Abstract
The genetic deletion of catechol O-methyltransferase (COMT) in mice produces a preeclampsia-like phenotype, with mice exhibiting hypertension, proteinuria, and histological changes, consistent with human pathological features. 2-Methoxyoestradiol, a metabolite of COMT, increases human trophoblast invasiveness in vitro under hypoxic conditions, providing further support that decreased COMT expression may have a role in preeclampsia. However, evidence confirming decreased COMT expression in human disease has been limited to small studies of placentas obtained from cases of term preeclampsia. We examined COMT expression in placentas obtained from healthy term pregnancies (n = 14), preterm normotensive pregnancies (n = 8), and pregnancies complicated by severe preterm preeclampsia (delivery at <34 weeks' gestation; n = 22). Among our preeclamptic cohort were 10 pregnancies further complicated by HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets); and one pregnancy complicated by an eclamptic seizure. COMT expression was analyzed by RT-PCR, Western analysis, and IHC. COMT was mainly expressed in the syncytiotrophoblast. We did not find a significant difference in placental COMT expression in severe preeclampsia compared with either term or preterm normotensive cohorts. Our results suggest that severe preeclampsia may not be associated with a decrease in placental COMT expression.
Preeclampsia is a condition that affects 5% to 8% of all pregnant women and is a significant contributor to both maternal and perinatal morbidity and mortality.1,2 It arises from inadequate remodeling of the maternal spiral arterioles by the extravillous cytotrophoblast in early pregnancy.3,4 This results in chronic fetoplacental hypoxia,5 which leads to the release of antiangiogenic factors, such as soluble fms-like tyrosine kinase-1 (sFlt-1), into the maternal circulation.5–7 These antiangiogenic factors are postulated to cause endothelial dysfunction, producing the clinical characteristics of preeclampsia.5–10
Recently, mice with a genetic deletion of the enzyme catechol O-methyltransferase (COMT) were reported to develop a preeclampsia-like phenotype when pregnant.11 The mechanism causing the preeclamptic phenotype is believed to be mediated through a lack of 2-methoxyoestradiol (2-ME), a product of COMT.11 In preeclampsia, hypoxia leads to the stabilization of hypoxia-inducible factor (HIF)-1α, a transcription factor that initiates the transcription of hypoxia-inducible genes, including sFlt-1.12–14 2-ME inhibits the stabilization of HIF-1α, promoting its degradation and preventing its activation.15,16 Therefore, the COMT knockout leads to decreased 2-ME production, resulting in HIF-1α activation and sFlt-1 release.11 Interestingly, the preeclamptic phenotype in the COMT knockout mouse was reversed when exogenous 2-ME was administered,11 pointing to a possible role of 2-ME as a therapeutic agent.
Furthermore, under hypoxic conditions, 2-ME induces the invasion of cytotrophoblasts in vitro,17 suggesting a putative mechanism whereby reduced COMT expression and 2-ME production might lead to inadequate placental invasion during early pregnancy.
Although these studies provide an attractive hypothesis implicating reduced COMT expression in the pathogenesis of preeclampsia, evidence confirming that reduced COMT expression occurs in human disease has been limited. By using results obtained using Western analysis of six placentas obtained from term gestations, Kanasaki et al11 concluded that there was decreased COMT expression during preeclampsia. These findings were likely from mild preeclamptic cases. Lee et al17 reported decreased COMT expression based on immunohistochemical (IHC) analysis of three preeclamptic placentas. Therefore, placental COMT expression has only been assessed in a limited cohort. Furthermore, placentas obtained from pregnancies complicated by severe preterm preeclampsia have not been investigated. Such cases are responsible for the largest burden of perinatal and maternal morbidity and mortality.
Therefore, we decided to determine the expression of placental COMT obtained from a significant cohort with severe early-onset preeclampsia.
