Abstract
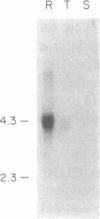
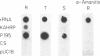
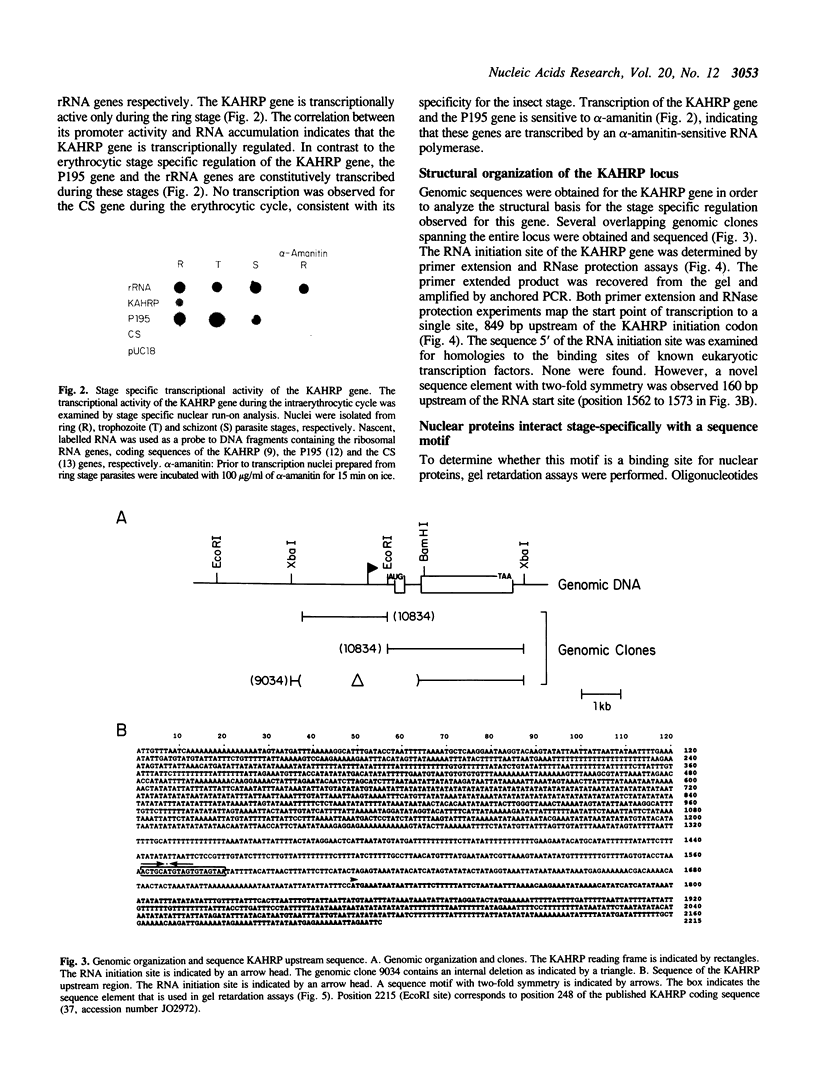
The Plasmodium falciparum gene encoding the knob associated histidine-rich protein (KAHRP) is shown to be transcriptionally regulated during its expression in the intraerythrocytic cycle as demonstrated by stage specific nuclear run-on analysis. The genomic organization of the KAHRP gene was determined and the structural basis for the stage specific transcription investigated. A sequence motif with two-fold symmetry was found 160 bp upstream of the RNA initiation site. This sequence element interacts with parasite derived nuclear extracts in a stage specific manner that correlates with the transcriptional activity of the KAHRP gene. These studies suggest a functional role for this structural element in the developmental regulation of a P. falciparum erythrocytic gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell J. W., Howard R. J., Miller L. H. Influence of the spleen on the expression of surface antigens on parasitized erythrocytes. Ciba Found Symp. 1983;94:117–136. doi: 10.1002/9780470715444.ch8. [DOI] [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Goman M., Langsley G., Hyde J. E., Yankovsky N. K., Zolg J. W., Scaife J. G. The establishment of genomic DNA libraries for the human malaria parasite Plasmodium falciparum and identification of individual clones by hybridisation. Mol Biochem Parasitol. 1982 Jun;5(6):391–400. doi: 10.1016/0166-6851(82)90012-3. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F., Walliker D. Genetic diversity in Plasmodium falciparum. Adv Parasitol. 1990;29:75–149. doi: 10.1016/s0065-308x(08)60105-0. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner S., Breuer W. V., Ginsburg H., Aley S. B., Cabantchik Z. I. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J Cell Physiol. 1985 Dec;125(3):521–527. doi: 10.1002/jcp.1041250323. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T., Motyl M. R., Trager W. Plasmodium falciparum: loss of knobs on the infected erythrocyte surface after long-term cultivation. Exp Parasitol. 1979 Oct;48(2):213–219. doi: 10.1016/0014-4894(79)90101-2. [DOI] [PubMed] [Google Scholar]
- Langsley G., Hyde J. E., Goman M., Scaife J. G. Cloning and characterisation of the rRNA genes from the human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 1983 Dec 20;11(24):8703–8717. doi: 10.1093/nar/11.24.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. P. Sequence analysis upstream of the gene encoding the precursor to the major merozoite surface antigens of Plasmodium yoelii. Mol Biochem Parasitol. 1990 Mar;39(2):285–288. doi: 10.1016/0166-6851(90)90068-w. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Chien S., Usami S. Decreased deformability of Plasmodium coatneyi-infected red cells and its possible relation to cerebral malaria. Am J Trop Med Hyg. 1972 Mar;21(2):133–137. doi: 10.4269/ajtmh.1972.21.133. [DOI] [PubMed] [Google Scholar]
