Abstract
Purpose of review
To summarize current evidence in the association of imprinting disorders and assisted reproductive technology.
Recent findings
The worldwide usage of assisted reproductive technology (ART) has continued to increase since the first successful birth of a human after IVF. Since 2002, several reports have raised concerns that children conceived by ART are at increased risk of having imprinting disorders. The majority of published studies have examined DNA methylation in children conceived by ART, but results are conflicting. Beckwith–Wiedemann syndrome and Angelman syndrome are the most extensively studied imprinting disorders and multiple case series and reports have been published on ART-conceived children with these syndromes. Overall the majority of reports suggest that ART might be associated with Beckwith–Wiedermann syndrome and Angelman syndrome, but larger collaborative studies need to be performed.
Summary
The current data suggest an association between imprinting disorders and ART although the absolute risk appears to be low. However, animal studies have established biologic plausibility and there is continuing concern about the possibility of epigenetic changes resulting from ART.
Keywords: Angelman syndrome, assisted reproductive technology, Beckwith–Wiedemann syndrome, DNA methylation, imprinting disorders
Introduction
Since the first report of IVF in 1978, assistd reproductive technologies have grown to encompass all methods used to achieve pregnancy by artificial means during which handling of eggs and sperm occurs, including IVF and intracytoplasmic sperm injection (ICSI) [1]. The 138 198 assisted reproductive technology (ART) cycles performed in 2006 resulted in 41 343 live births (deliveries of one or more living infants) and 54 656 infants, encompassing 1% of all births in the USA [1]. Three million babies worldwide have been born after conception with ART [2]. Concern within the scientific community exists regarding the potential lasting consequences of ART on imprinting in the developing fetus.
Epigenetics and reprogramming mechanisms
The term ‘epigenetics’ refers to stably heritable phenotypes ‘resulting from changes in a chromosome without alterations in the DNA sequence’ [3]. Epigenetic changes include post-translational modifications of histone tails (acetylation, methylation, phosphorylation, etc.), DNA methylation and higher order packaging of DNA around nucleosomes [4].
The most widely studied epigenetic mechanism is DNA methylation. Methylation of DNA occurs via the enzymatic addition of a methyl group from S-adenosylmethionine (SAM) to the carbon-5 position of the cytosine ring of the dinucleotide sequence CpG. This reaction is catalyzed by DNA methyltransferases [5]. Much of what is known about the roles and importance of genomic methylation has come from studies on DNA (cytosine-5)-methyltransferases and phenotypes resulting from mutations in the genes encoding these enzymes [6]. DNA methyltransferase (Dnmt) 1 serves to maintain methylation patterns [7], whereas Dnmt3a and Dnmt3b are responsible for de-novo methylation [8]. Genetic analysis of these Dnmts has established that DNA methylation is essential for vertebrate development. Loss of methylation has a profound effect, resulting in apoptosis in embryos and widespread depression of ectopic gene expression [9].
Genomic imprinting and assisted reproductive technology
Since 2002, several reports have raised concerns that children conceived by ART are at an increased risk of having imprinting disorders. Genomic imprinting is an epigenetic mechanism resulting in parental expression of certain genes [10•]. Imprinting is regulated by DNA methylation resulting in expression of either the maternal or paternal allele [11]. Most imprinted genes contain differentially methylated regions (DMRs), where methylation differs between the maternal and paternal alleles [12]. This variation allows for differential regulation of these alleles dependent on parental origin of the allele and may result in either active transcription or preferential silencing of genes [13]. Imprinted genes in particular are important in the regulation of the developing fetus [13,14] (Fig. 1).