Materials and Methods
Tissue Collection
Women presenting to two tertiary women's hospitals in Melbourne, Australia, between February 2008 and December 2009, consented to placental tissue collection. Placental tissue was obtained from normal-term pregnancies (n = 14), preterm pregnancies not complicated by preeclampsia (n = 8), and pregnancies complicated by severe early-onset preeclampsia (n = 22). Severe preeclampsia was defined as the presence of hypertension >160/110 mm Hg on two occasions longer than 6 hours apart, proteinuria >5 g/day, oliguria >500 mL/day, visual disturbance, pulmonary edema, right upper quadrant pain, abnormal liver function, thrombocytopenia, or fetal growth restriction.18 Early-onset preeclampsia was defined as requiring delivery at <34 weeks' gestation based on maternal indications.
Placental tissue was obtained immediately after delivery by caesarean section. Samples of placental tissue were removed and washed briefly in sterile PBS. Samples were then frozen within 15 minutes of delivery and stored at −80°C.
RT-PCR
RNA was extracted from placental tissue using Trizol (Invitrogen, Carlsbad, CA) and isolated using a mirVana kit (Ambion, Austin, TX). RNA (0.2 μg) was then reverse transcribed to cDNA using SuperScript III (Invitrogen) and random hexamers (Invitrogen), as per the manufacturer's guidelines. Primers were designed for COMT using AmplifX 1.5.4 and tested in primer-BLAST (National Center for Biotechnology Information, Bethesda, MD) to ensure specificity (forward 5′-ACACACTGGACATGGTCTTCCT-3′; reverse 5′-ATCACGTTGTCAGCCAGTAGCA-3′). Glyceraldehyde-3-phosphate dehydrogenase was used as a housekeeping gene (forward 5′-GCAAATTCCATGGCACCGT-3′; reverse 5′-TCGCTCCTGGAAGATGGTGAT-3′), and sFlt-1 was used as a positive indicator of preeclampsia (forward 5′-ACAATCAGAGGTGAGCACTGCAA-3′; reverse 5′-TCCGAGCCTGAAAGTTAGCAA-3′). PCR was performed on the 7900 HT (Applied Biosystems, Carlsbad, CA) using power SYBR (Applied Biosystems), with the following run conditions: 50°C for 2 minutes, 95°C for 10 minutes; 95°C for 15 seconds, 58°C for 1 minute, and 72°C for 30 seconds (45 cycles); followed by 95°C for 15 seconds, 60°C for 15 seconds, and 95°C for 15 seconds. The PCR product was confirmed by gel electrophoresis. Relative quantification was determined using the comparative CT method.
Western Blot Analysis
Placental tissue, 10 mg, was homogenized in cold radioimmunoprecipitation assay buffer [150 mmol/L NaCl, 50 mmol/L Tris (pH 8.0), 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS], with protease inhibitor cocktail set 1 (Calbiochem, La Jolla, CA). The protein concentration was determined using a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL) with an albumin standard. Protein, 30 μg, was heated to 95°C in 4× Laemmli buffer before separation on 12% polyacrylamide gels and wet transfer to a nitrocellulose membrane (BioRad, Hercules, CA). Membranes were then blocked with Tris-buffered saline containing 0.1% Tween-20 (TBST) and 5% skim milk for 1 hour. Membranes were briefly washed in TBST before blotting overnight with antibodies targeting COMT (1:5000; Millipore, Billerica, MA) or actin (1:500; Thermo Scientific, Fremont, CA) in TBST at 4°C. Membranes were then washed three times with TBST before exposure to a secondary goat anti-rabbit horseradish peroxidase conjugate antibody (1:2500; Promega, Madison, WI) at room temperature for 1 hour. Membranes were then washed in TBST a further six times before the application of the enhanced chemiluminescence detection system (Pierce Biotechnology) and visualization of bands using a commercially available system (ChemiDoc XRS system; BioRad). Equal loading was confirmed with the use of the actin antibody. A positive control (293 T-cell lysate) was also run. Relative densitometry was determined using ImageJ software (NIH, Bethesda, MD).
