Adolescents with oxidoreductase deficiency present with impaired pubertal development, manifesting in girls with hypergonadotropic hypogonadism and ovarian cysts while boys may show delayed but spontaneous pubertal progression.
Abstract
Context:
P450 oxidoreductase (POR) is a crucial electron donor to all microsomal P450 cytochrome (CYP) enzymes including 17α-hydroxylase (CYP17A1), 21-hydroxylase (CYP21A2) and P450 aromatase. Mutant POR causes congenital adrenal hyperplasia with combined glucocorticoid and sex steroid deficiency. P450 oxidoreductase deficiency (ORD) commonly presents neonatally, with disordered sex development in both sexes, skeletal malformations, and glucocorticoid deficiency.
Objective:
The aim of the study was to describe the clinical and biochemical characteristics of ORD during puberty.
Design:
Clinical, biochemical, and genetic assessment of seven ORD patients (five females, two males) presenting during puberty was conducted.
Results:
Predominant findings in females were incomplete pubertal development (four of five) and large ovarian cysts (five of five) prone to spontaneous rupture, in some only resolving after combined treatment with estrogen/progestin, GnRH superagonists, and glucocorticoids. Pubertal development in the two boys was more mildly affected, with some spontaneous progression. Urinary steroid profiling revealed combined CYP17A1 and CYP21A2 deficiencies indicative of ORD in all patients; all but one failed to mount an appropriate cortisol response to ACTH stimulation indicative of adrenal insufficiency. Diagnosis of ORD was confirmed by direct sequencing, demonstrating disease-causing POR mutations.
Conclusion:
Delayed and disordered puberty can be the first sign leading to a diagnosis of ORD. Appropriate testosterone production during puberty in affected boys but manifest primary hypogonadism in girls with ORD may indicate that testicular steroidogenesis is less dependent on POR than adrenal and ovarian steroidogenesis. Ovarian cysts in pubertal girls may be driven not only by high gonadotropins but possibly also by impaired CYP51A1-mediated production of meiosis-activating sterols due to mutant POR.
P450 oxidoreductase (POR) deficiency (ORD) is a variant of congenital adrenal hyperplasia caused by mutations in the POR gene (1–3). POR encodes the electron donor enzyme POR that transfers electrons from reduced nicotinamide adenine dinucleotide phosphate to all microsomal cytochrome P450 (CYP) enzymes; mutations result in impaired steroid and sterol synthesis and metabolism. Clinically, ORD manifests with disordered sex development (DSD), adrenal insufficiency, and skeletal malformations (1–5).
The skeletal malformations in ORD patients resemble the malformation phenotype of Antley-Bixler syndrome (OMIM 207410) (6). The malformation phenotype in ORD is thought to reflect impaired activities of POR-dependent enzymes involved in cholesterol synthesis and retinoic acid metabolism (7–10), including lanosterol 14α-demethylase (CYP51A1), squalene epoxidase, and CYP26 subfamily members. In ORD patients, combined deficiencies of the steroidogenic enzymes CYP17A1 and CYP21A2 represent the most striking biochemical findings. Steroid 21-hydroxylase (CYP21A2) catalyzes crucial steps of adrenal gluco- and mineralocorticoid production. CYP17A1 is expressed in adrenals and gonads and catalyzes 17α-hydroxylase and 17,20-lyase activities, required for glucocorticoid and sex steroid synthesis, respectively. Notably, ORD patients of both sexes may present at birth with DSD. Male undervirilization, i.e. 46,XY DSD, can be readily explained by inhibition of CYP17A1, which results in decreased androgen production. Masculinization of affected females, i.e. 46,XX DSD, is a more puzzling finding because circulating androgen levels are usually low. This apparent contradiction could possibly be explained by the presence of an alternative pathway toward active androgen synthesis, which is active only during human fetal development (2). Elements of this proposed alternative pathway have been previously found in the fetal gonad of the tammar wallaby (11). P450 aromatase (CYP19A1), the microsomal CYP enzyme responsible for the conversion of androgens to estrogens, also depends on electron transfer from POR. In the fetus, loss of aromatase activity due to inactivating CYP19A1 mutations is associated with maternal virilization due to placental aromatase deficiency, virilization of the female fetus (46,XX DSD), and disordered pubertal development in females (12). The broad variability in phenotypic manifestation of ORD may well be explained by differential inhibition of the various POR-dependent CYP enzymes by distinct POR mutants (13, 14).
Neonatal presentation in ORD has been well described (2, 4, 5, 15, 16). However, there is a paucity of detailed data on the pubertal phenotype that is likely to be affected by impaired sex steroid synthesis. Here we present seven patients (five females, two males) with ORD and describe their clinical and biochemical characteristics during puberty.
Patients and Methods
Patients
The cohort consisted of five female and two male patients from six different countries. In all patients, appropriate written consent and assent, respectively, had been obtained in accordance with local Institutional Review Board guidelines. Patient characteristics including genetic and biochemical findings are summarized in Table 1 for the affected girls and in Table 2 for the two male patients.
Table 1.