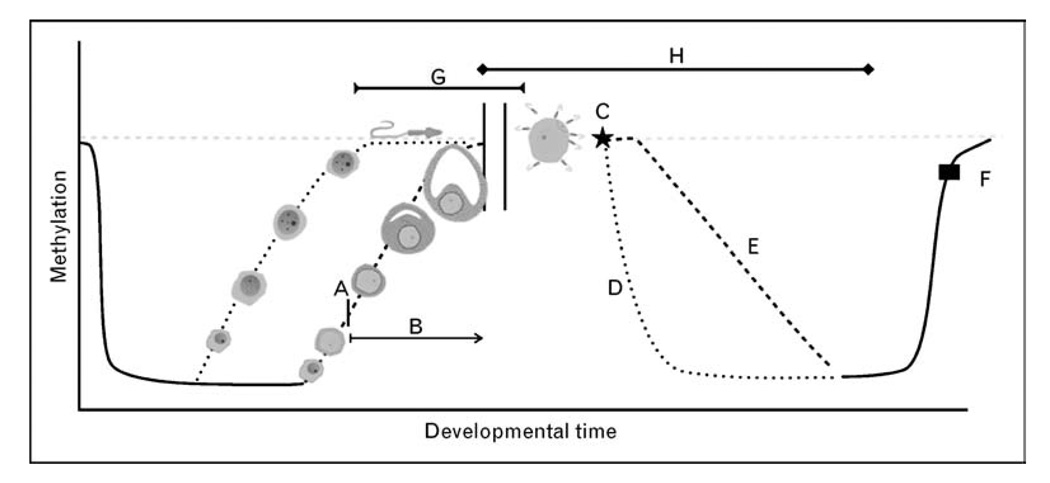
Figure 1. Epigenetic reprogramming and assisted reproductive technology.
Genomic methylation occurs in a cyclical fashion in mammals. Genomic imprints are erased and re-established during gametogenesis but re-establishment of methylation occurs much later in the development of oocytes. In spermatogenesis remethylation primarily occurs prior to birth, whereas in oogenesis remethylation begins after birth (a) and is not completed until metaphase of meiosis II [15]. This time (b) will vary for each oocyte and can last from birth until menopause. Once fertilization (c) occurs each haplogenome will again undergo demethylation, actively in the male haplogenome (d) and passively in the female haplogenome (e). Remethylation of the zygotic genome will then occur around the time of implantation (f) [15]. Assisted reproductive technology (ART) procedures occur during these vulnerable developmental periods and have been proposed to interact at certain points of the methylation cycle (g, ovulation induction and egg retrieval; h, IVF, ICSI, micromanipulation of gametes, exposure to culture medium, preimplantation genetic diagnosis, in-vitro oocyte maturation).  , baseline methylation; - - - -, male haplogenome; – – – –, female haplogenome.
, baseline methylation; - - - -, male haplogenome; – – – –, female haplogenome.
The possible influence of imprinting on ART was mentioned as early as 1998 when Tesarik et al. [16] suggested that reproductive centers wishing to use spermatids in assisted reproduction be prepared to offer diagnostic methods to control genomic imprinting abnormalites in the progeny. Several studies since have suggested that ART may lead to epigenetic changes in the offspring [17,18].
Current evidence on the association of imprinting disorders and assisted reproductive technology
Recent studies have investigated the association of imprinting disorders and ARTs (Table 1).
Table 1.
