Summary
Pseudomonas aeruginosa uses a type III secretion system to inject protein effectors into a targeted host cell. Effector secretion is triggered by host cell contact. How effector secretion is prevented prior to cell contact is not well understood. In all secretion systems studied to date, the needle-tip protein is required for controlling effector secretion, but the mechanism by which needle-tip proteins control effector secretion is unclear. Here we present data that the P. aeruginosa needle-tip protein, PcrV, controls effector secretion by assembling into a functional needle-tip complex. PcrV likely does not simply obstruct the secretion channel since the pore-forming translocator proteins can still be secreted while effector secretion is repressed. This finding suggests that PcrV controls effector secretion by affecting the conformation of the apparatus, shifting it from the default, effector secretion ‘on’ conformation, to the effector secretion ‘off’ conformation. We also present evidence that PcrG, which can bind to PcrV and is also involved in controlling effector export, is cytoplasmic and that the interaction between PcrG and PcrV is not required for effector secretion control by either protein. Taken together, these data allow us to propose a working model for control of effector secretion by PcrG and PcrV.
Introduction
Many Gram-negative pathogens use a type III secretion system (T3SS) to deliver effector proteins into the cytoplasm of targeted host cells in order to promote infection (Cornelis, 2006, Galan & Wolf-Watz, 2006, Troisfontaines & Cornelis, 2005). This is also the case for the opportunistic pathogen P. aeruginosa (Hauser, 2009, Yahr et al., 1996), where type III secretion is critical in several animal models of infection (Hauser et al., 1998, Kurahashi et al., 1999, Lee et al., 2003, Lee et al., 2005, Miyata et al., 2003, Pukatzki et al., 2002), and has been correlated with a more severe disease outcome in patients with ventilator associated pneumonia (Hauser et al., 2002, Roy-Burman et al., 2001).
One of the hallmarks of type III secretion systems is that effector secretion is up-regulated by cell-contact (Enninga et al., 2005, Pettersson et al., 1996, Schlumberger et al., 2005). How effector secretion is prevented prior to cell-contact, or indeed how cell contact results in triggering of effector secretion are not well understood (Cornelis, 2006). However, needle-tip proteins have been implicated in effector secretion control in every organism studied to date (these include PcrV in P. aeruginosa, LcrV in Yersinia sp., IpaD in Shigella sp. and SipD in Salmonella sp. (Veenendaal et al., 2007, Sani et al., 2007, Mueller et al., 2005, Lara-Tejero & Galan, 2009)). Deletion of the corresponding genes most commonly leads to up-regulation of effector secretion (Menard et al., 1993, McCaw et al., 2002, Kaniga et al., 1995a). The notable exception being Yersinia sp., where deletion of the gene encoding the needle-tip protein LcrV, results in a defect in effector secretion (see below)(Bergman et al., 1991, Price et al., 1991, Nilles et al., 1997).
The observation that deletion of the S. flexneri needle-tip protein gene ipaD, or the gene for the pore-forming translocator IpaB, results in deregulation of effector secretion led to the first model of effector secretion control, the ‘plug’ model (Menard et al., 1993). Here it was proposed that IpaD and IpaB form a structure at the tip of the type III secretion needle, which physically blocks secretion via the needle. Cell contact would then result in a conformational change in the needle-tip that opens the secretion channel. Consistent with this model, deletion of the export signal of IpaD results in a non-secreted protein that also fails to control effector export (Picking et al., 2005). Some support for the plug hypothesis also comes from modeling of the needle-tip based on the structure of an IpaD dimer found in one of the crystals used to solve the IpaD structure. The model predicts that five IpaD monomers assemble into a needle-tip structure that only has a small aperture at its center, much too small to allow the passage of proteins (Johnson et al., 2007). An earlier model in which the structure of LcrV had been inserted at the top of a T3SS needle resulted in a needle-tip with a large aperture (Deane et al., 2006). These two models have been interpreted to represent ‘open’ and ‘closed’ versions of the needle-tip (Blocker et al., 2008, Deane et al., 2006). Recent evidence suggests that the S. flexneri needle-tip may in fact consist of four IpaD molecules and one molecule of the pore-forming translocator protein IpaB (Johnson et al., 2007, Veenendaal et al., 2007). While some have only found IpaB associated with the needle-tip after treating bacteria with agents such as bile salts (Olive et al., 2007, Stensrud et al., 2008), it has been argued that IpaB may be queued within the needle and associated with the needle-tip even in the absence of these treatments and that bile-salts simply reconfigure the needle-tip to display a larger portion of IpaB (Espina et al., 2006). The great attraction of this model is that it immediately explains why in Shigella deletion of either ipaB or ipaD results in up-regulation of effector secretion. It also results in one of the clearest models for triggering of effector secretion on cell-contact, where insertion of needle tip-associated IpaB into the host cell membrane results in the conformational change that opens the needle-tip and allows secretion of the second pore-forming translocator, IpaC, as well as effectors to commence (Veenendaal et al., 2007). It is unclear, however, how far this model can be applied to other type III secretion systems. For one, no pore-forming translocators have been detected associated with the needle-tip of S. typhimurium, Y. enterocolitica or P. aeruginosa prior to cell contact ((Broz et al., 2007, Lara-Tejero & Galan, 2009, Mueller et al., 2005) and unpublished observation). Moreover, deletion of the pore-forming translocator proteins in P. aeruginosa or S. typhimuriurm, does not result in loss of effector secretion control (Broms et al., 2003, Cisz et al., 2008, Kaniga et al., 1995b).
Another variation of the plug model has been proposed, where a ‘sensor’ protein has been inserted into the type III secretion channel (Blocker et al., 2008). Its export is inhibited by the type III secretion needle-tip and its C-terminal portion binds to a part of the cytoplasmic face of the T3SS, effectively occluding an acceptor site for effector proteins. Once the T3SS has encountered the host-cell, a conformational change in the needle-tip permits export of the sensor protein, which frees an acceptor site for effectors and results in effector export. A possible candidate for such a sensor molecule are proteins of the YopN/MxiC family, which are required for preventing export of effector proteins prior to cell contact (Botteaux et al., 2008, Forsberg et al., 1991, Sundin et al., 2004). The best studied of these proteins is the Y. pestis YopN protein. YopN is thought to be tethered to the base of the apparatus via a C-terminal interaction with the small regulatory protein TyeA (Cheng & Schneewind, 2000, Day et al., 2003, Sundberg & Forsberg, 2003). Because YopN requires an intact type III secretion signal, as well as its export chaperone, to control effector secretion, it has been proposed that YopN is partially inserted into the T3SS before effector secretion is triggered (Day & Plano, 1998, Ferracci et al., 2005). The analogy here is the ruler model for type III secretion needle-length control, where YscP has been proposed to connect the growing needle-tip and the base of the apparatus (Journet et al., 2003, Wagner et al., 2009). One problem with the plug/sensor models, however, is that growing evidence from a variety of organisms, suggests that translocator proteins can be exported prior to cell contact, before effector secretion has been triggered (Cisz et al., 2008, Lee et al., 2001, Olive et al., 2007). In the case of the YscP ruler model, it has been proposed that the secretion channel is wide enough to accommodate two proteins in an extended conformation (Wagner et al., 2009). This line of reasoning could also be extended to the sensor model. Here the sensor would be inserted into the secretion channel in an extended conformation, allowing the simultaneous export of translocator proteins.