Summary of clinical, genetic, and hormonal findings in five female pubertal ORD patients
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Ethnicity | White Caucasian | White Caucasian | White Caucasian | White Caucasian | White Caucasian |
| Karyotype | 46,XX | 46,XX | 46,XX | 46,XX | 46,XX |
| POR mutations (p.) | A287P/R457H | A287P/A287P | A287P/A287P | T142A/Y376 LfsX74 | A287P/R223X |
| 46,XX DSD | Yes | Yes | Yes | No | Yes |
| Skeletal malformations | Mild | Mild | Mild | Overt at birth | Overt at birth |
| Age at investigation (yr) | 16 | 12 | 23 | 19 | 16 |
| Tanner stages | PH4, B4, M0 | PH2, B1, M0 | PH3, B3, M0 | PH3, B5, M1 | PH3, B3, M0 |
| Height (cm) | 168 (0.9 SDS) | 148 (−1.4 SDS) | 166 (0.4 SDS) | 182.5 (2.0 SDS) | 158.7 (−0.7 SDS) |
| Target height (cm) | 168 (−0.9 SDS) | 61 (0.9 SDS) | 1545 (−1.5 SDS) | 175 (+0.8 SDS) | 169.5 (+0.5 SDS) |
| Weight (kg) | 51.3 (−0.7 SDS) | 32.0 (−2.2 SDS) | 61.4 (−0.3 SDS) | 88.0 (3.1 SDS) | 40.2 (−2.4 SDS) |
| BMI (kg/m2) | 18.2 (−0.9 SDS) | 14.61 (−2.1 SDS) | 22.3 (0.5 SDS) | 26.3 (1.5 SDS) | 16.1 (−2.3 SDS) |
| ACTH (pmol/liter) | 3.1 (2.2–11) | 34.8 (2.2–11) | 22 (2.2–11) | 19 (2.2–11) | 5.3 (2.2–11) |
| Cortisol (nmol/liter) | |||||
| Baseline | 289 (150–780) | 607 (150–780) | 149 (150–780) | 375 (150–780) | 190 (120–780) |
| 60 min after 250 μg ACTH1-24 iv | 436 (>550) | 717 (>550) | 158 (>550) | 425 (>550) | 314 (>550) |
| Adrenal insufficiency | Yes | No | Yes | Yes | Yes |
| Progesterone (nmol/liter) | |||||
| Baseline | 8.8 (0.9–2.3) | 256 (0.3–14.1) | 45.8 (<3) | 58 (0.9–2.3) | 28.8 (0.1–5.1) |
| 60 min after 250 μg ACTH1-24 iv | 53.4 | 307 | 181.5 | 83 | >191 |
| 17OHP (nmol/liter) | |||||
| Baseline | 25.7 (0.45–2.9) | 72.7 (0.45–2.9) | 67.5 (<5) | 25 (0.45–2.9) | 15 (0.1–8.1) |
| 60 min after 250 μg ACTH1-24 iv | 53.4 | 86.8 | 82.3 | 36 | n.m. |
| Androstenedione (nmol/liter) | 2.6 (1.8–12.9) | 3.45 (2.8–6.6) | n.m. | 0.6 (3.0–9.6) | 1.7 (0.1–5.5) |
| DHEAS (μmol/liter) | 5.7 (1.1–6.2) | 2.41 (0.8–9.3) | 2 (2.5–5.25) | 1,2 (3.0–13.0) | 4.2 (0.4–7.9) |
| Testosterone (nmol/liter) | n.m. | n.m. | 0.48 (<2.4) | 1.1 (1.0–3.5) | 0.48 (0.1–1.7) |
| E2 (pmol/liter) | 53.5 (78–312) | Undetectable | n.m. | 90 (70–530) | 84 (66–246) |
| LH (U/liter) | 35.0 (1.1–3.7) | 19.0 (0.3–1.2) | 53.0 (0.7–4.7) | 6.1 (1.1–3.7) | 19.2 (0.7–4.7) |
| FSH (U/liter) | 12.6 (3.1–8.1) | 17.0 (1.6–7.3) | 29.0 (3.9–7.0) | 10.0 (3.1–8.1) | 13.1 (3.9–7.0) |
n.m., Not measured; SDS, sd score.
Table 2.
Summary of clinical, genetic, and hormonal findings in two male pubertal ORD patients
| Case 6 | Case 7 | |||
|---|---|---|---|---|
| Ethnicity | Hispanic/Asian | White Caucasian | ||
| Karyotype | 46,XY | 46,XY | ||
| POR mutations (p.) | R457H/Y576X | A287P/IVS7 + 2dupT | ||
| 46,XY DSD | No, but both testicles undescended (inguinal canal) | No, but right testicle undescended (inguinal canal) | ||
| Skeletal malformations | Overt at birth | Overt at birth | ||
| Age at investigation (yr) | 12 | 16 | 12 | 13.5 |
| Tanner stages | PH1, G1 | PH5, G5 | PH1, G1 | PH3, G2 |
| Testicular volume (right/left) (ml) | 1/2 | 20/25 | 4/12 | 10/25 |
| Height (cm) | 143 (−0.6 SDS) | 166.1 (−0.9 SDS) | 138.2 (−1.6 SDS) | 150.9 (−0.8 SDS) |
| Target height (cm) | 178.5 (+1.3 SDS) | 174 (−0.1 SDS) | ||
| Weight (kg) | 28.7 (−1.8 SDS) | 39.9 (−2.95 SDS) | 42.5 (0.2 SDS) | 56.6 (0.9 SDS) |
| BMI (kg/m2) | 14.1 (−2.3 SDS) | 15.0 (−3.3 SDS) | 22.3 (1.3 SDS) | 25.1 (1.60 SDS) |
| ACTH (pmol/liter) | 39.6 (2.2–11) | 16 (2.2–11) | 12.4 (2.2–11) | 99.1 (2.2–11) |
| Cortisol (nmol/liter) | ||||
| Baseline | 198 (150–780) | 217 (150–780) | 353 (150–780) | n.m. |
| 60 min after 250 μg ACTH1-24 iv | 229 (>550) | n.m. | 411 (>550) | n.m. |
| Adrenal insufficiency | Yes | Yes | ||
| 17OHP (nmol/liter) | ||||
| Baseline | 112 (<6) | 91.5 | 14 (0.45–2.9) | n.m. |
| 60 min after 250 μg ACTH1-24 iv | 124 | n.m. | 54 | n.m. |
| Androstenedione (nmol/liter) | 5.4 (1.8–12.9) | 6.0 (2.7–10) | 1.1 (1.8–12.9) | n.m. |
| DHEAS (μmol/liter) | 0.9 (1.1–6.2) | 1.9 (1.8–10) | 0.8 (0.5–4.1) | 1.3 (1.1–6.2) |
| Testosterone (nmol/liter) | 0.8 (0.2–1.5) | 26.6 (9–27) | 0.7 (0.07–0.8) | 10.7 (9–27) |
| LH (U/liter) | 0.4 (<1.7) | 37 (0.2–6.1) | 0.6 (<1.7) | 2.4 (0.4–4.6) |
| FSH (U/liter) | 1.7 (<2.5) | 12.5 (0.5–6.3) | 1.9 (<2.5) | 4.8 (2.7–4.4) |
n.m., Not measured; SDS, sd score.