Current data on the association of assisted reproductive technology and Beckwith–Wiedemann syndrome and Angelman syndrome
| Citation | Type of study |
Prevalence of IVF/ICSI in general population during study period |
Prevalence of IVF or ICSI in study syndrome populationa,b |
ART procedure(s) |
No. of case(s) tested for imprinting defect |
No. of case(s) with imprinting defect |
Percentage of syndrome pts post-IVF or ICSI with imprinting abnormalities |
|---|---|---|---|---|---|---|---|
| Angelman syndrome | |||||||
| Cox et al. [19] | Case series | NR | NR | 2 ICSI | 2 | 2 | 100.0% |
| Orstavik et al. [20] | Case report | NR | NR | 1 ICSI | 1 | 1 | 100.0% |
| Lidegaard et al. [21] | Cohort | NR | 0.0% | 162 SIVF/4372 ICSI | 0 | 0 | 0% |
| Ludwig et al. [22] | Survey | NR | 3.8% | 3 ICSI/5 OS | 3 | 1 | 33.3% |
| Sutcliffe et al. [23] | Survey | 0.8% | 0.0% | 1 OS/2 IUI | 0 | 0 | 0% |
| Doornbos et al. [24] | Survey | 0.5% | 0.0% | 3 OS/1 IUI | 4 | 0 | 0% |
| BWS | |||||||
| Lidegaard et al. [21] | Cohort | 1.3% | 0% | 162 SIVF/4372 ICSI | 0 | 0 | 0% |
| Sutcliffe et al. [23] | Survey | 0.8% | 2.9% | 1 IVF/5 ICSI | 6 | 6 | 100.0% |
| Doornbos et al. [24] | Survey | 0.9% | 5.6% | 4 IVF | 4 | 4 | 100.0% |
| DeBaun et al. [25] | Case series | 0.8% | 4.6% | 2 IVF/5 ICSI | 6 | 5 | 83.3% |
| Maher et al. [26] | Case series | 1.2% | 4.0% | 3 IVF/3 ICSI | 2 | 2 | 100.0% |
| Gicquel et al. [27] | Case series | 1.3% | 4.0% | 4 IVF/2 ICSI | 6 | 6 | 100.0% |
| Halliday et al. [28] | Case–control | 1.1% | 10.8% | 3 IVF/1 ICSI | 3 | 100.0% | |
| Chang et al. [29] | Case seriesc | NR | 5.6% | 5 IVF/5 ICSI | 6c | 5 | 83.3% |
| Rossignol et al. [30] | Cohortd | NR | 27.5%d | 8 IVF/3 ICSI | 11 | 11 | 100.0% |
| Bowdin et al. [31] | Cohort | NR | 0.1% | IVF/ICSI | 1 | 1 | 100.00% |
| Lim et al. [32••] | Cohortd | NR | 22.3%d | 12 IVF/13 ICSI | 25 | 24 | 96.0% |
| Strawn et al. [33•] | Case report | NR | NR | IVF | 1 | 0e | 0% |
ART, assisted reproductive technology; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; IVF, in-vitro fertilization; NR, not recorded; OS, ovarian stimulation; SIVF; singleton IVF.
Prevalence of Beckwith–Wiedemann syndrome (BWS) in general population is 1/13 700 and prevalence of BWS with imprinting defects in the general population is 1/30 000.
Prevalence of Angelman syndrome in general population is 1/15 000 and prevalence of Angelman syndrome with imprinting defect in the general population is 1/300 000.
Patients previously reported by Debaun et al. [25].
Cohort with known imprinting defect in KvDMR1.
Molecular analysis of one child performed but was inconclusive.
Angelman syndrome
Angelman syndrome is characterized by mental retardation, an inappropriate happy demeanor and dysmorphic facial features [34]. The incidence of Angelman syndrome is approximately 1 in 15 000 [19] and the syndrome is caused by abnormalities (imprinting defects, maternal deletions, point mutations and uniparental disomy) in the chromosome 15q11–13 region [35]. Fewer than 5% of Angelman syndrome cases are associated with an imprinting defect [35].
The first cases of ART-related Angelman syndrome were reported in 2002 and 2003 (Table 1) [19,20]. Cox et al. [19] described two patients conceived by intracytoplasmic sperm injection who were diagnosed with Angelman syndrome and found to have aberrant loss of methylation on chromosome 15. Orstavik et al. [20] described a similar methylation defect of the SNRPN locus in another patient conceived with ICSI. As imprinting accounts for less than 5% of Angelman syndrome cases, the discovery of these two cases raised concern about possible over-representation of imprinting defects among children conceived by ICSI.