The third, ‘allosteric’, model of effector secretion control also circumvents the problem of translocator secretion in the absence of effector export. In this model the needle-tip controls effector secretion by effecting a conformational change in the apparatus (Blocker et al., 2008, Kenjale et al., 2005, Torruellas et al., 2005). Here, cell-contact results in a conformational change in the needle-tip, which in turn results in a change in needle-conformation that propagates down the needle and changes the conformation of the base of the apparatus to permit effector export. In support of this model, needle-protein mutations have been isolated both in Shigella and Yersinia, that result in de-regulated effector secretion (Davis & Mecsas, 2007, Kenjale et al., 2005, Torruellas et al., 2005). While some of these mutations disrupt the assembly of the needle-tip, and are therefore compatible with all of the proposed models, others still allow assembly of the needle-tip, suggesting that the mutations lock the apparatus in the effector secretion ’on’ conformation (Kenjale et al., 2005, Zhang et al., 2007). Analysis of the helical pitch and axial rise of the needle-subunits in needles isolated from wild-type Shigella or locked ‘on’ mutants (that either still responded to congo red or were congo-red insensitive), however, could not discern a difference between the wild-type needles or the mutant needles (Cordes et al., 2005). These data were taken to mean that triggering of effector secretion cannot be associated with a large-scale conformational change of the needle, akin to the change in flagellar packing that has been detected upon changing the direction of rotation (Blocker et al., 2008, Cordes et al., 2005, Mueller et al., 2008). Instead, it was proposed that the signal is propagated by subtle and/or rapid changes in the interactions between head and tail portions of adjacent needle-subunits (Deane et al., 2006).
Among the well-studied type III secretion systems, the Yersinia sp. LcrV protein is the needle-tip protein that is most closely related to P. aeruginosa PcrV (Troisfontaines & Cornelis, 2005). However, as mentioned above, LcrV represents the curious exception to how needle-tip proteins control effector secretion. Unlike other needle tip proteins, the deletion of lcrV results in a defect in effector secretion. This is not the case for P. aeruginosa PcrV (McCaw et al., 2002, Rietsch et al., 2005). Yersinia LcrV interacts with a small export chaperone, LcrG, that is also required for effector secretion control (Nilles et al., 1997, DeBord et al., 2001, Matson & Nilles, 2001, Lawton et al., 2002, Matson & Nilles, 2002). Deletion of lcrG results in constitutive effector secretion. LcrG is cytoplasmic in all species of Yersinia, except Y. pestis, where a portion of it appears to be exported (DeBord et al., 2001, Matson & Nilles, 2001, Reina et al., 2008). However, fusions that prevent export do not abolish its activity, suggesting that it controls effector secretion in the cytoplasm in Y. pestis as well (Reina et al., 2008). Mutations that prevent the interaction between LcrV and LcrG prevent up-regulation of effector secretion (Matson & Nilles, 2001, Hamad & Nilles, 2007) and overexpression of LcrV stimulates effector secretion (Lee et al., 2000). Based on these data, it was proposed that LcrG blocks effector secretion at the cytoplasmic face of the T3SS. Upon cell-contact, LcrV expression is up-regulated and titrates LcrG from the apparatus, thereby negating the block imposed by LcrG (titration model). While this model explains some of the mutant data, differential up-regulation of LcrV over LcrG (lcrG and lcrV are part of the lcrGVHyopBD operon and are coordinately expressed) has, to our knowledge, never been demonstrated. Moreover, effector secretion in Y. pseudotuberculosis can be triggered in the presence of chloramphenicol, suggesting that no de novo protein synthesis is needed to up-regulate effector export (Lloyd et al., 2001).
Given the differences in effector secretion control by PcrV when compared to its nearest relative, LcrV, as well as the wealth of competing models of effector secretion control, we decided to examine the role of PcrV and PcrG in control of effector secretion by P. aeruginosa. We confirmed that deletion of pcrG or pcrV results in effector secretion even in the absence of cell contact. Moreover, the deregulation caused by the individual deletion mutations appears to be additive. Next we determined that PcrV export is required in order to control effector secretion. Specific mutants of PcrV and PcrG allowed us to determine that the interaction of PcrG and PcrV is not required for regulation of effector secretion, but rather that effector secretion control by PcrV is tied to its ability to form a functional needle-tip complex. A physical block of the secretion apparatus by PcrV seems unlikely, since translocator proteins are secreted prior to cell contact even in wild-type bacteria. PcrG, while required for efficient export of PcrV, controls effector secretion from the cytoplasm in a PcrV-independent manner. Taken together these results are consistent with a model in which PcrV acts as an allosteric regulator that helps shift the needle complex from its default, effector secretion ‘on’ conformation, to the effector secretion ‘off’ conformation.
Results
Deletion of pcrG and pcrV results in premature effector secretion
In P. aeruginosa, as in Yersinia sp., effector secretion can be triggered in vitro by removing calcium from the growth medium. As had been previously reported, deletion of pcrG or pcrV results in effector secretion in the presence of calcium (McCaw et al., 2002, Sundin et al., 2004, Rietsch et al., 2005). We decided to test these phenotypes in our strain of P. aeruginosa using two assays: indirectly, by monitoring control of exoS transcription, and directly, by assaying effector secretion by western blot.
In P. aeruginosa, expression of all type III secretion-related genes (both apparatus and effector genes) is controlled by the master regulator ExsA (Frank & Iglewski, 1991, Yahr & Frank, 1994, Wolfgang et al., 2003). The activity of ExsA is related to triggering of effector secretion by virtue of the small type III secreted protein, ExsE (Rietsch et al., 2005, Urbanowski et al., 2007, Urbanowski et al., 2005). When effector secretion is up-regulated (either by cell contact or, in vitro, by removing calcium from the medium), ExsE is exported via the type III secretion apparatus, which, through a series of protein-protein interactions, results in the release of ExsA from an inactive complex with the negative regulator ExsD, thereby allowing ExsA to bind to its cognate promoters and to activate transcription (Brutinel et al., 2009, McCaw et al., 2002, Thibault et al., 2009). As a result, transcription of T3SS related genes (in our case, the effector gene, exoS) can be used as a convenient readout for detecting effector export.
Our second assay involves a modified secretion assay. Since gene expression is co-regulated with effector secretion, we designed our secretion experiment to first up-regulate gene expression (and secretion) by growing the bacteria in the absence of calcium and then pelleting the cells and resuspending them in medium with or without calcium to determine if re-addition of calcium can block effector export (reestablishing calcium control, RECC assay) (Cisz et al., 2008).