Case 1
The neonatal presentation of this case, virilized genitalia (46,XX DSD) and low circulating androgens, has been described previously (2). At the age of 14 yr, the patient presented with acute abdominal pain. Investigations revealed rupture of a large (4.5 cm) left ovarian cyst requiring surgical intervention, with removal of a largely degenerative polycystic ovary. Physical examination also revealed mild dysmorphic features (depressed nasal bridge, arachnodactyly, camptodactyly, restricted elbow extension bilaterally, syndactyly of toes 2/3). Tanner stages were PH4, B4, and M0. However, serum estradiol (E2) was prepubertal and gonadotropins were high (Table 1). 17-Hydroxyprogesterone (17OHP) and progesterone were elevated, cortisol showed a subnormal response to ACTH1-24 (Table 1), and hydrocortisone cover during stress was recommended. After 6 months of treatment with synthetic progestin, E2 levels slightly increased, but gonadotropins were still high (E2, 106 pmol/liter; LH, 15.3 U/liter; FSH, 8 U/liter). Treatment was changed to an E2/progestin combination. Follow-up 1 yr later showed low E2 levels and elevated gonadotropins (E2, 63 pmol/liter; LH, 40 U/liter; FSH, 16 U/liter), possibly reflecting compliance problems. At the age of 15.8 yr, spontaneous rupture of a right ovarian cyst occurred that required emergency surgical intervention resulting in the removal of the residual right ovary. The patient now receives continuous estrogen/progestin replacement therapy.
Case 2
The patient (46,XX) was born after 42 wk gestation as the third child of nonconsanguineous parents of Polish origin. Her genitalia was virilized at birth (clitoromegaly and urogenital sinus). At the age of 11.5 yr, bilateral ovarian masses were diagnosed by ultrasound and computed tomography and subsequently surgically removed. Histopathology confirmed ovarian follicular cysts. Endocrine assessment revealed undetectable E2 and elevated LH and FSH (Table 1). After 10 months, ovarian cysts on both sides recurred, and the patient was referred for further endocrine investigations. Tanner stages were B1, PH2, and M0. Several dysmorphic features were noted, including midface hypoplasia, brachycephaly, dysplastic ears, arachno- and camptodactyly, and bilaterally restricted elbow extension. Hormonal assessment showed elevated 17OHP, progesterone, and ACTH concentrations; however, cortisol was high-normal at baseline and after iv cosyntropin (Table 1). Medical treatment of the ovarian cysts with a combination of E2 (1 mg/d), GnRH superagonist (Diphereline, 3.75 mg/month), and dexamethasone (0.25 mg/d; reduction to 0.125 mg/48 h after 2 months) was initiated. On this regimen, the ovarian cysts partially regressed in size after 12 months of treatment. At 13.5 yr, the patient's pubertal staging was B4, PH3; she was then started on a combination of triamcinolone 2 mg/d, 17β-E2, and synthetic progestin, resulting in regular withdrawal bleeds. The ovarian cysts further decreased in size, and until the present age of 18 yr there have been no recurrences.
Case 3
The patient was born with ambiguous genitalia after an uneventful pregnancy to consanguineous parents of Italian origin. The baby was raised as a boy until the age of 5 when her gender assignment was reconsidered after the birth of a sister with obvious 46,XX DSD. A diagnostic laparotomy showed the presence of normal internal female genital organs. Multiple genital corrective surgeries including vaginoplasty and partial clitoral reduction were performed between 5 and 12 yr of age. Normal psychomotor development, bone age, and growth velocity (50th to 75th percentile) were documented. Her younger sister later died at age 8 yr after developing signs and symptoms of shock shortly after undergoing an appendectomy.
Pubertal development was induced at the age of 17 yr by treatment with equine estrogens and progesterone. At the age of 23 yr, she was assessed as having normal female appearance but with only moderate breast development (Table 1). Several mild dysmorphic features were noted (narrow hard palate, low-set ears, clinodactyly, camptodactyly, and brachydactyly of the fourth toe bilaterally). Genetic analysis confirmed a 46,XX karyotype. Hormonal evaluation showed elevated serum progesterone and 17OHP levels, whereas serum androgens were low; serum cortisol was low at baseline and did not increase appropriately after iv cosyntropin (Table 1). After 3 months of estrogen withdrawal, the patient's gonadotropins were increased (LH, 53 U/liter; FSH, 29 U/liter). Concurrently, large ovarian cysts up to 7 cm in diameter developed, which regressed partially after reintroduction of estrogen/progestin treatment, but only resolved after the addition of glucocorticoid treatment (dexamethasone, 0.375 mg/d).
Case 4
The patient was the first child born to nonconsanguineous parents of Dutch origin. There was no genital ambiguity, but she underwent early postnatal assessment for dysmorphic features, including frontal bossing, midface hypoplasia, pear-shaped nose, low-set ears with flat helices, impaired supination of the forearms, arachnodactyly, and synostosis of several distal and interphalangeal joints. However, no precise diagnosis was established, and she was subsequently lost to follow-up.
At the age of 19 yr, she presented with irregular menses. Pubertal development was partially delayed (Table 1). Hormonal assessment revealed low E2 with normal gonadotropins; 17OHP and progesterone were elevated; baseline cortisol was normal but did not increase sufficiently after iv cosyntropin, and adrenal androgens were low (Table 1). A magnetic resonance imaging scan of the pelvic region revealed cystic enlargement of the right ovary (3 × 4 cm) with normal appearance of the left ovary and normal female anatomy of the reproductive system. Combined treatment with E2 (2 mg daily) and dydrogesterone (10 mg/d) was initiated. At present, the patient has been on hormone replacement therapy for 12 months, and repeat imaging revealed complete regression of the ovarian cyst.
Case 5
The girl was born at 36 wk gestation after an uneventful pregnancy to nonconsanguineous parents of German origin. Postnatally, the girl needed ventilation due to severe respiratory distress associated with bilateral choanal stenosis. At birth there were obvious dysmorphic features including brachycephaly, midface hypoplasia, exophthalmus, retrognathia, depressed nasal bridge, low-set ears, and bilateral radiohumeral synostosis. The external genitalia were ambiguous with clitoral hypertrophy and an urogenital sinus (Prader stage III). Clitoral reduction and vulvo- and vaginoplasty were performed at 2 yr of age. During childhood, the girl had multiple reconstructive surgeries for correction of the craniofacial abnormalities.
At 16 yr of age, she presented to the endocrine clinic because of primary amenorrhea. Tanner staging revealed PH3, B3, and M0. Bone age was delayed by 2 yr. Pelvic ultrasound revealed an infantile uterus and bilateral polycystic ovaries with the largest cyst measuring 1 cm in diameter and with increased total ovarian volumes of 8.6 and 10 ml, respectively. Hormonal analysis showed a low E2 with up-regulated gonadotropins; baseline cortisol was low and did not increase sufficiently after iv cosyntropin, and adrenal androgens were normal (Table 1). She was started on E2 valerate for hormone replacement.