In 2005, a cohort study was published investigating the occurrence of imprinting disorders after IVF in the Danish population [21]. Using a Denmark National Registry of 25 000 children born after IVF, no Angelman syndrome cases were identified, arguing against an association of IVF with Angelman syndrome [21]. However, in the same year a German cohort study (n = 79) [22] reported imprinting defects in four (25%) of 16 Angelman syndrome syndrome children born to subfertile couples. The relative risk (RR 6.25) of an imprinting defect was noted to be the same in subfertile patients who underwent no therapy (n = 8) as compared to subfertile patients who underwent ICSI or hormone treatment (n = 8) [22]. These data (Table 1) were the first to suggest that the increased prevalence of imprinting in Angelman syndrome children born after IVF might be linked to subfertility rather than treatments for subfertility [22].
A British survey further examined the correlation between ART and imprinting disorders [23]. Questionnaires on conception history were mailed to 384 families of children with Angelman syndrome and 81 replies were received [23]. Six children were excluded based on a family history of Angelman syndrome, none of which were conceived by ART. Of the remaining 75 children, three (4%) were conceived by ART. One family used artificial insemination by donor, another used intrauterine insemination (IUI) by donor and one had previously used IVF (suggesting a history of ovarian stimulation), thus resulting in a minimum prevalence of 0.8% in this ART group [23]. Molecular analysis of these three children (Table 1) revealed one child (conceived by IUI with donor sperm) with loss of maternal allele methylation at the SNRPN locus. Epigenetic changes at this imprinting control region are rare with an estimated prevalence of 1 in 300 000 births [23]. The finding of such an imprinting abnormality in one of three ART-related Angelman syndrome cases led the authors to conclude that ART-related Angelman syndrome is associated with aberrant methylation in a critical imprinting control region [23].
The following year a Dutch study using a nationwide survey of the Netherlands was published [24]. This survey was sent to 135 families with Angelman syndrome children and a response of 72.6% was obtained. Thirty-five children were excluded based on conception prior to 1983, the first year IVF children were born in the Netherlands [24]. Notably, the maternal age of the Angelman syndrome group was significantly higher than the Dutch population (30.64 versus 29.68, P<0.05). The Angelman syndrome group also had an increased prevalence of fertility problems compared to the Dutch population (19% versus 5.9%, RR 3.4) [24]. Of the 63 Angelman syndrome cases included, 4 (6.3%) were conceived with ART: 1 case from IUI/donor insemination and 3 cases after ovulation induction. The number of Angelman syndrome children born after ovulation induction with medications (n = 3) was significantly higher than that in the Dutch population (4.8% versus 0.39%, RR = 12.3, P < 0.05). Molecular analysis of three Angelman syndrome cases revealed two cases with maternal UBE3A deletion and one case with a confirmed but unspecified mutation (Table 1) [24]. Overall, this study supported the conclusion that infertility and ovulation induction are risk factors for ART-related Angelman syndrome cases.
Beckwith–Wiedemann syndrome
Beckwith–Wiedemann syndrome (BWS) is a growth disorder caused by methylation defects and uniparental disomy of chromosome 11p15 [36]. The incidence of BWS is approximately one in 13 700 [36]. Cases of BWS associated with ART were first reported in 2003 (Table 1) [25,27]. DeBaun et al. [25] identified seven children with sporadic BWS conceived with ART. Six of the seven children were conceived with IVF using the biological mother’s egg and biological father’s sperm (the remaining with donor egg). Four of the seven children were from ICSI using ejaculated sperm, one with ICSI using testicular sperm extraction (TESE) and two did not involve ICSI [25]. Prevalence of ART in the Washington University BWS registry was 4.6% (3/65), presenting a six-fold increase in ART among BWS cases as compared to the US population (0.76%) [25]. Molecular analysis of five of the six children showed hypomethylation of LIT1 and the remaining child with additional hypermethylation of H19 [25]. As a result of these findings, the authors [25] concluded that this specific imprinting defect might be increased in children conceived by ART.