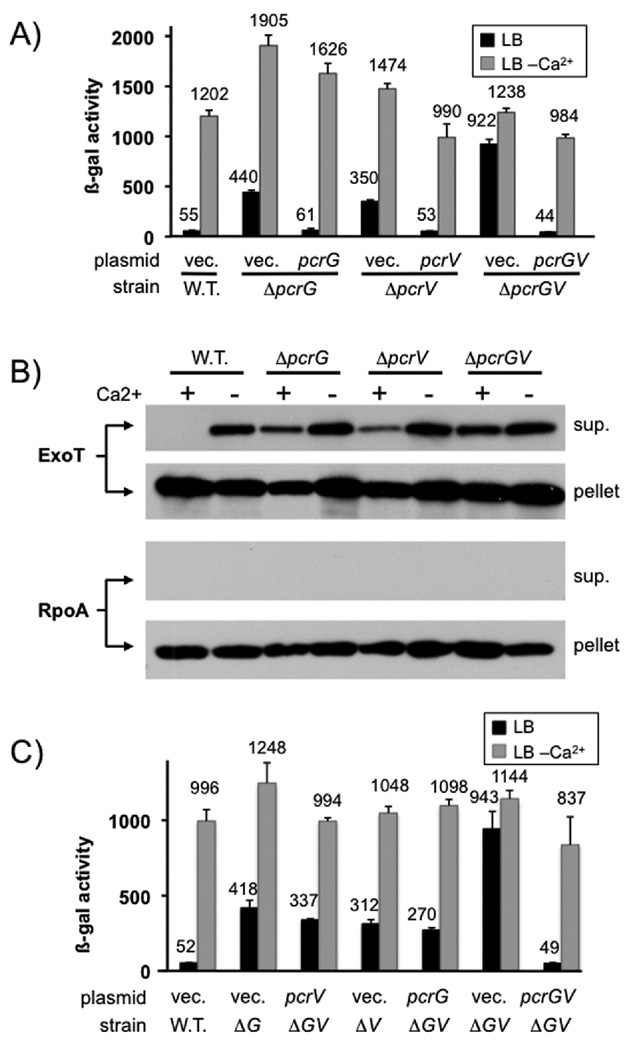
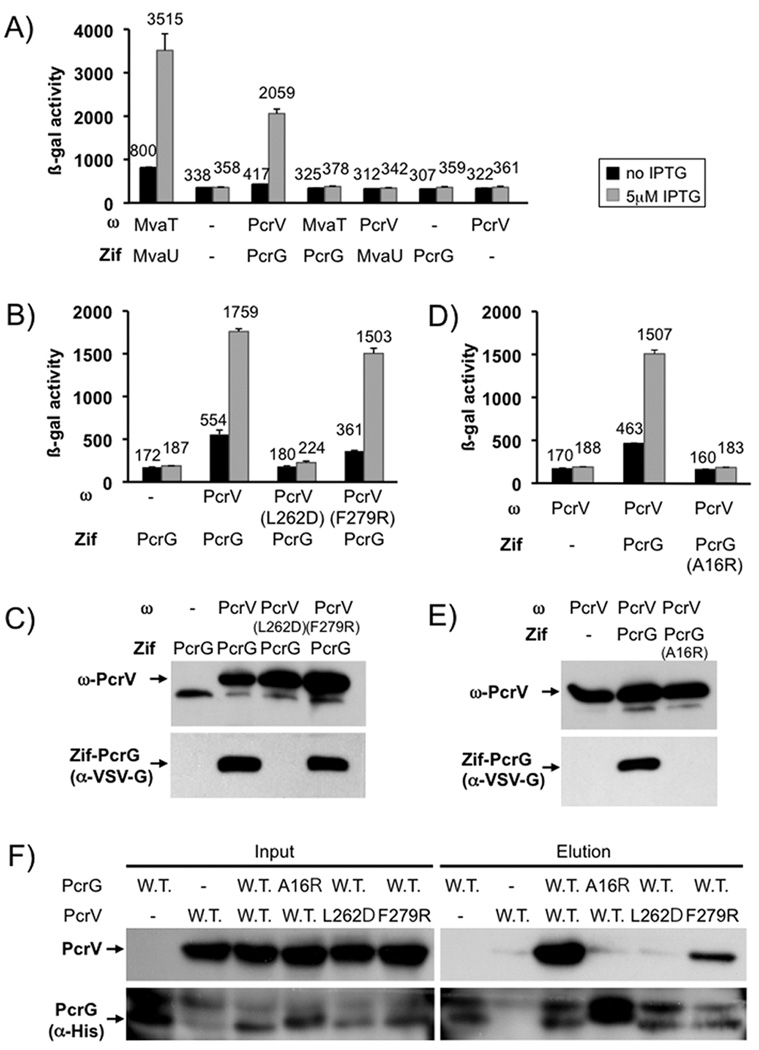
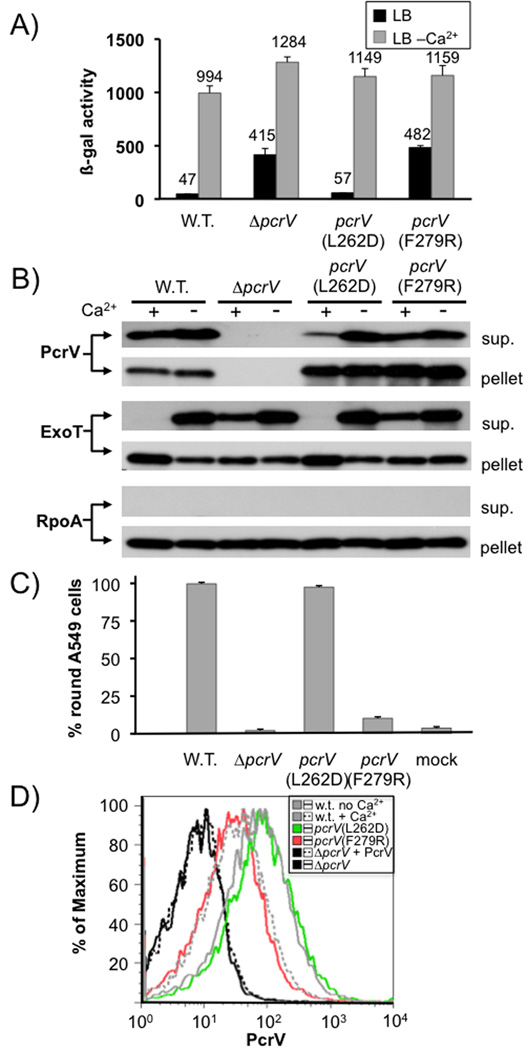
Both deletion of pcrG and pcrV resulted in up-regulation of effector secretion in the presence of calcium (Fig. 1A+B). Interestingly, the effect of deleting pcrG and pcrV appears to be additive, in that the pcrGV double mutant has a more severe regulatory defect than either of the single mutants. This observation was evident both by monitoring exoS transcription (which is dependent on export of the negative regulator ExsE), as well as monitoring the export of the effector protein ExoT directly. Indeed, using the exoS transcriptional reporter, we were able to recapitulate the single mutant phenotypes by expressing either pcrV or pcrG from a plasmid in the ΔpcrGV mutant background, indicating that this is not an aberrant side-effect of the pcrGV double null mutant strain, but rather a property of the two regulatory proteins (Fig. 1C). The remaining low-calcium dependent up-regulation of exoS expression seen in these experiments is likely not the result of increased secretion. This type of up-regulation can even be seen in mutants where the secretion-dependent regulatory system has been inactivated, such as exsE or exsD null mutants (McCaw et al., 2002, Rietsch et al., 2005, Urbanowski et al., 2005). It is likely the result of global changes in gene expression (e.g. low-calcium dependent up-regulation of cAMP (Wolfgang et al., 2003), which is a positive regulator of type III secretion gene expression). However, the presence of additional regulatory factors that work in conjunction with PcrG and PcrV cannot be ruled out entirely.
Fig. 1. pcrG and pcrV mutant phenotype.
(A) The effect of deleting pcrG and/or pcrV was assayed by measuring β-galactosidase activity of P. aeruginosa PAO1 in which the chromosomal copy of exoS has been replaced by a tandem set of translationally coupled reporters (GFP-lacZ). Where indicated, a wild-type copy of pcrG or pcrV was supplied in trans from a plasmid (vec = vector control). (B) export of ExoT was measured by RECC assay. Supernatant and pellet fractions are indicated. The RNA polymerase alpha subunit (RpoA) served as fractionation control. (C) a ΔpcrGV double null mutant was complemented by either expressing pcrG alone, pcrV alone or both open reading frames together. The pcrG plasmid was induced with 10µM IPTG, the pcrV plasmid with 125 µM IPTG and the pcrGV plasmid with 10µM IPTG. The success of the complementation was monitored using the chromosomal exoS reporter fusion described in (A).