IHC
Paraffin-embedded placental tissues (4-μm thick) were deparaffinized and rehydrated using standard protocols. Antigen retrieval was performed using microwave heating in citric acid buffer (0.01 mol/L, pH 6.0). Endogenous peroxidase activity was blocked by incubating the sections in 0.3% H2O2 in 50% methanol for 10 minutes. Sections were thoroughly washed in PBS (0.1 mol/L, pH 7.4); nonspecific binding was blocked by incubating the sections with a serum-free protein block (Dako, Carpinteria, CA), followed by overnight incubation at 4°C with rabbit anti-human COMT antibody (1:400; Chemicon International, Billerica, MA). Rabbit IgG was used as the negative control. The sections were thoroughly washed in PBS with 0.5% Tween-20 before the sections were incubated with a horseradish peroxidase conjugate polymer detection kit according to manufacturer's instructions (SuperPicture; Invitrogen). The sections were then incubated with diaminobenzidine for 5 minutes to visualize the immunoreactive structures. Subsequently, all sections were counterstained with hematoxylin before being dehydrated and cleared for coverslipping. The investigator (B.S.) who performed the staining was blinded to the identity of the samples.
Statistical Analysis
Continuous variables were assessed using either an unpaired t-test for assessing parametric data or a U-test for assessing nonparametric data. IHC staining was scored on a scale from 0 to 3 at 0.5 intervals, with 0.0 representing no staining and 3.0 representing heavy staining. Six blinded assessors (K.P., C.W., S.T.) scored the slides, with scores then averaged and taken as the final score for that sample. The scores for each sample/slide were then compared using a U-test. All statistical analysis was performed using software (GraphPad Prism; GraphPad Software, La Jolla, CA).
Results
Study Participants
We recruited 22 women with pregnancies complicated by severe early-onset preeclampsia, 8 women with preterm pregnancies not affected by preeclampsia, and 14 women with uncomplicated term pregnancies.
The baseline characteristics for these populations are outlined in Table 1. There was no difference between the two control groups and our preeclamptic cohort regarding maternal age, ethnicity, and body mass index. Gestational age at delivery was no different between preterm controls and the preeclamptic cohort. There were significantly more primigravids in our preeclamptic population compared with preterm controls (P < 0.05) and term controls (P < 0.001). There was a marked elevation in systolic (177 mm Hg) and diastolic (107 mm Hg) blood pressure in our preeclamptic population around the time of delivery, significantly higher than the two control groups that were normotensive (Table 1). Our preeclamptic cohort also included 10 women (45.5%) whose conditions were further complicated by HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets); and one woman (4.5%) who experienced an eclamptic fit.
Table 1.
Baseline Characteristics
| Characteristics | Preterm controls (n = 8) | Term controls (n = 14) | Preeclamptic patients (n = 22) |
|---|---|---|---|
| Maternal age (years) | 30.5 ± 2.3 | 32.9 ± 1.1 | 31.4 ± 1.4 |
| Gestational age at delivery (weeks) | 30.0 ± 1.5 | 38.5 ± 0.2⁎ | 31.0 ± 0.8 |
| Ethnicity | |||
| White | 7 (88%) | 10 (71%) | 16 (73%) |
| Asian | 1 (12%) | 3 (21%) | 5 (23%) |
| African | 0 | 1 (7%) | 0 |
| Hispanic | 0 | 0 | 1 (5%) |
| Body mass index (kg/m2) | 25.0 ± 1.8 | 32.8 ± 3.0 | 28.1 ± 1.8 |
| Primiparous | 2 (25%)† | 2 (14%)⁎ | 15 (68%) |
| Blood pressure at delivery (mm/Hg) | |||
| Systolic | 111.9 ± 4.5⁎ | 118.2 ± 3.7⁎ | 177.3 ± 5.8 |
| Diastolic | 68.1 ± 3.3⁎ | 78.4 ± 3.0⁎ | 107.5 ± 2.9 |
| Birth weight (g) | 1552 ± 258.3 | 3400 ± 112.1⁎ | 1549 ± 155.4 |
Data are given as mean ± SEM unless percentage is shown. Comparisons were performed between each control group and the preeclamptic cohort.
P < 0.001.
P < 0.05.
IHC Indicates Preserved COMT Expression in Preeclampsia
IHC analysis showed that COMT was mainly expressed in the syncytiotrophoblast layer of the placenta, which forms the maternal-fetal interface (Figure 1). No significant difference in COMT staining was seen between either preterm (n = 6) or term (n = 7) controls and those with a preeclamptic placenta (n = 7; P ≥ 0.5828 for both comparisons; Figure 1). Supplemental Figure S1 (available at http://ajp.amjpathol.org) provides immunostaining images from all samples.