Case 6
This boy was born at 39 wk gestation as the first child of a Chinese mother and a Costa Rican father. Both testes were undescended but palpable in the inguinal canal; the scrotum was hypoplastic. Bilateral orchidopexies were performed at the age of 12 months. In addition, at birth, choanal stenosis, facial dysmorphism (brachycephaly, frontal bossing, mid-face hypoplasia, preauricular skin tags), and multiple skeletal abnormalities, including arachnodactyly, shoulder and knee contractures, radioulnar synostosis, bilateral coronal synostosis, and partial fusions of thoracic vertebrae T3/T4, were noted. No conclusive diagnosis was made, and the patient was lost to follow-up.
The patient was referred for endocrine assessment at the age of 10.5 yr because of poor genital development (Table 2). His genitalia were undermasculinized for age (phallus of 15 mm length with little erectile tissue). Serum gonadotropins and androgen levels were prepubertal (Table 2). Cortisol was normal at baseline but insufficiently stimulated after 250 μg ACTH1–24 revealing partial adrenal insufficiency (Table 2). Hydrocortisone replacement therapy (10 mg/m2 daily) and early pubertal induction with oral testosterone (40 mg/d) were commenced, but the family then dropped out of care and ceased medications.
At age 16 yr, the patient presented again after several years of no medication. However, pubertal development had spontaneously progressed, and the phallus was 8.5 cm with adequate erectile tissue, appropriate testicular volumes, and advanced pubertal staging (Table 2). Hormonal investigations revealed normal androgens but up-regulated gonadotropins (Table 2).
Case 7
This boy was born after an uneventful pregnancy to nonconsanguineous parents of British origin. At birth, several dysmorphic features were noted including choanal stenosis, brachycephaly, and joint contractures due to radioulnar synostosis. There was no genital ambiguity; however, his right testicle was undescended but palpable in the inguinal channel. Right-sided orchidopexy was undertaken at the age of 12 months, and the boy underwent a series of osteotomies for coronal craniosynostosis.
He presented at 12 yr of age to the endocrine clinic with lack of pubertal development and testicular volumes of 4 and 12 ml, respectively. Adrenal androgen concentrations were low; testosterone was high-normal in comparison to age-specific reference (Table 2). Baseline cortisol was normal, but response to iv cosyntropin was insufficient (Table 2). Hydrocortisone treatment was initiated (10 mg/m2 daily), and further pubertal development was monitored at 6-month intervals. Puberty progressed spontaneously, and when assessed at the age of 13.5 yr testicular volumes had increased to 10 and 25 ml, respectively (Table 2). Dehydroepiandrosterone sulfate (DHEAS), testosterone, and baseline gonadotropins were within age-specific reference ranges (Table 2).
Urinary steroid metabolite analysis
Analysis of urinary steroid metabolite excretion was performed as described previously by a quantitative gas chromatography/mass spectrometry (GC/MS) selected ion-monitoring method (2, 17). In brief, steroids were enzymatically released from conjugation and, after extraction, chemically derivatized before GC/MS selected ion-monitoring analysis. Steroids quantified included corticosterone metabolites [tetrahydrocorticosterone (THB), 5αTHB, tetrahydro-11-dehydrocorticosterone (THA), and 5αTHA)], the progesterone metabolite pregnanediol (PD), 17OHP metabolites [pregnanetriol (PT), and 17-hydroxypregnanolone (17HP)], the 17-hydroxypregnenolone metabolite pregnenetriol (5-PT), the 21-desoxycortisol metabolite pregnanetriolone (P'TONE), cortisol metabolites [tetrahydrocortisol (THF), 5αTHF, tetrahydrocortisone (THE)], and androgen metabolites [androsterone, etiocholanolone, dehydroepiandrosterone (DHEA), and 16-hydroxy-DHEA].
After quantification of steroid metabolites by GC/MS, we calculated substrate/product ratios to determine the approximate in vivo net activity of specific steroidogenic enzymes: 21-hydroxylase, 100 × P'TONE/(THF + 5αTHF + THE), (17HP + PT)/(THF + 5αTHF + THE); 17α-hydroxylase, (THA + 5αTHA + THB + 5αTHB)/(THF + 5αTHF + THE); 17,20-lyase, (17HP + PT)/(androsterone + etiocholanolone); ORD-specific ratio, PD/(THF + 5αTHF + THE). These diagnostic ratios and the overall secretion patterns were compared with urinary steroid profiles obtained from a normal reference cohort of boys and girls between 12 and 16 yr of age (n = 12).
Genetic analysis
The coding sequence of the POR gene including exon-intron boundaries was amplified in 13 PCR fragments as previously described (2). Direct sequencing was carried out employing an automated ABI3730 Sequencer (Applied Biosystems Inc., Foster City, CA). Sequences were analyzed using the DNAStar Lasergene software package (DNASTAR Inc., Madison, WI). Sequence variants were designated according to Human Genome Variation Society recommendations (www.hgvs.org/rec.html) using the reference sequences GenBank NC_000007 (g.DNA), GenBank NM_000941.2 (c.DNA), and GenBank NP_000932.3 (protein). For DNA numbering, the nucleotide designated +1 was the A of the ATG start codon.
Results
Clinical and serum biochemical characteristics
All seven patients presented with absent or incomplete pubertal development leading to the diagnosis of ORD. Three female patients had presented neonatally with 46,XX DSD, and five of the seven patients had overt skeletal malformations noted immediately after birth. However, in all cases this had not prompted the establishment of a conclusive diagnosis before puberty.
Generally, female patients presented with significant pubertal impairment and ovarian cysts. In case 1, spontaneous cyst rupture required two surgical emergency interventions, resulting in loss of both ovaries despite ongoing estrogen/progestin therapy. Ovarian cysts in cases 2 and 3 only resolved after the addition of glucocorticoids and GnRH agonists to ongoing estrogen/progestin therapy. Four of the five female patients presented with primary amenorrhea and biochemical evidence of primary hypogonadism (Table 1). By contrast, both boys showed spontaneous, albeit slightly delayed, pubertal progression, with testicular volumes appropriate for age, and biochemical signs of compensated hypogonadism, with normal testosterone but up-regulated gonadotropins (Table 2).
In all patients, adrenal androgens were either normal or low (Table 1). All cases had elevated 17OHP and progesterone concentrations, accompanied by increased ACTH levels in five patients. Baseline cortisol secretion was normal in five patients and borderline low in two. In all but one patient, cortisol secretion was not sufficiently stimulated by iv cosyntropin, indicative of partial adrenal insufficiency (Table 1).