It should be noted that the start codon for the pcrG open reading frame was mis-annotated in the genome (Stover et al., 2000). Selective inactivation of the annotated ATG (ATG1) or the ATG four codons downstream of the annotated ATG (ATG2) demonstrated that, the correct translation initiation codon of pcrG is ATG2 (Fig. S1). This also places the start codon in a better context with regard to the proposed pcrG promoter (ATG1 overlaps with the proposed −10 box) (Brutinel et al., 2008). Indeed, mutating ATG1 to GGG (as opposed to ATC) results in a slight deregulation of the system, suggesting that the proposed site of the pcrG promoter is correct (data not shown).
PcrV export is required to control effector secretion
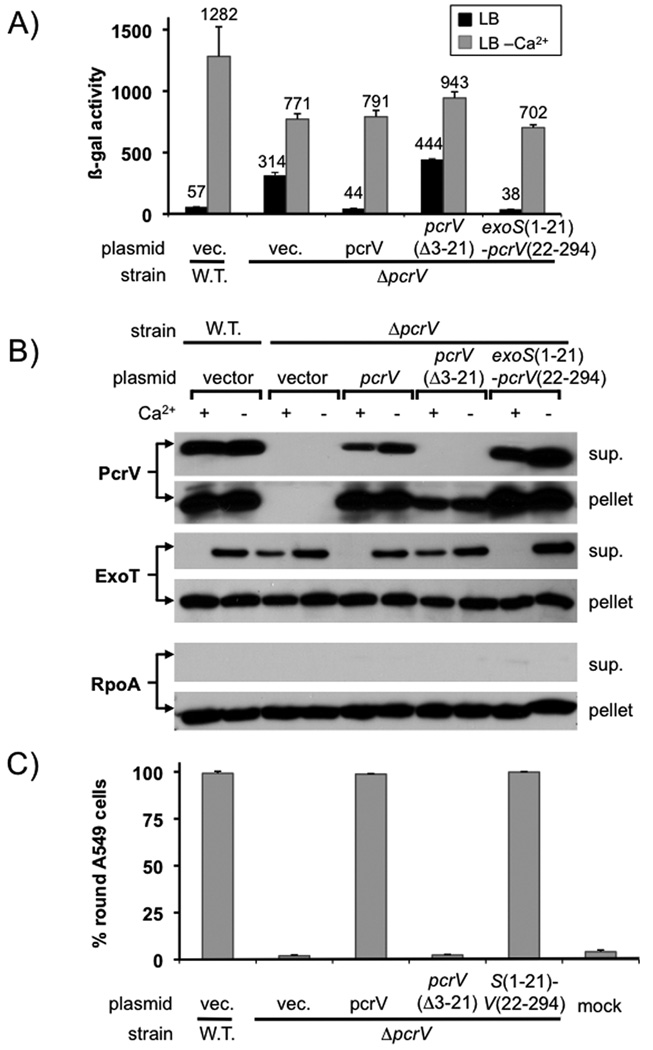
Current models in the Yersinia field suggest that LcrV controls effector secretion when located in the cytoplasm through its interaction with LcrG. Overexpression of LcrV, even a non-secreted form of LcrV, can up-regulate effector export in this organism. We decided to test whether PcrV must be exported in order to control effector secretion by expressing a truncated version of the protein in which the signal sequence had been deleted, PcrV(Δ3–21). To create this protein, we relied on experiments performed in Y. pseudotuberculosis in which deletion of amino-acids 3–20 of LcrV prevented its export (Broms et al., 2007).
The mutant protein, lacking the secretion signal, was expressed but not secreted (Fig. 2B). Consistent with these data, expression of the deletion mutant was also not able to restore cytotoxicity to a pcrV null mutant strain (Fig. 2C). Cytotoxicity relies on the presence of PcrV at the needle tip to help form and presumably dock to the translocation pore formed by PopB and PopD (Dacheux et al., 2001, Goure et al., 2004, Sawa et al., 1999). The signal sequence deletion mutant also failed to control effector export (Fig. 2A+2B). Replacement of the signal sequence of PcrV with that of the effector ExoS was able to restore export, cytotoxicity and effector secretion control, demonstrating that PcrV requires an intact export signal in order to control effector secretion (Fig 2). The signal sequence deletion mutant was expressed at a somewhat lower level than the wild-type protein in our experiment (with 10µM IPTG induction across the board). However, performing the experiment at a higher concentration of IPTG (25µM) yielded the same result (data not shown), suggesting that the level of expression cannot explain the mutant phenotype.
Fig. 2. PcrV requires an intact secretion signal to control effector export.
(A) a pcrV deletion mutant was complemented with either wild-type pcrV, a pcrV deletion mutant lacking codons 3–21, or a fusion in which codons 1–21 of exoS were fused to codons 22–294 of pcrV. The ability of the indicated ORF to complement the pcrV null mutant phenotype was monitored using a lacZ reporter inserted in the exoS locus (A) or by RECC assay (B). The ability of the indicated open reading frames to complement the defect in cytotoxicity of a pcrV null mutant was assayed by monitoring rounding of A549 cells after 5 hours of infection (MOI 10)(C).
We considered two possible scenarios to explain the lack of secretion control by PcrV(Δ3–21). On the one hand, PcrV could exert its influence on effector secretion control by assembling at the needle-tip (perhaps controlling effector secretion by influencing the conformation of the apparatus). On the other hand, PcrV could require its secretion signal in order to be targeted to the secretion apparatus and control effector export from the cytoplasm (perhaps in a complex with PcrG, or independently of PcrG via a second interaction with the apparatus). In Y. enterocolitica, overexpression of wild-type or a non-secreted form of LcrV resulted in deregulation of effector secretion (Lee et al., 2000). This observation was interpreted to mean that LcrV can relieve the block of secretion imposed by LcrG by titrating it from the apparatus. We decided to test if overexpression of signal-sequenceless PcrV is dominant negative. We reasoned that if PcrV exerts its influence in the cytoplasm, the signal-sequence deletion mutant could be dominant negative by either titrating PcrG into an inactive complex or forming a non-productive interaction with the apparatus that precludes the interaction of wild-type PcrV with the T3SS.