Figure 1.
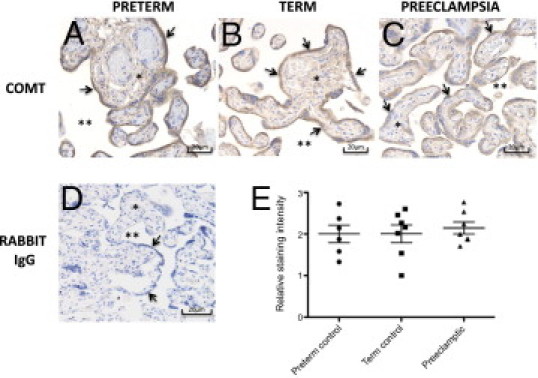
IHC of COMT expression in preterm control (A), term control (B), and preeclamptic (C) placentas. D: Negative treatment control. E: Staining intensity assessed by six blinded assessors on six preterm controls, seven term controls, and seven preeclamptic samples. The averaged score for each slide (see Supplemental Figure S1 at http://ajp.amjpathol.org for all slides) is shown, with horizontal lines representing the mean ± SEM. A Mann-Whitney U-test was used to compare either preterm or term controls with preeclamptic samples (P ≤ 0.5974). Arrows indicate the syncytiotrophoblast; asterisk, villous stroma; and double asterisks, intervillous space.
COMT Protein Expression Is Unchanged in the Presence of Preeclampsia
Total COMT protein expression using Western blot was similar between both term (n = 8) and preterm (n = 8) controls compared with preeclamptic (n = 8) placentas (P = 0.8850 and P = 0.0925, respectively; Figure 2). Supplemental Figure S2 (available at http://ajp.amjpathol.org) provides Western blots of all samples.
Figure 2.
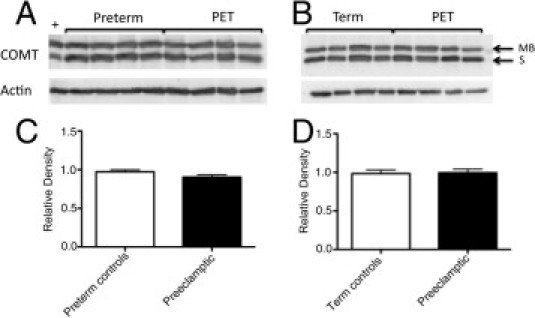
Western analysis of placental COMT expression. Representative Western blots comparing preterm controls with preeclamptic placenta (PET) (A) and term controls with preeclamptic placenta (B), with actin used as a loading control (see Supplemental Figure S2 at http://ajp.amjpathol.org for the full Western blot for all samples analyzed). MB indicates membrane-bound COMT at 30 kDa; and S, soluble COMT at 25 kDa. Densitometry of Western blots using preterm controls (C) and term controls (D) was performed, with relative density of total COMT expressed as mean ± SEM (P = 0.0925 and P = 0.8850, respectively).
mRNA Expression of sFtl-1, but Not COMT, Is Increased in Placentas Complicated by Severe Early-Onset Preeclampsia
To confirm the phenotype of our preeclamptic placentas, we assessed the expression of sFlt-1, an antiangiogenic factor well established to be highly expressed in the preeclamptic placenta.5,19 We found increased sFlt-1 mRNA expression in the placentas from preeclamptic pregnancies compared with controls (P < 0.05, Figure 3A). In contrast, there was no difference in COMT mRNA expression in these two groups (P = 0.65, Figure 3B).
Figure 3.
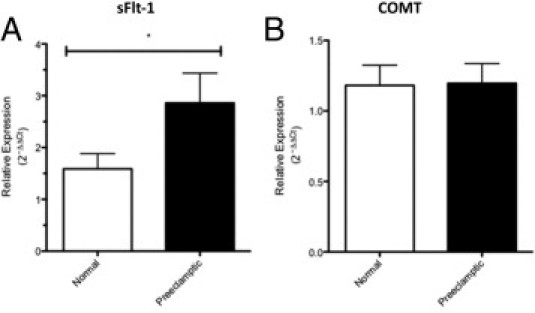
sFlt1 (A) and COMT (B) mRNA expression levels in the placentas of control (n = 14) and preeclamptic (n = 22) women. The relative expression was normalized against glyceraldehyde-3-phosphate dehydrogenase. Results are expressed as mean ± SEM and analyzed using an unpaired t-test. *P < 0.05.