Gicquel et al. [27] made a similar observation and reported six cases of sporadic BWS children conceived with ART, in a cohort of 149 BWS patients. These six patients were found to exhibit isolated demethylation of KvDMR1 [27]. The 4% (6/149) representation of BWS was significantly higher than the reported 1.3% prevalence of BWS in France at the time [27]. With a calculated odds ratio (OR) of 3.2 (95% confidence interval (CI) 1.4–7.3) of BWS after ART, the authors concluded that ART is potentially associated with aberrant imprinting [27].
A review of 149 BWS patients in the UK yielded similar results (Table 1) [26]. Six of 149 BWS children (4%) were born after ART (three with IVF and three with ICSI). Two of the children were found to have hypomethylation of KvDMR1 while all four were negative for uniparental disomy [26]. This prevalence was significantly higher than the reported 1.2% use of ART at the time suggesting a possible association of ART with imprinting abnormalities.
In 2004, Halliday et al. [28] published a case–control study of 1 316 500 live births documented in the Victorian Perinatal Data Collection unit between 1983 and 2003. Thirty-seven cases of BWS were detected, giving an overall incidence of BWS of 1 in 35 580 live births for the period investigated [28]. IVF was the method of conception of four of 37 BWS cases (10.8%) and in one control resulting in an OR of 17.8 (95% CI 1.8–432.9, P < 0.006) [28]. However, the authors [28] advised caution in considering the OR due to the wide CI. The absolute risk of BWS in this population was less than 1% (4 in 14 894), but was still nine times greater than the general population [28].
In the previously mentioned ‘Danish National IVF Cohort Study’ [21], a database of all singleton children born in Denmark from 1995 to 2001 was queried for BWS cases. During these 7 years, 442 349 singleton non-IVF and 6052 IVF children were born [21]. In total, 54 children with imprinting diseases were identified in the non-IVF cohort: 44 with renal cancer, five with retinoblastoma, three with Prader–Willi syndrome and two with Russell–Silver syndrome. However, no cases of imprinting disorders were detected in the IVF cohort (Table 1) [21]. Based on the non-IVF cohort an expected incidence of 0.74% in the IVF cohort was expected [21]. These findings do not support an increased risk of BWS in children conceived with ART.
In February 2005, a retrospective case series from the USA was published [29]. Using a BWS registry, 19 of 341 BWS cases resulted from ART [29]. Both maternal and paternal age was significantly higher in the BWS–ART group. When the type of ART, the type of IVF media and timing of embryo transfer was considered no significant association was detected [29]. Epigenetic analysis had previously been reported in six of the patients [25] and the molecular data were unavailable in the remaining patients [29]. These findings led the authors [29] to conclude that the findings were limited by study size and larger studies would be needed to detect a significant correlation between ART and BWS.
In 2006, Rossignol et al. [30] investigated the incidence of imprinting in 11 patients with BWS conceived with ART who had known imprinting defects in KCNQ1OT1. Multiple genes including IGF2R, PEG1/MEST, KCNQ1OT1, H19 and SNRPN were assessed for methylation status in these patients and 29 controls with a similarly known imprinting defect [30]. Three of the 11 (27%) patients conceived with ART displayed a demethylation anomaly at multiple loci [30]. Comparison of ART procedures among the 11 revealed no differences [30]. Molecular analysis was performed in 29 BWS syndrome patients conceived naturally revealing a similar incidence (24%) of methylation abnormalities (Table 1) [30] and suggesting the incidence of aberrant methylation was not increased in BWS patients conceived by ART.