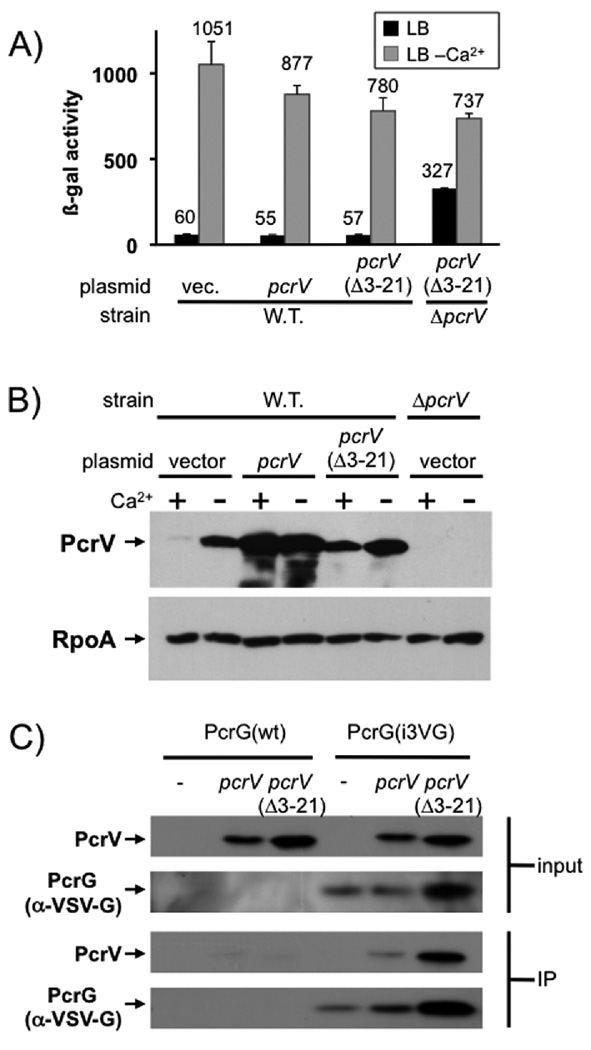
We overexpressed either wild-type or signal-sequenceless PcrV in an otherwise wild-type strain of P. aeruginosa. Neither overexpression of wild-type PcrV nor the signal-sequenceless mutant resulted in de-regulation of effector secretion (Fig. 3). We considered the possibility that PcrV(Δ3–21) could be defective in its interaction with PcrG. To this end we modified the chromosomal copy of pcrG to express an internally VSV-G (Vesicular Stomatitis Virus G-protein epitope) tagged version of PcrG in order to be able to track the protein. Importantly this tagged version of the protein is essentially fully functional (94% active, Fig. S2). Both versions of PcrV could be co-precipitated with PcrG from cytoplasmic extracts, demonstrating that the two proteins interact in P. aeruginosa and that the interaction is not perturbed by removal of the signal sequence of PcrV (Fig. 3C). The lack of overexpression phenotype, together with the inability of signal-sequenceless PcrV to control effector secretion, suggested to us that PcrV has to be exported to control effector secretion. However, as mentioned above, the absence of a dominant negative phenotype could also be explained by arguing that the regulatory interaction of PcrG or wild-type PcrV with the apparatus is too strong to be perturbed by excess non-secreted PcrV.
Fig. 3. Overexpression of PcrV or PcrV (Δ3–21).
PcrV or the signal-sequenceless version PcrV(Δ3–21) were overexpressed in a wild-type strain background either in the presence or absence of calcium. The effect on the export of effectors was monitored using a lacZ reporter inserted in the exoS locus (A). Overexpression of PcrV or PcrV (Δ3–21) was confirmed by western blot on cell pellets of overexpressing strains (B, RpoA served as loading control). (C) Wild-type PcrV or a mutant lacking the export signal (Δ3–21) were expressed from a plasmid in a strain in which the chromosomal copy of pcrV had been deleted and in which expression of the type III secretion genes was uniformly up-regulated (ΔexsE). Where indicated, the strain also harbored a chromosomal copy of pcrG, which had been modified to express an internally VSV-G tagged version of the protein, PcrG(i3VG). Both input cell-lysates and output fractions after immunoprecipitation with the anti-VSV-G tag antibody were separated by SDS-PAGE and probed for the presence of PcrV or the VSV-G-tagged PcrG as indicated.
Efector secretion control correlates with needle-tip function, not the interaction between PcrG and PcrV
The interaction between LcrG and LcrV is crucial for effector secretion control in the homologous Yersinia T3SS. The interaction with PcrG and PcrV has been demonstrated in vitro (Nanao et al., 2003), and we have now demonstrated it in vivo. We therefore set out to determine if the PcrG-PcrV interaction is important for effector secretion control in P. aeruginosa.
As mentioned in the introduction, the data regarding the localization of LcrG in Yersinia sp. is somewhat confusing. While experiments performed in Y. pseudotuberculosis and Y. enterocolitica demonstrate that LcrG is cytoplasmic, evidence gathered in Y. pestis suggests that the protein is exported. This export is not, however, required for effector secretion control since a non-secreted Gal4-LcrG fusion was still capable of complementing a lcrG null mutant (Reina et al., 2008).
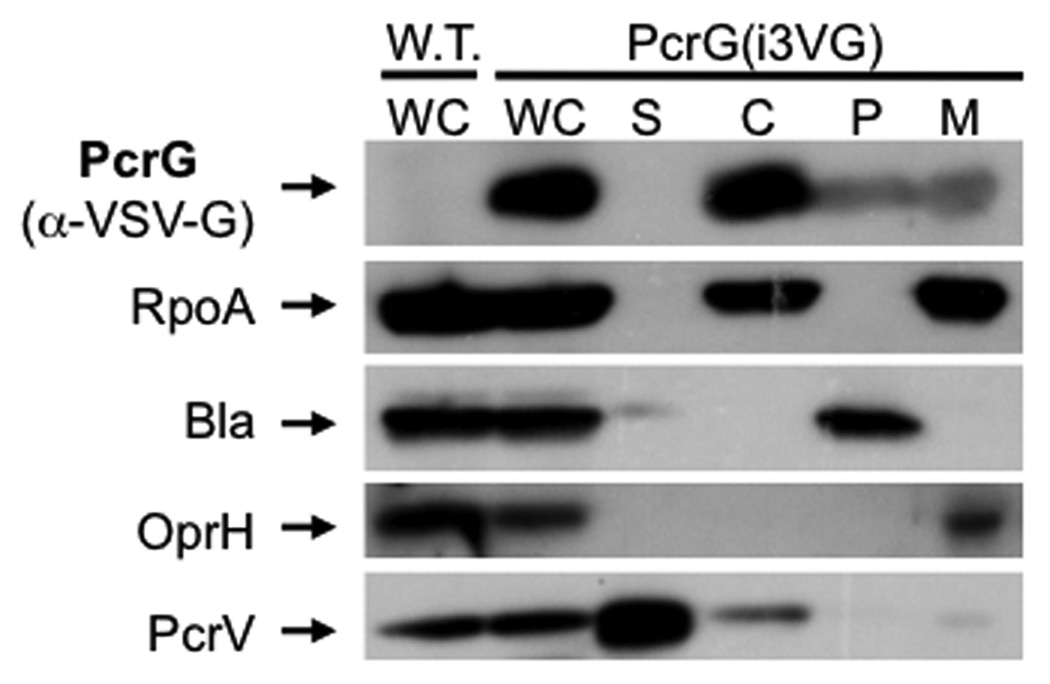
We decided to test whether PcrG is exported in order to determine if it could be involved in controlling effector export in conjunction with PcrV at the needle-tip. A standard fractionation experiment, however, demonstrated that, unlike PcrV, PcrG is a cytoplasmic protein and not secreted (Fig. 4). While we detected a small amount of PcrG in the membrane and periplasmic fractions in this experiment, the presence of PcrG in these fractions was not affected by the presence or absence of an intact T3SS, suggesting that this represents the resolution limit of the technique rather than an association with the T3SS (data not shown).
Fig. 4. Co-immunoprecipitation of PcrG and PcrV.
A functional, VSV-G-tagged version of PcrG (PcrG(i3VG)) was used to determine the localization of PcrG in the cell. Bacteria were fractionated using the lysozyme/EDTA method. Fractions are indicated above each lane (wc = whole cell, S = supernatant, C = cytoplasm, P = periplasm, M = membrane). Fractionation was controlled using markers for secreted proteins (PcrV), membrane proteins (OprH), periplasmic proteins (beta-lactamase) and the cytoplasm (RpoA).
Next we determined if the interaction between PcrG and PcrV is required for controlling effector secretion. While PcrV appears to exert its influence at the needle-tip, the interaction could still be required to stabilize PcrG or to influence its conformation in a way that allows it to interact with the apparatus.