Discussion
By using placental samples obtained from a significant cohort of women, we were unable to confirm an association between reduced COMT expression and severe early-onset preeclampsia.
Kanasaki et al11 first proposed a possible role for reduced placental COMT expression in the pathophysiology characteristics of preeclampsia, showing that the COMT knockout mouse develops a preeclamptic phenotype when pregnant. This provided a novel mechanism through which preeclampsia could be initiated. Lee et al17 demonstrated that 2-ME, a metabolite of COMT, enhanced invasion of HTR-8 cells (a human trophoblast cell line) under hypoxic conditions in vitro. Placental invasion during the first trimester occurs under hypoxic conditions.20 Thus, these studies provided evidence supporting a possible role for reduced COMT expression and decreased 2-ME in the development of preeclampsia.
However, evidence confirming that reduced COMT expression is relevant to human disease has been limited. Kanasaki et al11 assessed COMT expression in six mild term preeclamptic placentas, and Lee et al17 reported results based on three preeclamptic placentas. In contrast, our study explored placental COMT expression levels from 22 women with severe preeclampsia and compared them with those of 14 term controls and 8 preterm controls. The inclusion of preterm controls enabled comparisons of placentas at the same gestational ages. Although the preterm placentas will not be from entirely normal pregnancies, they are valid controls to test the hypothesis that reduced COMT expression occurs specifically during preeclampsia.
Our cohort represents placentas complicated by severe clinical disease. Of these placentas, 50% were further complicated by either HELLP syndrome or eclampsia; and all required delivery before 34 weeks' gestation on maternal grounds. Placental COMT expression was investigated at the mRNA and protein levels (using both Western blot and IHC). Finally, we confirmed the preeclamptic nature of the placental samples by confirming elevated expression of sFlt-1.5,19
There is significant evidence suggesting that HIF-1α plays an important role in the pathogenesis of preeclampsia. Observational studies12–14,21 showing HIF-1α is up-regulated in preeclamptic placentas have recently been complemented by mechanistic data,22 showing that adenoviral delivery of HIF-1α to pregnant mice at gestational day 8 (without 2-ME coadministration) results in a preeclamptic phenotype, including hypertension, renal and liver abnormalities, and elevations in serum sFlt-1 and endoglin. Thus, a possible explanation reconciling our observations with those of Kanasaki et al11 is that the COMT knockout phenocopies clinical preeclampsia by stabilizing HIF-1α (via decreased 2-ME); however, in human disease, HIF-1α stabilization occurs independently of decreased COMT expression.
The observation of Lee et al17 that 2-ME facilitates invasion of HTR-8 cells in vitro under hypoxic conditions may also be explained by the fact that 2-ME facilitates HIF-1α degradation (HIF-1α possibly inhibits placental invasion17). Again, this may not necessarily be a feature of human disease. There has been no demonstration of differential COMT expression or 2-ME levels around the time of placental invasion in early pregnancy among those destined to develop preeclampsia.
In conclusion, we were not able to confirm that COMT expression is reduced in placentas destined to develop early-onset severe preeclampsia.
Acknowledgment
We acknowledge the study participants.
Footnotes
Supported by a Postgraduate Medical Research Scholarship (grant 607219 to K.P.), an R.D. Wright Fellowship (grant 454777 to M.L.), and a Career Development Award (grant 490970 to S.T.) from the National Health and Medical Research Council.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.02.029.
Supplementary data
Immunohistochemistry of COMT expression in preterm, term, and preeclamptic placentas (PETs), with sections shown for all samples analyzed.
Western blots of COMT expression in preterm (n = 8), term (n = 8), and preeclamptic (n = 8) placentas, with actin as a loading control. Blots for all samples were analyzed. P indicates severe preeclampsia; and H, a syndrome that included hemolysis, elevated liver enzymes, and low platelets.