- Oquendo P., Hundt E., Lawler J., Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989 Jul 14;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Perkins M. Stage-dependent processing and localization of a Plasmodium falciparum protein of 130,000 molecular weight. Exp Parasitol. 1988 Feb;65(1):61–68. doi: 10.1016/0014-4894(88)90107-5. [DOI] [PubMed] [Google Scholar]
- Pollack Y., Katzen A. L., Spira D. T., Golenser J. The genome of Plasmodium falciparum. I: DNA base composition. Nucleic Acids Res. 1982 Jan 22;10(2):539–546. doi: 10.1093/nar/10.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Pavlovec A., Shio H., Ravetch J. V. Primary structure and subcellular localization of the knob-associated histidine-rich protein of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7139–7143. doi: 10.1073/pnas.84.20.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Raventos-Suarez C., Kaul D. K., Macaluso F., Nagel R. L. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3829–3833. doi: 10.1073/pnas.82.11.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Kochan J., Perkins M. Isolation of the gene for a glycophorin-binding protein implicated in erythrocyte invasion by a malaria parasite. Science. 1985 Mar 29;227(4694):1593–1597. doi: 10.1126/science.3883491. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Ozaki L. S., Gwadz R. W., Godson G. N. Organization and expression of the Plasmodium knowlesi circumsporozoite antigen gene. Mol Biochem Parasitol. 1987 Apr;23(3):233–245. doi: 10.1016/0166-6851(87)90030-2. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Vernot-Hernandez J. P., Heidrich H. G. Time-course of synthesis, transport and incorporation of a protein identified in purified membranes of host erythrocytes infected with a knob-forming strain of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jul;12(3):337–350. doi: 10.1016/0166-6851(84)90090-2. [DOI] [PubMed] [Google Scholar]
- Wallach M. Efficient extraction and translation of Plasmodium falciparum messenger RNA. Mol Biochem Parasitol. 1982 Dec;6(6):335–342. doi: 10.1016/0166-6851(82)90023-8. [DOI] [PubMed] [Google Scholar]
- Waters A. P., Syin C., McCutchan T. F. Developmental regulation of stage-specific ribosome populations in Plasmodium. Nature. 1989 Nov 23;342(6248):438–440. doi: 10.1038/342438a0. [DOI] [PubMed] [Google Scholar]
- Weber J. L. Analysis of sequences from the extremely A + T-rich genome of Plasmodium falciparum. Gene. 1987;52(1):103–109. doi: 10.1016/0378-1119(87)90399-4. [DOI] [PubMed] [Google Scholar]
- Weber J. L. Molecular biology of malaria parasites. Exp Parasitol. 1988 Aug;66(2):143–170. doi: 10.1016/0014-4894(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Wesseling J. G., Snijders P. J., van Someren P., Jansen J., Smits M. A., Schoenmakers J. G. Stage-specific expression and genomic organization of the actin genes of the malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 1989 Jun 15;35(2):167–176. doi: 10.1016/0166-6851(89)90119-9. [DOI] [PubMed] [Google Scholar]