To this end, we constructed mutants of PcrV that either disrupt the PcrG-PcrV interaction without influencing needle-tip function or disrupt needle-tip function and still allow binding of PcrG. The L262D and F279R mutations were originally chosen because the homologous residues are thought to be involved in the interaction with LcrG in Y. pestis (Hamad & Nilles, 2007). The L262D amino-acid substitution had been constructed previously in P. aeruginosa and was reported to allow intoxication of host cells (albeit with a slight defect) (Gébus et al., 2008). The effect of an F279R mutation on PcrV function has not been reported, nor has the effect of either mutation on binding of PcrV to PcrG.
We first examined the ability of these mutant forms of PcrV to interact with PcrG. To this end we used an E. coli two-hybrid system in which one interaction partner is fused to the omega subunit of RNA polymerase, the other interaction partner is fused to the monomeric zinc-finger DNA binding domain of murine Zif268 (Zif) (Vallet-Gely et al., 2005). An interaction between the two proteins results in recruitment of RNA polymerase to a test promoter, thereby activating lacZ transcription, which can be readily measured by β-galactosidase assay. PcrG interacts strongly with PcrV in this system and neither protein interacts with the unrelated nucleoid proteins MvaT and MvaU (Fig. 5A). In subsequent experiments we used a modified version of the Zif-PcrG fusion, in which a VSV-G tag was inserted between the fusion partners in order to be able to monitor the stability of the fusion protein. The insertion of the tag did not interfere with the PcrG-PcrV interaction (Fig. 5B and 5D). Introduction of the L262D amino-acid substitution into PcrV abolished the interaction with PcrG, whereas introduction of the F279R substitution had no effect on binding in this assay. Interestingly, the Zif-PcrG fusion was unstable when co-expressed with the ω-PcrV(L262D) fusion protein, or omega alone, suggesting that PcrG is unstable in this background when not interacting with PcrV (Fig. 5C). This observation is consistent with published reports that binding of PcrG to PcrV results in greater resistance of PcrG to thermal denaturation in vitro (Nanao et al., 2003). We also tested these interactions by determining whether PcrV can be co-purified with a His-tagged version of PcrG from cytoplasmic extracts of E. coli BL21 expressing either wild-type or mutant versions of the proteins (Fig. 5F). While the F279R mutation had a deleterious effect on PcrG-binding when compared to wild-type PcrV in this assay (only barely detectable in the two-hybrid data), it was not as severe as the interaction-defect seen with PcrG(A16R) or PcrV(L262D).
Fig. 5. The interaction between PcrG and PcrV.
The interaction between PcrG and PcrV was assayed by two-hybrid analysis. PcrV was fused to the omega-subunit of RNA polymerase (ω). PcrG was fused to the monomeric DNA binding protein Zif. Interaction of the fusion proteins results in recruitment of RNA polymerase to a test promoter, which can be readily assayed by β-galactosidase assay. PcrG and PcrV interact with each other and not the unrelated histone-like proteins MvaT and MvaU (A). The effect of mutations in PcrV (B) or PcrG (D) on the interaction was monitored by using the same two-hybrid system, the only modification being that the Zif-fusion had been modified by inserting a VSV-G tag into the linker between Zif and PcrG. Production of the ω and Zif fusion proteins was monitored by western blot (C, E). Interaction data was confirmed by pull-down in E. coli (F). PcrV and a His-tagged version of PcrG, as well as mutants of either protein, were co-expressed in E. coli BL21. Co-purification of PcrG and PcrV was monitored by detecting PcrV in the elution fraction after purification.
We next crossed the L262D and F279R mutations into the chromosomal copy of pcrV and assayed the effect of these mutations on effector secretion control, export of the protein and cytotoxicity. All forms of PcrV were stably expressed and secreted, although secretion of the L262D mutant was somewhat reduced when compared to that of wild-type PcrV or the F279R mutant (Fig. 6B). Consistent with the proposed role in needle-tip function, the F279R mutation led to a severe defect in cytotoxicity (Fig. 6C). In order to assess the ability of the mutant PcrV to assemble at the needle-tip we employed a FACS assay in which we stained intact P. aeruginosa with an affinity purified antiserum directed against PcrV. Purified PcrV added to our null mutant bacteria did not interfere with the assay, suggesting that any PcrV protein secreted by these strains should not interfere with the detection of needle-tip associated PcrV (Fig. 6D). In addition, analysis of the FACS samples by immunofluorescence microscopy demonstrated a punctate localization, consistent with the detection of needle-tip complexes as opposed to non-specifically associated PcrV (Fig. S3). The pcrV(F279R) mutation resulted in a ~5 fold reduction of PcrV displayed at the bacterial cell surface as assayed by FACS (Fig. 6D). Taken together, these data demonstrate that the PcrV(F279R) mutant protein is indeed defective at assembling into a needle tip. The L262D mutation, on the other hand did not interfere with cytotoxicity or assembly at the needle tip (Fig. 6C + 6D). Interestingly, the L262D mutant of PcrV, which had lost the ability to interact with PcrG, was still able to control effector secretion. The F279R mutant of PcrV, on the other hand, lost the ability to control effector secretion (Fig. 6A + 6B).
Fig. 6. Phenotypic characterization of pcrV point mutants.
The effect of point mutations L262D and F279R in pcrV was assayed by β-galactosidase assay using a chromosomal lacZ reporter inserted in the exoS locus (A). It was also examined by RECC assay (B). Secretion of PcrV, ExoT and RpoA (control) was monitored by Western blot (the respective protein detected is indicated to the left of each blot, the fraction (cell pellet or supernatant) is indicated to the right). The ability to intoxicate cells was determined by monitoring rounding of A549 cells after 5 hours of infection (MOI10). Surface localization of PcrV was monitored by FACS using an affinity-purified anti-PcrV antiserum and an anti-rabbit IgG APC-conjugated secondary antibody. To normalize expression of PcrV, all strains were grown in the absence of calcium, except the wild-type, which was grown both in the absence and presence of calcium (indicated in the legend). Purified PcrV protein was added to one sample of the pcrV null mutant before performing the antibody staining procedure in order to rule out the possibility that secreted protein being non-specifically cross-linked to the cells interfered with our assay.
Taken together, these data suggest that the ability of PcrV to control effector secretion correlates with its function as the needle-tip protein, and not with its ability to form a complex with PcrG. These data are consistent with our observation that PcrV must be exported to control effector secretion and suggest that assembling into a functional needle-tip is required for controlling effector secretion. Since the phenotype of the pcrV null mutant is deregulated effector secretion, it would suggest that the default state of the core secretion apparatus is effector secretion ‘on’ and that assembly of PcrV at the needle tip shifts the overall conformation of the apparatus to the effector secretion ‘off’ position.