Auxological investigations in all seven cases showed a normal height at investigation within the familial target height range (Table 1).
In vivo assessment of steroidogenesis by urinary steroid profiling
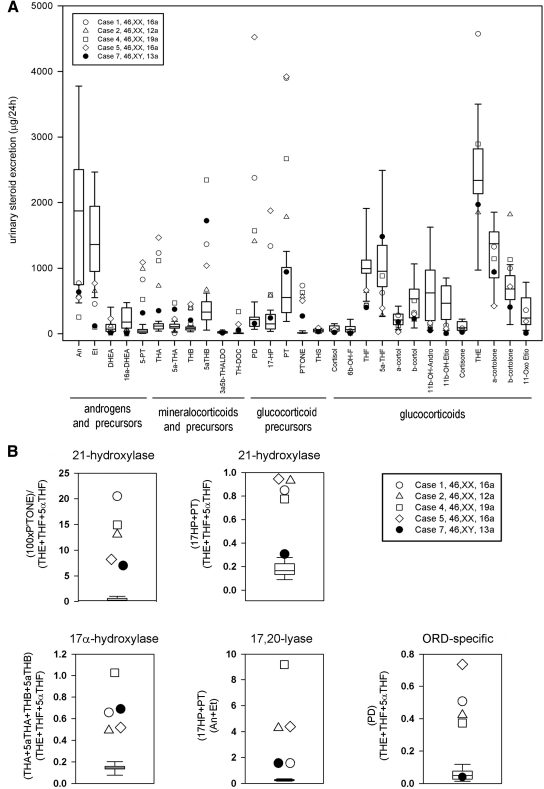
Steroid profiling by GC/MS revealed a typical pattern for ORD in all patients studied (Fig. 1). Twenty-four-hour excretion analysis revealed a characteristically increased excretion of metabolites of 17-hydroxypregnenolone (5-PT), progesterone (PD), and 17OHP (17HP, PT, P'TONE) and a mild increase in mineralocorticoid precursor metabolites (e.g. THB, tetrahydrodeoxycorticosterone) (Fig. 1A), which reflects the concurrent impairment of 17α-hydroxylase and 21-hydroxylase activities. In keeping with impaired 17,20-lyase activity, androgen and androgen precursor metabolite excretion was decreased or low normal (Fig. 1A). Cortisol metabolites were low or in the lower normal reference range, indicative of impaired 21-hydroxylase activity (Fig. 1A). Substrate/product ratios clearly demonstrated attenuation of 17α-hydroxylase, 17,20-lyase, and 21-hydroxylase activities in all cases examined (Fig. 1B).
Fig. 1.
In vivo steroidogenic enzyme activities in pubertal ORD patients as determined by total 24-h urinary steroid metabolite excretion (A) and diagnostic steroid substrate/product ratios (B) measured by GC/MS and shown in comparison to an age-matched reference cohort (n = 12). Box plots represent the interquartile ranges (25th to 75th percentiles), and whiskers represent the 5th and 95th percentiles, respectively, of the reference cohort; each pubertal ORD case is represented by specific symbols as indicated in the legend.
Genetic confirmation of diagnosis
Sequencing analysis of the POR gene revealed compound heterozygous missense mutations p.A287P/p.R457H in case 1. Cases 2 and 3 were homozygous for p.A287P, and case 4 showed compound heterozygous mutations comprising a missense mutation (p.T142A) and a novel frameshift mutation resulting in a premature stop codon (p.Y376LfsX74). Case 5 was compound heterozygous, with a p.A287P mutation on one allele, and a novel p.R223X nonsense mutation on the other allele.
Case 6 was compound heterozygous for p.R457H and p.Y567X, whereas case 7 was compound heterozygous for p.A287P and a novel duplication disrupting the splice donor site of intron 7, IVS7 + 2dupT. The duplication does not yield an alternative splice site according to splice site prediction tool http://www.cbs.dtu.dk/services/NetGene2/, thus resulting in translation of intron 7 until a premature stop codon 64 aa downstream of the last exon 7 codon (p.P277PfsX65).
Discussion
Here, we describe the clinical phenotype as well as biochemical and genetic findings in seven adolescents with ORD all presenting with disturbed pubertal development, manifesting with insufficient pubertal development and primary amenorrhea in four of the five girls and delayed, albeit spontaneously progressive, puberty in the two boys.
All five females presented with ovarian cysts. This is a common finding in females affected by steroidogenic disorders and has been reported in 21-hydroxylase deficiency (18, 19), 17α-hydroxylase deficiency (20–22), congenital lipoid adrenal hyperplasia due to mutant steroidogenic acute regulatory protein (23–25), P450 aromatase deficiency (26, 27), and also recently in ORD (5, 28). Of note, two infants with ORD have been described who even developed large ovarian cysts at 2 and 4 months of age, respectively (15, 29). Excessive LH-mediated ovarian stimulation as a consequence of primary hypogonadism is an obvious underlying mechanism. Interestingly, girls with congenital lipoid adrenal hyperplasia often spontaneously undergo puberty and have normal circulating E2 levels (23) but still present with large ovarian cysts. It has been suggested that this may be the consequence of chronic anovulation rather than LH overstimulation (24), a concept that is supported by a study in a patient with amenorrhea, ovarian cysts, and inactivating mutations of the LH receptor gene (30). Similarly, multiple ovarian cysts can be found in women with steroidogenic disorders resulting in androgen excess such as 21-hydroxylase deficiency (19, 31), which again could be a consequence of chronic anovulation or potentially direct androgen effects.