In the previously mentioned nationwide survey of the Dutch population [24], surveys were sent to 138 families with BWS children known to BWS support groups. There was a 78% response rate (n = 75) and 71 of these were eligible for the study based on inclusion criteria of birth in the Netherlands between 1983 and 2003 [24]. The maternal age of the BWS group was significantly higher than that of the Dutch population (30.59 versus 29.68, P = 0.03) and fertility problems were significantly increased in the BWS group compared to the Dutch population (11.1 versus 5.9%, RR = 2.0) [24]. Four BWS children were born after IVF with an incidence of 5.6% compared to 0.92% in the Dutch population (RR = 6.1, P < 0.01). Six cases of BWS syndrome were found in a group of patients with fertility problems and all six children had hypomethylation of LIT1 (four of six were after IVF, one of six after hormonal stimulation of ovulation alone and one of six after IUI) [24]. After correcting for fertility problems in patients, no increased incidence of BWS was noted after ART [24]. These data (Table 1) suggest that the increase in imprinting anomalies after ART might be due to infertility and not ART specifically.
Another questionnaire-based study on the risk of imprinting disorders after ART was reported in 2007 [31]. A survey was sent to 1559 families with children born after ART with the goal of identifying cases of BWS and Angelman syndrome that had previously been nondiagnosed secondary to mild phenotypes. Seventy children were identified and 47 accepted inclusion into the study (67% acceptance rate) [31]. Four children with BWS phenotypes were discovered but only one was found to have aberrant methylation [31]. The authors [31] concluded that the absolute risk of imprinting disorders in children conceived by ART is small (<1%) and recommended further investigation [31].
A study published in 2009 compared molecular features and clinical phenotype of 25 ART related and 87 non-ART related BWS children with known KvDMR1 demethylation (Table 1) [32••]. Twenty-four of the 25 (96%) BWS–ART children had KvDMR1 loss of methylation, but there was no significant difference in the mean methylation index of BWS–ART children and the non-ART BWS children (4.6 versus 7.6%, P = 0.6) [32••]. The authors also investigated loss of methylation at other imprinting control regions in both groups. Investigation of DMRs at 6q24 (ZAC locus associated with Transient Neonatal Diabetes Mellitus), 7q32 (PEG1) and 15q13 (SNRPN commonly associated with Angelman syndrome and Prader–Willi syndrome) found that additional demethylation occurred in 37.5% of the ART-related BWS patients and 6.4% of the naturally conceived BWS patients [32••]. These findings led the authors [32••] to conclude that an increased risk of methylation abnormalities after ART is possible.
Conclusion
Multiple case reports and case series have suggested an association between imprinting disorders and ART but recent cohort studies have failed to confirm the association. Studies have been performed with other imprinting disorders, including Prader–Willi syndrome, Silver–Russell syndrome, transient neonatal diabetes mellitus and maternal hypomethylation syndrome, but an association with ART is either weak or nonexistent for these conditions [37]. Studies on global methylation changes in patients with the reported syndromes after ART are conflicting [38••,39••]. Tierling et al. [38••] recently reported no association with ART and imprinting in a study of 10 loci known to be imprinted, whereas Katari et al. [39••] observed aberrant genomic imprinting of greater than 2sd after analysis of 1536 CpG sites suggesting an association of ART with altered gene transcription. Multiple studies and reports [33•,40–42] have not clearly related ART and imprinting disorders, but one recent study [43••] showed aberrant imprinting in clinically normal children conceived with ART, suggesting that the impact of ART on the epigenome is not yet completely understood. Additional studies are needed in order to establish the level of risk of imprinting disorders in patients pursuing ART.
Acknowledgement
This study was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 569).
- 1.2006 assisted reproductive technology success rates: national summary and fertility clinic reports. Atlanta: U.S. Department of Health and Human Services, Center for Disease Control and Prevention; 2008. Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. [Google Scholar]
- 2.International Committee for Monitoring Assisted Reproductive Technology (ICMART) World collaborative report on assisted reproductive technology, 2002. Hum Reprod. 2009;24:2310–2320. doi: 10.1093/humrep/dep098. [DOI] [PubMed] [Google Scholar]
- 3.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. [PubMed] [Google Scholar]
- 5.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 6.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 7.Yoder JA, Soman N, Verdine GL, Bestor TH. DNA methyltransferases in mouse tissues and cells. Studies with a mechanism-based probe. J Mol Biol. 1997;270:385–395. doi: 10.1006/jmbi.1997.1125. [DOI] [PubMed] [Google Scholar]
- 8.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 9.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33 Suppl:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 10. Koerner MV, Barlow DP. Genomic imprinting-an epigenetic gene-regulatory model. Curr Opin Genet Dev. 2010;20:164–170. doi: 10.1016/j.gde.2010.01.009. This article is an up-to-date review of genomic imprinting and emphasizes the need for continued research to identify new epigenetic mechanisms.