PcrG controls effector secretion in a PcrV-independent manner
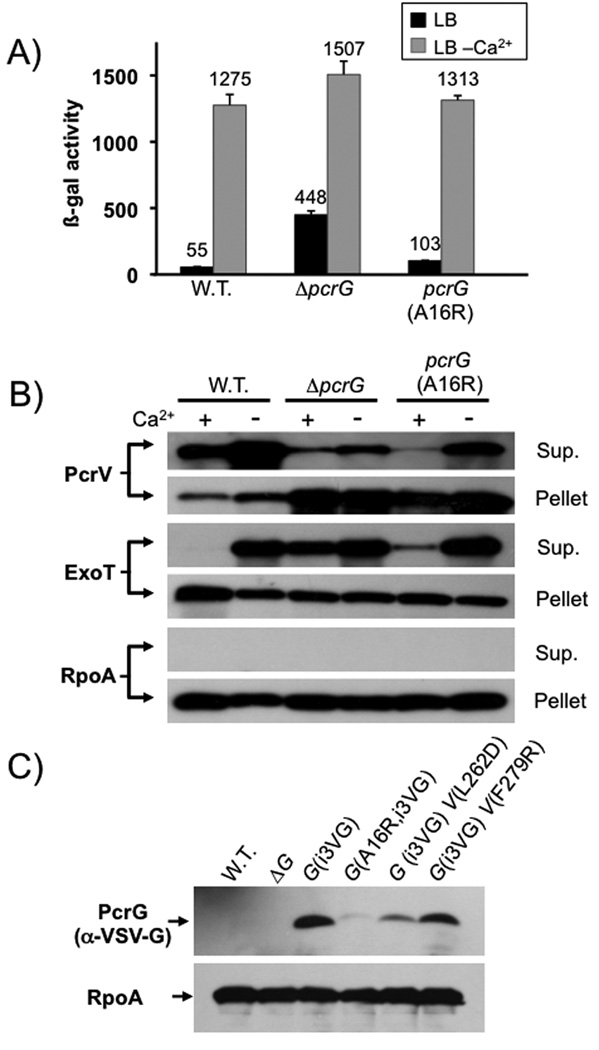
The above data also suggest that PcrG can exert its influence without being in a complex with PcrV. To further confirm these data we modified the chromosomal copy of pcrG to express an A16R mutant of PcrG based on the homologous mutation in LcrG that had been demonstrated to abrogate the LcrG-LcrV interaction (Matson & Nilles, 2001). This mutant also failed to interact with PcrV (Fig. 5D + 5F), however, it retained most of its regulatory capacity (Fig. 7A and 7B). Interestingly, the Zif-PcrG(A16R) fusion was also unstable, even when co-expressed with wild-type PcrV (Fig. 5E). We therefore decided to determine if PcrG also depends on PcrV-binding for stability in P. aeruginosa. Indeed, introduction of the A16R mutation into our VSV-G tagged PcrG, or co-expression of the tagged PcrG with the PcrV(L262D) mutant protein (all expressed in their native chromosomal context) resulted in destabilization of PcrG (Fig. 7C). It is interesting that the PcrG(A16R) mutant is still able to control effector export in P. aeruginosa despite this instability. In fact, what little deregulation we observed in this mutant can likely be attributed to the reduction in PcrG stability.
Fig. 7. Phenotypic characterization of pcrG point mutants.
The effect of mutating the chromosomal copy of pcrG was assayed by β-galactosidase assay, using a lacZ reporter inserted in the exoS locus (A), or by RECC assay (B). Proteins detected in the RECC assay are indicated to the left of each blot, the fraction to the right. (C) Stability of PcrG was assayed by modifying the chromosomal copy of pcrG to express an internally VSV-G tagged version of PcrG (PcrG(i3VG)). The western blot was performed on cell pellets of bacteria grown under T3SS inducing (low calcium) conditions.
In Yersinia sp., LcrG serves as an export chaperone of LcrV. We decided to use the A16R mutant of PcrG to test if the interaction with PcrV is required for PcrV export. Both deletion of pcrG, as well as introduction of the A16R mutation, resulted in a significant defect in PcrV export (Fig. 7B), suggesting that the PcrG-PcrV interaction, although not absolutely required, does facilitate PcrV export. Consistent with this finding, the PcrV(L262D) mutant protein, which is impaired in its ability to interact with PcrG, also displayed a slight defect in export (Fig. 6B). Notably, in all cases, PcrV is still secreted in the presence and absence of calcium (as we had observed for all translocator proteins (Cisz et al., 2008)), demonstrating that PcrG is not specifically required for PcrV export prior to triggering of effector secretion.
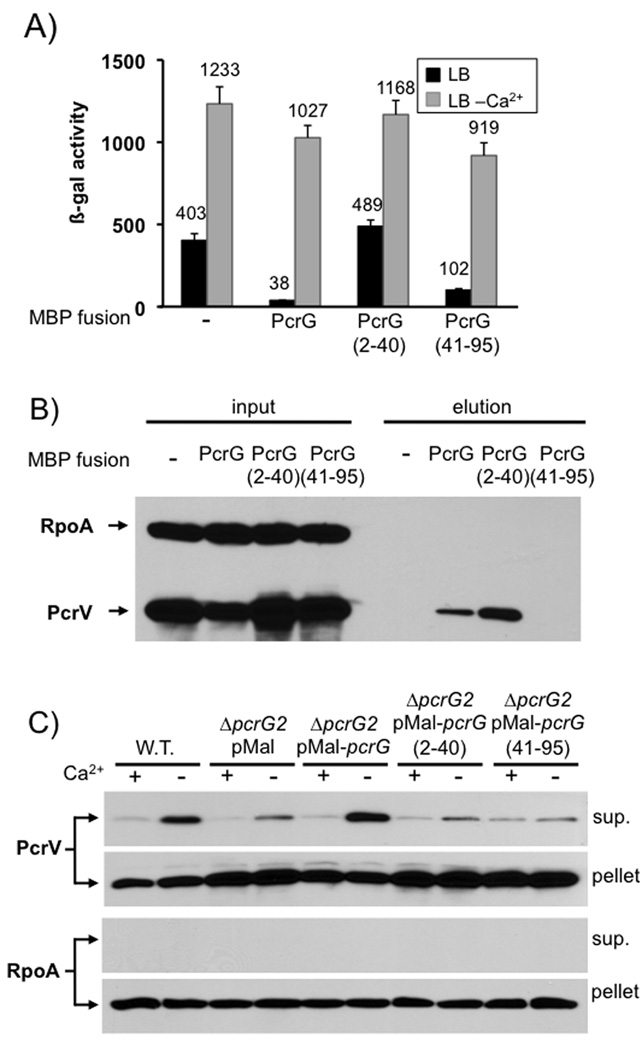
In a final set of experiments we decided to attempt to completely separate the PcrV-binding and regulatory activities of PcrG. To this end we created fusions of either full-length PcrG, amino acids 2–40 or 41–95 to maltose binding protein (MBP) and assayed them for their ability to control effector secretion or mediate PcrV-binding. Both full-length PcrG and PcrG(41–95) were able to control effector secretion (exoS expression was only slightly increased in the strain expressing PcrG(41–95)), while expression of the MBP-PcrG(2–40) fusion protein resulted in deregulated effector secretion similar to that observed in the vector control (Fig. 8A). However, pull-down of PcrG using an amylose resin demonstrated that only full-length PcrG and PcrG(2–40) were able to interact with PcrV (Fig. 8B). These two activities of PcrG can therefore be separated and binding of PcrV to PcrG is not required for effector secretion control, or low-calcium dependent triggering of effector export. Interestingly, both halves of PcrG are required to restore efficient export of PcrV (Fig. 8C). Since the regulatory activity of PcrG likely requires a specific interaction with the T3SS, these data suggest that the interaction also serves to bring PcrV to the apparatus to facilitate its export.
Fig. 8. PcrV-binding and regulatory functions of PcrG can be separated.
Full length PcrG or truncated versions (aa 2–40 or 41–95) were fused to the C-terminus of MBP and expressed in a mutant of P. aeruginosa lacking pcrG. The ability to control effector export was monitored by β-galactosidase assay using a lacZ reporter inserted into the chromosomal exoS locus (A). The ability of the MBP-fusions to bind to PcrV was determined by pull-down from cytoplasmic extracts using an amylose-resin, followed by Western blot analysis (B). Secretion of PcrV was analyzed by RECC assay (C). The genotype and plasmid is noted above each set of lanes. Supernatant and cell pellet fractions, as well as the protein being detected are listed to the side of the relevant panel.
