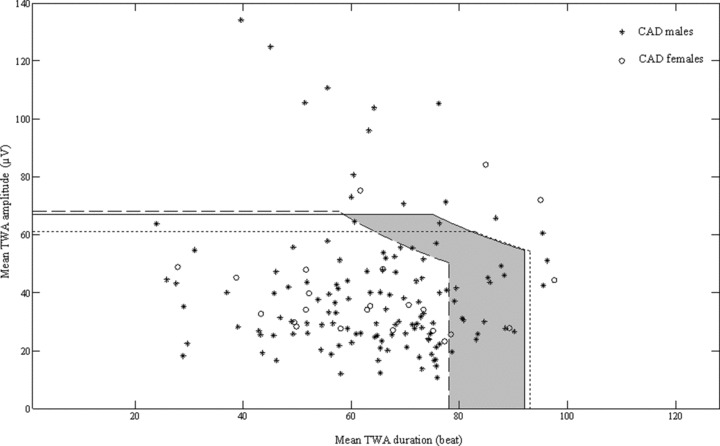
Superimposition of the TWA normality regions, respectively, defined over the entire control population (solid line), the control‐male subgroup (dashed line) and the control‐female subgroup (dotted line). Compared to the control‐population normality region, the control‐male normality region is characterized by a significant reduction of TWA duration threshold which gives rise to the shaded zone where 17 extra CAD‐male patients are identified as TWA+ with a gender‐related analysis.
The fact that these extra cases are characterized by a prolonged TWA duration (i.e., more stable TWA) meets a previous finding by others 17 that increased TWA stability is more likely to associate with cardiac instability.
Conclusions
In healthy male subjects, TWA is characterized by significantly shorter duration than in healthy female. This finding justifies the definition of separate TWA normality regions, for males and females, which enable us to unmask extra TWA+ cases not detectable after definition of a gender‐independent TWA normality region. Our finding that all these unmasked cases are CAD males meets the common knowledge in clinics that males are at higher risk of cardiac instability than females.
Conflict of Interest Statement: All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
REFERENCES
- 1. Bigger JT, Bloomfield DM. Microvolt T‐wave alternans: An effective approach to risk stratification in ischemic cardiomyopathy? Nat Clin Pract Cardiovasc Med 2007;4:300–301. [DOI] [PubMed] [Google Scholar]
- 2. Zacks ES, Morin DP, Ageno S, et al Effect of oral beta‐blocker therapy on microvolt T‐wave alternans and electrophysiology testing in patients with ischemic cardiomyopathy. Am Heart J 2007;153:392–397. [DOI] [PubMed] [Google Scholar]
- 3. Narayan SM. T‐wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol 2006;47:269–281. [DOI] [PubMed] [Google Scholar]
- 4. Bloomfield DM, Bigger JT, Steinman RC, et al Microvolt T‐wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;47:456–463. [DOI] [PubMed] [Google Scholar]
- 5. Ikeda T, Yoshino H, Sugi K, et al Predictive value of microvolt T‐wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: Results of a collaborative cohort study. J Am Coll Cardiol 2006;48:2268–2274. [DOI] [PubMed] [Google Scholar]
- 6. Verrier RL, Nearing BD, La Rovere MT, et al Ambulatory electrocardiogram‐based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol 2003;14:705–711. [DOI] [PubMed] [Google Scholar]
- 7. Klingenheben T, Zabel M, D’Agostino RB, et al Predictive value of T‐wave alternans for arrhythmic events in patients with congestive heart failure. Lancet 2000;356:651–652. [DOI] [PubMed] [Google Scholar]
- 8. Rosenbaum DS, Jackson LE, Smith JM, et al Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 1994;330:235–241. [DOI] [PubMed] [Google Scholar]
- 9. Adam DR, Smith JM, Akselrod S, et al Fluctuations in T‐wave morphology and susceptibility to ventricular fibrillation. J Electrocardiol 1984;17:209–218. [DOI] [PubMed] [Google Scholar]
- 10. Smith JM, Clancy EA, Valeri CR, et al Electrical alternans and cardiac electrical instability. Circulation 1988;77:110–121. [DOI] [PubMed] [Google Scholar]
- 11. Nearing BD, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T wave. Science 1991;252:437–440. [DOI] [PubMed] [Google Scholar]
- 12. Burattini L, Zareba W, Moss AJ. Correlation method for detection of transient T‐wave alternans in digital Holter ECG recordings. Ann Noninvasive Electrocardiol 1999;4:416–424. [Google Scholar]
- 13. Nearing BN, Verrier RL. Modified moving average analysis of T‐wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol 2002;92:541–549. [DOI] [PubMed] [Google Scholar]
- 14. Burattini L, Zareba W, Burattini R. Automatic detection of microvolt T‐wave alternans in holter recordings: Effect of baseline wandering. Biomed Signal Process Control 2006;1:162–168. [Google Scholar]
- 15. Martínez JP, Olmos S, Wagner G, et al Characterization of Alternans during ischemia: Time‐course and spatial analysis. IEEE Trans Biomed Eng 2006;53:701–711. [DOI] [PubMed] [Google Scholar]
- 16. Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol 2002;13:502–512. [DOI] [PubMed] [Google Scholar]
- 17. Pruvot EJ, Katra RP, Rosenbaum DS, et al Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 2004;94:1083–1090. [DOI] [PubMed] [Google Scholar]
- 18. Burattini L, Zareba W, Burattini R. Assessment of physiological amplitude, duration and magnitude of ECG T‐wave alternans. Ann Noninvasive Electrocardiol 2009;14:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burattini L, Zareba W, Burattini R. Adaptive match filter based method for time versus amplitude characterization of microvolt ECG T‐wave alternans. Ann Biomed Eng 2008;36:1558–1564. [DOI] [PubMed] [Google Scholar]
- 20. Lilliefors HW. On the Kolmogorov‐Smirnov test for normality with men and variance unknown. J Am Stat Assoc 1967;62:399–402. [Google Scholar]
- 21. Bland JM, Altman DG. Multiple significance tests: The Bonferroni method. BMJ 1995;310:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burattini L, Zareba W, Burattini R. Identification of time‐varying T‐wave alternans from 20‐Minute ECG recordings. In: Proceedings of BIOSTEC 2008, International Joint Conference on Biomedical Engineering Systems and Technologies, Funchal, Madeira, Portugal, January 28–31, 2008;186–192.
- 23. Burattini L, Bini S, Burattini R. Comparative analysis of methods for automatic detection and quantification of microvolt T‐wave alternans. Med Eng Phys 2009;31:1290–1298. [DOI] [PubMed] [Google Scholar]
- 24. Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovasc Res 1994;28:1440–1449. [DOI] [PubMed] [Google Scholar]
- 25. Klingenheben T, Ptaszynski P, Hohnloser SH. Quantitative assessment of microvolt T‐wave alternans in patients with congestive heart failure. J Cardiovasc Electrophysiol 2005;16:620–624. [DOI] [PubMed] [Google Scholar]
- 26. Kaufman ES, Bloomfield DM, Steinman RC, et al. “Indeterminate” microvolt T‐wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;48:1399–1404. [DOI] [PubMed] [Google Scholar]
- 27. Minkkinen M, Kähönen M, Viik J, et al Enhanced predictive power of quantitative TWA during routine exercise testing in the Finnish Cardiovascular Study. J Cardiovasc Electrophysiol 2009;20:408–415. [DOI] [PubMed] [Google Scholar]