- 11.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 12.Neumann B, Kubicka P, Barlow DP. Characteristics of imprinted genes. Nat Genet. 1995;9:12–13. doi: 10.1038/ng0195-12. [DOI] [PubMed] [Google Scholar]
- 13.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 14.Morison IM, Paton CJ, Cleverley SD. The imprinted gene and parent-of-origin effect database. Nucleic Acids Res. 2001;29:275–276. doi: 10.1093/nar/29.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucifero D, Mertineit C, Clarke HJ, et al. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–538. doi: 10.1006/geno.2002.6732. [DOI] [PubMed] [Google Scholar]
- 16.Tesarik J, Sousa M, Greco E, Mendoza C. Spermatids as gametes: indications and limitations. Hum Reprod. 1998;13 Suppl 3:89–107. doi: 10.1093/humrep/13.suppl_3.89. [DOI] [PubMed] [Google Scholar]
- 17.Shieve LA, Meikle SF, Ferre C, et al. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 18.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injections and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 19.Cox GF, Burger J, Lip V, et al. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orstavik KH, Eiklid K, van der Hagen CB, et al. Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet. 2003;72:218–219. doi: 10.1086/346030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lidegaard O, Pinborg A, Anderson AN. Imprinting diseases and IVF: Danish National IVF cohort study. Hum Reprod. 2005;20:950–954. doi: 10.1093/humrep/deh714. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig M, Katalinic A, Grob S, et al. Increased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couples. J Med Genet. 2005;42:289–291. doi: 10.1136/jmg.2004.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutcliffe AG, Peters CJ, Bowdin S, et al. Assisted reproductive therapies and imprinting disorders: a preliminary British survey. Hum Reprod. 2006;21:1009–1011. doi: 10.1093/humrep/dei405. [DOI] [PubMed] [Google Scholar]
- 24.Doornbos ME, Maas SM, McDonnell J, et al. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod. 2007;22:2476–2480. doi: 10.1093/humrep/dem172. [DOI] [PubMed] [Google Scholar]
- 25.DeBaun MR, Niemitz EL, Fienberg AP. Association of in vitro fertilization with Beckwith–Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maher ER, Brueton LA, Bowdin SC, et al. Beckwith–Wiedemann syndrome and assisted reproduction technology (ART) J Med Genet. 2003;40:62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gicquel C, Gaston V, Mandelbaum J, et al. In vitro fertilization may increase the risk of Beckwith–Wiedemann syndrome related to the abnormal imprinting of the KCN10T gene. Am J Hum Genet. 2003;72:1338–1341. doi: 10.1086/374824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliday J, Oke K, Breheny S, et al. Beckwith–Wiedemann syndrome and IVF: a case–control study. Am J Hum Genet. 2004;75:526–528. doi: 10.1086/423902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang AS, Moley KH, Wangler M, et al. Association between Beckwith–Wiedemann syndrome and assisted reproductive technology: a case series of 19 patients. Fertil Steril. 2005;83:349–354. doi: 10.1016/j.fertnstert.2004.07.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossignol S, Steunou V, Chalas C, et al. The epigenetic imprinting defect of patients with Beckwith–Wiedemann syndrome born after assisted reproductive technology is not restricted to the 11p15 region. J Med Genet. 2006;43:902–907. doi: 10.1136/jmg.2006.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowdin S, Allen C, Kirby G, et al. A survey of assisted reproductive technology births and imprinting disorders. Hum Reprod. 2007;22:3237–3240. doi: 10.1093/humrep/dem268. [DOI] [PubMed] [Google Scholar]
- 32. Lim D, Bowdin SC, Tee L, et al. Clinical and molecular genetic features of Beckwith–Wiedemann syndrome associated with assisted reproductive technologies. Hum Reprod. 2009;24:741–747. doi: 10.1093/humrep/den406. This study shows an increased incidence of aberrant imprinting in BWS–ART patients at regions other than KvDMR1. These findings suggest that global hypomethylation is more frequent in this subset of patients.