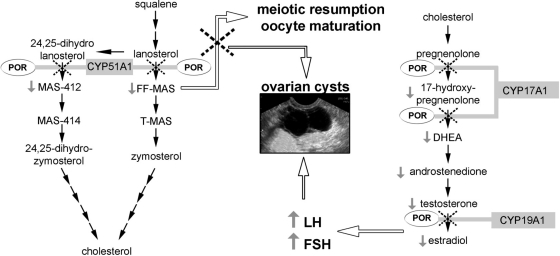
A unique mechanism driving ovarian cyst development in females affected by ORD in addition to high gonadotropins resulting from estrogen deficiency might be the disruptive impact of mutant POR on sterol synthesis and metabolism (Fig. 2). CYP51A1 requires electron transfer from POR for catalytic activity and catalyzes the conversion of lanosterol to meiosis-activating sterols (MAS). Follicular fluid MAS (FF-MAS) has been shown to be crucial for the resumption of oocyte meiosis at puberty and also supports oocyte maturation (32–34). The detailed mechanism underlying the effects of FF-MAS on oocyte maturation is not clear yet but may involve the protein kinase C pathway (35) or hedgehog signaling pathways (36, 37). FF-MAS is further converted to testicular MAS, which has been found to be highly expressed in mammalian testis, especially in spermatozoa, suggesting a role in male fertility (37); however, its precise role in male human reproduction remains to be elucidated. Importantly, CYP51A1 is highly expressed in murine and human gonads (38), and its inhibition in vitro results in oocyte arrest (39). Interestingly, it has recently been shown that gonadotropins induce up-regulation of CYP51A1 in mice and rabbits (39, 40), suggesting that POR-dependent CYP51A1 may be a crucial contributor to oocyte maturation at puberty. Thus, it is conceivable that female ORD patients have to take a “double hit” driving ovarian cyst development, disrupted MAS synthesis, and high gonadotropins due to low estrogen production (Fig. 2). This may possibly explain why the cysts appeared to be more difficult to control in our patients than in patients with other steroidogenic disorders, with two of our patients requiring long-acting glucocorticoids in addition to sex steroid replacement to control the effects of excess LH secretion.
Fig. 2.
Schematic representation of the possible mechanisms leading to ovarian cysts in female patients with ORD. Impaired sex steroid synthesis results in low E2 levels (right) causing hypergonadotropic hypogonadism with subsequent stimulation of the ovaries by the up-regulated gonadotropins. In addition, mutant POR affects the cholesterol synthesis pathway due to impairment of CYP51A1 activity (left) leading to decreased generation of MAS that are important for meiotic resumption and oocyte maturation. T-MAS, Testicular MAS. Each black arrow indicates an enzymatic reaction; dotted crosses indicate impairment of pathway reactions; gray arrows indicate increased (or decreased) serum levels.
In contrast to the findings in the five female cases, pubertal development in our two male ORD cases was more mildly affected. Case 6 presented with poor development at the age of 10 yr, but subsequently he and the other male patient spontaneously progressed through puberty with near normal testosterone production. This may indicate that sex steroid production in the testicles during puberty is less dependent on fully functional POR than ovaries or adrenals. Fukami et al. (5) previously reported that three of six male ORD patients had a normal pubertal development, whereas all seven pubertal-age girls in their cohort had impaired pubertal development; however, only limited detail on the pubertal findings and no longitudinal data were provided. The only other paper previously reporting on pubertal age ORD patients described two young women aged 17 and 19 yr, one of them presenting with normal breast development but primary amenorrhea, the other with complete lack of pubertal development (29).
Interestingly, circulating androgens in our pubertal patients were relatively normal, although low androgen levels in infants and younger children with ORD are well documented (1, 2, 5). The relative increase in androgen production at pubertal age despite POR-dependent inhibition of sex steroid synthesis by CYP17A1 could possibly be explained by increased expression of cytochrome b5 (CYB5), which acts as an allosteric facilitator of POR and CYP17A1 (41) thereby facilitating CYP17A1 17,20-lyase activity. CYB5 is expressed pre- and postnatally in human adrenal and testicular tissues (42). However, its expression increases in the adrenal zona reticularis around the age of adrenarche (43), and a similar increase in testicular Leydig cells could enhance 17,20-lyase activity significantly to rectify androgen production during adrenarche and puberty despite partially impaired POR function. A recently reported ORD case with severe neonatal 46,XY DSD, who presented with normal adrenal androgens and phallic catch-up growth during adrenarche, supports this hypothesis (16).
A diagnosis of adrenal insufficiency was established in all but one of our cases at presentation during puberty, generally displaying normal baseline cortisol levels but failure to respond appropriately to ACTH. Of note, despite neonatal presentation with DSD of unknown origin, none of the patients had previously undergone assessment of adrenal function, which had put them at considerable risk of adrenal crisis in case of intercurrent illness, inflammatory stress, or surgery. The untimely death of the younger sister of case 3, who also presented with 46,XX DSD and died at the age of 8 yr of unexplained shock after a routine appendectomy, illustrates the dramatic consequences that overlooking adrenal insufficiency can have.
Some of the phenotypic variability we observed in our patients may be explained by the differential effects of distinct POR mutations on electron-accepting CYP enzymes (13). Interestingly, the three patients with either homozygous or compound heterozygous missense mutations presented with rather mild skeletal malformations and lack of pubertal progression. By contrast, the four patients carrying a major loss of function mutation in addition to a missense mutation on the other allele presented with severe skeletal malformations but only relatively mildly impaired pubertal development. This finding will require further genotype-phenotype analysis based on larger cohorts. Data from the Japanese ORD cohort (5) support the notion that the presence of a null mutation on one allele is associated with a higher incidence of severe malformations; however, pubertal data of this cohort are not described in sufficient detail to compare them to our findings. The most common POR mutation in Caucasians, p.A287P (44), was found on seven alleles in five of our patients. p.R457H, the most common POR mutation in Japan (5, 45), was found in two of our patients, who were of Polish and Chinese origin, respectively. p.A287P decreases 17α-hydroxylase to 20–40% of wild-type activity but retains 70% of 21-hydroxylase wild-type activity (13); p.R457H equally disrupts CYP17A1 and CYP21A2 activities (13). p.T142A has been reported to only mildly impair CYP17A1 activity (4) and was found in our case 4, the only patient with spontaneous menarche and a considerable degree of spontaneous pubertal development.
In summary, pubertal development of children with ORD is impaired and frequently manifests with primary hypogonadism in girls, requiring medical induction of puberty and monitoring for ovarian cyst development. Affected boys may have the capacity for spontaneous pubertal development justifying a primarily observatory approach. Importantly, adequate hydrocortisone replacement and stress dose cover to prevent life-threatening adrenal crisis is crucial in these patients. The late diagnosis of adrenal insufficiency in our patients and the death of a sibling likely to have suffered from ORD emphasizes the need for concurrent assessment of adrenal and gonadal function not only in ORD but also in all DSD patients with an unclear diagnosis. This will facilitate early diagnosis of partial adrenal insufficiency, thus hopefully preventing adrenal crisis-related deaths in ORD, and timely establishment of a conclusive diagnosis in affected patients.