References
- 1.Sibai B.M., Caritis S., Hauth J. What we have learned about preeclampsia. Semin Perinatol. 2003;27:239–246. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 2.Walker J.J. Pre-eclampsia. Lancet. 2000;356:1260–1265. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 3.Brosens I. Morphological changes in the uteroplacental bed in pregnancy hypertension. Clin Obstet Gynecol. 1977;4:573–593. [PubMed] [Google Scholar]
- 4.Roberts J.C., Cooper D.W. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 5.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., Epstein F.H., Sukhatme V.P., Karumanchi S.A. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesha S., Toporsian M., Lam C., Hanai J., Mammoto T., Kim Y.M., Yuan H.T., Libermann T.A., Stillman I.E., Roberts D., D'Amore P.A., Epstein F.H., Sellke F.W., Romero R., Sukhatme V.P., Letarte M., Karumanchi S.A. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 7.Silasi M., Cohen B., Karumanchi S.A., Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37:239–253. doi: 10.1016/j.ogc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Mutter W.P., Karumanchi S.A. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine R.J., Lam C., Qian C., Yu K.F., Maynard S.E., Sachs B.P., Sibai B.M., Epstein F.H., Romero R., Thadhani R., Karumanchi S.A. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg G., Khankin E.V., Karumanchi S.A. Angiogenic factors and preeclampsia. Thromb Res. 2009;123(Suppl 2):S93–S99. doi: 10.1016/S0049-3848(09)70020-9. [DOI] [PubMed] [Google Scholar]
- 11.Kanasaki K.P.K., Sugimoto H., Ahmad S., Hamano Y., Xie L., Parry S., Augustin H.G., Gattone V.H., Jr, Folkman J., Strauss J.F., Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Gu B., Zhang Y., Lewis D.F., Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;26:210–217. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Nevo O., Soleymanlou N., Wu Y., Xu J., Kingdom J., Many A., Zamudio S., Carniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–R1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas C.P., Andrews J.I., Raikwar N.S., Kelley E.A., Herse F., Dechend R., Golos T.G., Liu K.Z. A recently evolved novel trophoblast-enriched secreted form of fms-like tyrosine kinase-1 variant is up-regulated in hypoxia and preeclampsia. J Clin Endocrinol Metab. 2009;94:2524–2530. doi: 10.1210/jc.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S.H., Cho H.T., Devi S., Zhang Z., Escuin D., Liang Z., Mao H., Brat D.J., Olson J.J., Simons J.W., Lavallee T.M., Giannakakou P., Van Meir E.G., Shim H. Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res. 2006;66:11991–11997. doi: 10.1158/0008-5472.CAN-06-1320. [DOI] [PubMed] [Google Scholar]
- 16.Mabjeesh N.J., Escuin D., LaVallee T.M., Pribluda V.S., Swartz G.M., Johnson M.S., Willard M.T., Zhong H., Simons J.W., Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 17.Lee S.B., Wong A.P., Kanasaki K., Xu Y., Shenoy V.K., McElrath T.F., Whitesides G.M., Kalluri R. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176:710–720. doi: 10.2353/ajpath.2010.090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ACOG Committee on Practice Bulletins–Obstetrics ACOG Practice bulletin No. 33: diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 19.Levine R.J., Qian C., Maynard S.E., Yu K.F., Epstein F.H., Karumanchi S.A. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006;194:1034–1041. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 20.Jauniaux E., Poston L., Burton G.J. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajakumar A., Brandon H.M., Daftary A., Ness R., Conrad K.P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–769. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Tal R., Shaish A., Barshack I., Polak-Charcon S., Afek A., Volkov A., Feldman B., Avivi C., Harats D. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am J Pathol. 2010;177:2950–2962. doi: 10.2353/ajpath.2010.090800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry of COMT expression in preterm, term, and preeclamptic placentas (PETs), with sections shown for all samples analyzed.
Western blots of COMT expression in preterm (n = 8), term (n = 8), and preeclamptic (n = 8) placentas, with actin as a loading control. Blots for all samples were analyzed. P indicates severe preeclampsia; and H, a syndrome that included hemolysis, elevated liver enzymes, and low platelets.