- 33. Strawn EY, Bick D, Swanson A. Is it the patient or the IVF? Beckwith–Wiedemann syndrome in both spontaneous and assisted reproductive conceptions. Fertil Steril. 2010;94:754.e1–754.e2. doi: 10.1016/j.fertnstert.2010.01.067. This case presents two BWS children born to the same parents after ART. One child was conceived naturally (after exposure to ovarian stimulation in the past) and one child was conceived with IVF. This case suggests both infertility and ovarian stimulation as possible risk factors for imprinting disorders.
- 34.Williams CA, Driscoll DJ. GeneReviews at GeneTests: Medical Genetics Information Resource (database online) Seattle: University of Washington; 2007. Angelman syndrome. [Google Scholar]
- 35.Clayton-Smith J, Laan L. Angelman syndrome: a review of the clinical and genetic aspects. J Med Genet. 2003;40:87–95. doi: 10.1136/jmg.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuman C, Smith AC, Weksberg R. GeneReviews at GeneTests: Medical Genetics Information Resource (database online) Seattle: University of Washington; 2005. Beckwith–Wiedemann syndrome. [Google Scholar]
- 37.Armor DJ, Halliday J. A review of known imprinting syndromes and their association with assisted reproduction technologies. Hum Reprod. 2008;23:2826–2834. doi: 10.1093/humrep/den310. [DOI] [PubMed] [Google Scholar]
- 38. Tierling S, Souren NY, Gries J, et al. Assisted reproductive technologies do not enhance the variability of DNA methylation imprints in human. J Med Genet. 2010;47:371–376. doi: 10.1136/jmg.2009.073189. This article presents data suggesting ART does not cause an increased risk in imprinting defects.
- 39. Katari S, Turan N, Bibikova M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18:3769–3778. doi: 10.1093/hmg/ddp319. This study analyzed a subset of imprinted and nonimprinted genes in children with a normal phenotype conceived both in vitro and in vivo. Results show altered expression in several genes implicated in metabolic disorders, such as obesity and diabetes. The study concludes that ART may have an effect on global patterns of DNA methylation and these changes may have long-term effects.
- 40.Manning M, Lissens W, Bonduelle M, et al. Study of DNA-methylation patterns at chromosome 15q11–q13 in children born after ICSI reveals no imprinting defects. Mol Hum Reprod. 2000;6:1049–1053. doi: 10.1093/molehr/6.11.1049. [DOI] [PubMed] [Google Scholar]
- 41.Neri QV, Takeuchi T, Palermo GD. An update of assisted reproductive technologies results in the United States. Ann NY Acad Sci. 2008;1127:41–48. doi: 10.1196/annals.1434.017. [DOI] [PubMed] [Google Scholar]
- 42.Pinborg A, Loft A, Aaris Henningson AK, et al. Infant outcome of 957 singletons born after frozen emryo replacement: The Danish National Cohort Study 1995–2006. Fertil Steril. 2009;94:1320–1327. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 43. Gomes MV, Huber J, Ferriani RA, et al. Abnormal methylation at the KvDMR1 imprinting control region in clinically normal children conceived by assisted reproductive technologies. Mol Hum Reprod. 2009;15:471–477. doi: 10.1093/molehr/gap038. This study investigated the effect of ART on KvDMR1 in children with a normal phenotype. Hypomethylation was observed in three of 18 clinically normal patients conceived with ART. This study suggests a possible association of abnormal imprinting and ART.