Acknowledgments
This work was supported by the Medical Research Council UK (Program Grant 0900567, to W.A.), the European Society for Pediatric Endocrinology (research fellowship, to J.I.), the European Community's Seventh Framework Program (Collaborative Research Project EuroDSD GA-2008-201444, to W.A.; Marie Curie Intra-European Fellowship PIEF-GA-2008-221058, to N.R.), and the Wellcome Trust (Clinician Scientist Fellowship GR079865MA, to N.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CYP
- Cytochrome P450
- CYP51A1
- lanosterol 14α-demethylase
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- DSD
- disordered sex development
- E2
- estradiol
- FF-MAS
- follicular fluid MAS
- GC/MS
- gas chromatography/mass spectrometry
- 17HP
- 17-hydroxypregnanolone
- 5-PT
- pregnenetriol
- MAS
- meiosis-activating sterol
- 17OHP
- 17-hydroxyprogesterone
- ORD
- POR deficiency
- PD
- pregnanediol
- POR
- P450 oxidoreductase
- PT
- pregnanetriol
- P'TONE
- pregnanetriolone
- THA
- tetrahydro-11-dehydrocorticosterone
- THB
- tetrahydrocorticosterone
- THE
- tetrahydrocortisone
- THF
- tetrahydrocortisol.
References
- 1. Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K, Miller WL. 2004. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 36:228–230 [DOI] [PubMed] [Google Scholar]
- 2. Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CHL. 2004. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet 363:2128–2135 [DOI] [PubMed] [Google Scholar]
- 3. Krone N, Dhir V, Ivison HE, Arlt W. 2007. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf) 66:162–172 [DOI] [PubMed] [Google Scholar]
- 4. Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, Miller WL. 2005. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukami M, Nishimura G, Homma K, Nagai T, Hanaki K, Uematsu A, Ishii T, Numakura C, Sawada H, Nakacho M, Kowase T, Motomura K, Haruna H, Nakamura M, Ohishi A, Adachi M, Tajima T, Hasegawa Y, Hasegawa T, Horikawa R, Fujieda K, Ogata T. 2009. Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab 94:1723–1731 [DOI] [PubMed] [Google Scholar]
- 6. Antley R, Bixler D. 1975. Trapezoidocephaly, midfacial hypoplasia and cartilage abnormalities with multiple synostoses and skeletal fractures. Birth Defects Orig Artic Ser 11:397–401 [PubMed] [Google Scholar]
- 7. Schmidt K, Hughes C, Chudek JA, Goodyear SR, Aspden RM, Talbot R, Gundersen TE, Blomhoff R, Henderson C, Wolf CR, Tickle C. 2009. Cholesterol metabolism: the main pathway acting downstream of cytochrome P450 oxidoreductase in skeletal development of the limb. Mol Cell Biol 29:2716–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roux C, Wolf C, Mulliez N, Gaoua W, Cormier V, Chevy F, Citadelle D. 2000. Role of cholesterol in embryonic development. Am J Clin Nutr 71:1270S–1279S [DOI] [PubMed] [Google Scholar]
- 9. Ribes V, Otto DM, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, Blomhoff R, Wolf CR, Tickle C, Dollé P. 2007. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev Biol 303:66–81 [DOI] [PubMed] [Google Scholar]
- 10. Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, Adams RH, Tickle C, Wolf CR. 2003. Identification of novel roles of the cytochrome p450 system in early embryogenesis: effects on vasculogenesis and retinoic acid homeostasis. Mol Cell Biol 23:6103–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 2003. 5α-Androstane-α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology 144:575–580 [DOI] [PubMed] [Google Scholar]
- 12. Bulun SE. 1996. Clinical review 78: aromatase deficiency in women and men: would you have predicted the phenotypes? J Clin Endocrinol Metab 81:867–871 [DOI] [PubMed] [Google Scholar]
- 13. Dhir V, Ivison HE, Krone N, Shackleton CH, Doherty AJ, Stewart PM, Arlt W. 2007. Differential inhibition of CYP17A1 and CYP21A2 activities by the P450 oxidoreductase mutant A287P. Mol Endocrinol 21:1958–1968 [DOI] [PubMed] [Google Scholar]
- 14. Huang N, Agrawal V, Giacomini KM, Miller WL. 2008. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci USA 105:1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukami M, Hasegawa T, Horikawa R, Ohashi T, Nishimura G, Homma K, Ogata T. 2006. Cytochrome P450 oxidoreductase deficiency in three patients initially regarded as having 21-hydroxylase deficiency and/or aromatase deficiency: diagnostic value of urine steroid hormone analysis. Pediatr Res 59:276–280 [DOI] [PubMed] [Google Scholar]
- 16. Idkowiak J, Malunowicz EM, Dhir V, Reisch N, Szarras-Czapnik M, Holmes DM, Shackleton CH, Davies JD, Hughes IA, Krone N, Arlt W. 2010. Concomitant mutations in the P450 oxidoreductase and androgen receptor genes presenting with 46,XY disordered sex development and androgenization at adrenarche. J Clin Endocrinol Metab 95:3418–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shackleton C, Marcos J, Malunowicz EM, Szarras-Czapnik M, Jira P, Taylor NF, Murphy N, Crushell E, Gottschalk M, Hauffa B, Cragun DL, Hopkin RJ, Adachi M, Arlt W. 2004. Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am J Med Genet A 128A:223–231 [DOI] [PubMed] [Google Scholar]
- 18. New MI. 1993. Nonclassical congenital adrenal hyperplasia and the polycystic ovarian syndrome. Ann NY Acad Sci 687:193–205 [DOI] [PubMed] [Google Scholar]
- 19. Hague WM, Adams J, Rodda C, Brook CG, de Bruyn R, Grant DB, Jacobs HS. 1990. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf) 33:501–510 [DOI] [PubMed] [Google Scholar]
- 20. Rosa S, Duff C, Meyer M, Lang-Muritano M, Balercia G, Boscaro M, Topaloglu AK, Mioni R, Fallo F, Zuliani L, Mantero F, Schoenle EJ, Biason-Lauber A. 2007. P450c17 deficiency: clinical and molecular characterization of six patients. J Clin Endocrinol Metab 92:1000–1007 [DOI] [PubMed] [Google Scholar]
- 21. ten Kate-Booij MJ, Cobbaert C, Koper JW, de Jong FH. 2004. Deficiency of 17,20-lyase causing giant ovarian cysts in a girl and a female phenotype in her 46,XY sister: case report. Hum Reprod 19:456–459 [DOI] [PubMed] [Google Scholar]
- 22. Mallin SR. 1969. Congenital adrenal hyperplasia secondary to 17-hydroxylase deficiency. Two sisters with amenorrhea, hypokalemia, hypertension, and cystic ovaries. Ann Intern Med 70:69–75 [DOI] [PubMed] [Google Scholar]
- 23. Fujieda K, Tajima T, Nakae J, Sageshima S, Tachibana K, Suwa S, Sugawara T, Strauss JF., 3rd 1997. Spontaneous puberty in 46, XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest 99:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shima M, Tanae A, Miki K, Katsumata N, Matsumoto S, Nakajima S, Harada T, Shinagawa T, Tanaka T, Okada S. 2000. Mechanism for the development of ovarian cysts in patients with congenital lipoid adrenal hyperplasia. Eur J Endocrinol 142:274–279 [DOI] [PubMed] [Google Scholar]
- 25. Tanae A, Katsumata N, Sato N, Horikawa R, Tanaka T. 2000. Genetic and endocrinological evaluations of three 46, XX patients with congenital lipoid adrenal hyperplasia previously reported as having presented spontaneous puberty. Endocr J 47:629–634 [DOI] [PubMed] [Google Scholar]
- 26. Belgorosky A, Pepe C, Marino R, Guercio G, Saraco N, Vaiani E, Rivarola MA. 2003. Hypothalamic-pituitary-ovarian axis during infancy, early and late prepuberty in an aromatase-deficient girl who is a compound heterocygote for two new point mutations of the CYP19 gene. J Clin Endocrinol Metab 88:5127–5131 [DOI] [PubMed] [Google Scholar]
- 27. Mullis PE, Yoshimura N, Kuhlmann B, Lippuner K, Jaeger P, Harada H. 1997. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J Clin Endocrinol Metab 82:1739–1745 [DOI] [PubMed] [Google Scholar]
- 28. Fukami M, Horikawa R, Nagai T, Tanaka T, Naiki Y, Sato N, Okuyama T, Nakai H, Soneda S, Tachibana K, Matsuo N, Sato S, Homma K, Nishimura G, Hasegawa T, Ogata T. 2005. Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab 90:414–426 [DOI] [PubMed] [Google Scholar]
- 29. Sahakitrungruang T, Huang N, Tee MK, Agrawal V, Russell WE, Crock P, Murphy N, Migeon CJ, Miller WL. 2009. Clinical, genetic, and enzymatic characterization of P450 oxidoreductase deficiency in four patients. J Clin Endocrinol Metab 94:4992–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Latronico AC, Anasti J, Arnhold IJ, Rapaport R, Mendonca BB, Bloise W, Castro M, Tsigos C, Chrousos GP. 1996. Brief report: testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormone-receptor gene. N Engl J Med 334:507–512 [DOI] [PubMed] [Google Scholar]
- 31. Falhammar H, Thorén M, Hagenfeldt K. 2008. A 31-year-old woman with infertility and polycystic ovaries diagnosed with non-classic congenital adrenal hyperplasia due to a novel CYP21 mutation. J Endocrinol Invest 31:176–180 [DOI] [PubMed] [Google Scholar]
- 32. Byskov AG, Andersen CY, Nordholm L, Thøgersen H, Xia G, Wassmann O, Andersen JV, Guddal E, Roed T. 1995. Chemical structure of sterols that activate oocyte meiosis. Nature 374:559–562 [DOI] [PubMed] [Google Scholar]
- 33. Grøndahl C, Ottesen JL, Lessl M, Faarup P, Murray A, Grønvald FC, Hegele-Hartung C, Ahnfelt-Rønne I. 1998. Meiosis-activating sterol promotes resumption of meiosis in mouse oocytes cultured in vitro in contrast to related oxysterols. Biol Reprod 58:1297–1302 [DOI] [PubMed] [Google Scholar]
- 34. Grondahl C, Hansen TH, Marky-Nielsen K, Ottesen JL, Hyttel P. 2000. Human oocyte maturation in vitro is stimulated by meiosis-activating sterol. Hum Reprod 15(Suppl 5):3–10 [DOI] [PubMed] [Google Scholar]
- 35. Jin S, Zhang M, Lei L, Wang C, Fu M, Ning G, Xia G. 2006. Meiosis activating sterol (MAS) regulate FSH-induced meiotic resumption of cumulus cell-enclosed porcine oocytes via PKC pathway. Mol Cell Endocrinol 249:64–70 [DOI] [PubMed] [Google Scholar]
- 36. Nguyen NT, Lin DP, Yen SY, Tseng JK, Chuang JF, Chen BY, Lin TA, Chang HH, Ju JC. 2009. Sonic hedgehog promotes porcine oocyte maturation and early embryo development. Reprod Fertil Dev 21:805–815 [DOI] [PubMed] [Google Scholar]
- 37. Mann RK, Beachy PA. 2004. Novel lipid modifications of secreted protein signals. Annu Rev Biochem 73:891–923 [DOI] [PubMed] [Google Scholar]
- 38. Rozman D, Cotman M, Frangez R. 2002. Lanosterol 14α-demethylase and MAS sterols in mammalian gametogenesis. Mol Cell Endocrinol 187:179–187 [DOI] [PubMed] [Google Scholar]
- 39. Wang C, Xu B, Zhou B, Zhang C, Yang J, Ouyang H, Ning G, Zhang M, Shen J, Xia G. 2009. Reducing CYP51 inhibits follicle-stimulating hormone induced resumption of mouse oocyte meiosis in vitro. J Lipid Res 50:2164–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang F, Yang J, Wang H, Xia G. 2010. Gonadotropin-regulated expressions of lanosterol 14α-demethylase, sterol Delta14-reductase and C-4 sterol methyl oxidase contribute to the accumulation of meiosis-activating sterol in rabbit gonads. Prostaglandins Other Lipid Mediat 92:25–32 [DOI] [PubMed] [Google Scholar]
- 41. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 42. Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR., Jr 2004. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod 71:83–88 [DOI] [PubMed] [Google Scholar]
- 43. Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. 2000. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 53:739–747 [DOI] [PubMed] [Google Scholar]
- 44. Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. 2010. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol 163:919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adachi M, Asakura Y, Matsuo M, Yamamoto T, Hanaki K, Arlt W. 2006. POR R457H is a global founder mutation causing Antley-Bixler syndrome with autosomal recessive trait. Am J Med Genet A 140:633–635 [DOI] [PubMed] [Google Scholar]