Taken together, these data illustrate that the interaction between PcrG and PcrV is not required for effector secretion control. PcrV controls effector export by assembling into a functional needle tip, while PcrG controls effector secretion in the cytoplasm by an as-yet to be discovered interaction with the T3SS.
Discussion
Cell-contact dependent delivery of effector proteins is a core feature of most virulence-related type III secretion systems. How this process is regulated, however, is not well understood. In most cases where it has been tested, the needle-tip protein prevents premature secretion of effector proteins.
We have presented several lines of evidence that PcrV controls effector secretion by assembling at the needle-tip. PcrV has to be export competent to control effector secretion. Similarly, effector secretion control was specifically abrogated in a point mutant (F279R) that interfered with the ability of PcrV to assemble into a functional needle-tip (without interfering with the ability to bind to PcrG or be targeted for secretion). A complimentary mutant (L262D) that was still able to assemble at the needle-tip, but had lost the ability to bind to PcrG, still controlled effector secretion. The effector secretion control defect of the non-secreted PcrV mutant, as well as the needle-tip assembly mutant (F279R), is of a similar magnitude as that of the complete null. Finally, unlike Yersinia LcrV, overexpression of wild-type PcrV or a non-secreted mutant of PcrV had no effect on effector secretion control. Taken together these data argue strongly that PcrV controls effector secretion by assembling into a functional needle-tip complex, and argue against models where PcrV physically obstructs access of effectors to the cytoplasmic face of the T3SS. Our data on PcrV function are consistent with published work in Shigella. Small (as little as 3 amino acid) deletions at the C-terminus of IpaD were able to prevent surface assembly and resulted in loss of effector secretion control (Espina et al., 2006). Similarly, deletion of the secretion signal of IpaD also resulted in constitutive effector secretion (Picking et al., 2005). While the data was interpreted to corroborate the plug model of effector secretion control, the authors did note that all of their internal deletion mutants resulted in loss of effector secretion control, but not, for example, the ability to invade tissue culture cells, suggesting that the needle senses the overall conformation of the needle-tip protein (Picking et al., 2005).
Of the three models for effector secretion control that have been proposed, our data best fits the allosteric model of effector secretion control. An outright block of the secretion channel by the needle-tip protein as proposed in the ‘plug’ model seems unlikely, since we have demonstrated that translocator proteins can be secreted even when effector protein secretion is off (Cisz et al., 2008). The sensor model also doesn’t fit our data well, since the partial secretion control defect of the pcrV null mutant is difficult to reconcile with this model where loss of tethering of the sensor at the needle-tip should result in unrestricted effector export. Assuming that PcrV controls effector secretion by influencing the conformation of the apparatus, then our data imply that the default state of the apparatus, in the absence of PcrG and PcrV, is the effector secretion ‘on’ conformation. Assembly of PcrV at the needle-tip (and perhaps binding of PcrG to a cytoplasmic component(s) of the apparatus) shifts the conformation of the apparatus to the effector secretion ‘off’ state (Fig. 9). Cell contact, in turn, would likely result in a conformational change in the needle-tip (perhaps by interacting with the assembled translocation pore) which is then propagated to the base of the apparatus resulting in release of PcrG and triggering of effector secretion.
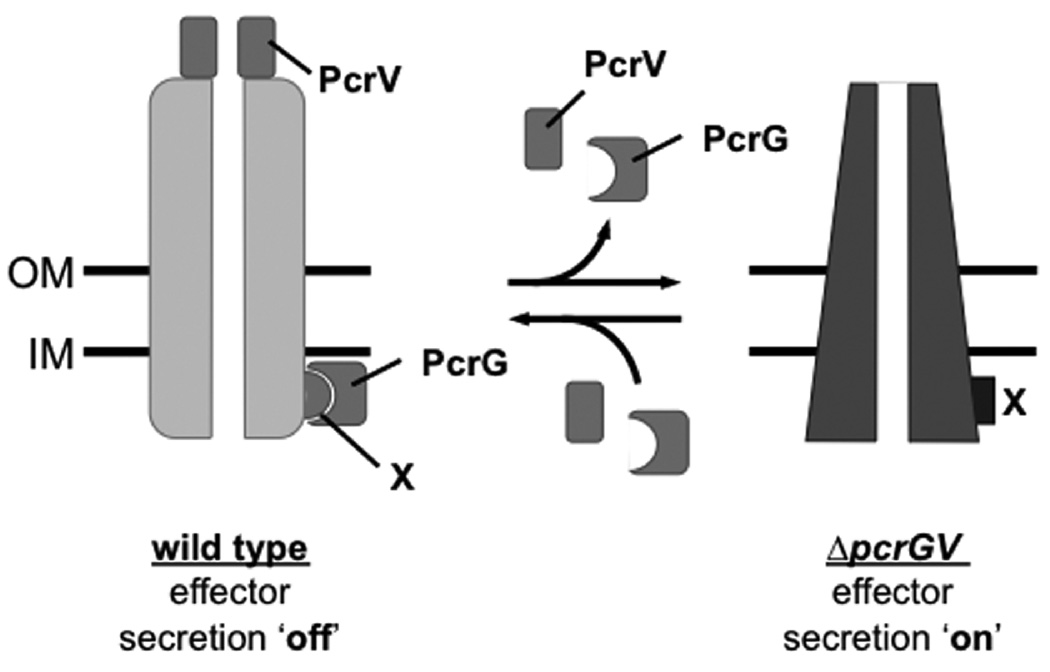
Fig. 9. Model of PcrV function in type III secretion regulation.
We propose that the T3SS is in the effector secretion ‘on’ position in the absence of PcrG and PcrV (e.g. in the case of the pcrGV null mutant). The assembly of PcrV at the needle-tip (and perhaps binding of PcrG to a cytoplasmic component(s) of the T3SS [X]) shifts the conformation of the apparatus to the effector secretion ‘off’ conformation.
While we favor an allosteric model of effector secretion control, some reason for caution remains. As mentioned in the introduction, one problem with the conformational control model is that the biophysical characterization of isolated wild-type and effector secretion ‘on’ mutant needles failed to discern a large scale conformational difference (Cordes et al., 2005). One potential explanation for this result, is that the isolated wild-type needles used in this study may in fact have switched to the effector secretion ‘on’ conformation once separated from the base of the apparatus. This assumes that the Shigella T3SS has a similar propensity to be in the effector secretion ‘on’ conformation as we are proposing for the P. aeruginosa T3SS. Perhaps the analysis of mutants locked in the effector secretion ‘off’ state could be used to trap the second conformation proposed by the allosteric control model. It could also be argued, for example, that the translocator secretion we detect in our RECC assays is the result of translocator proteins being secreted from assembling T3SS, which haven’t yet assembled the PcrV needle-tip. However, this seems unlikely since export of the pore-forming translocator proteins in our RECC assays is quite robust. Moreover, the pore-forming translocator proteins have to be exported before effector proteins and in cis for triggering of effector secretion to occur on cell-contact (Cisz et al., 2008), arguing that translocator export has to occur before effector export and likely by the same apparatus. In other systems it has been reported that translocator export requires a specific trigger (e.g. serum albumin in the case of Y. enterocolitica, or bile salts in the case of S. flexneri (Lee et al., 2001, Olive et al., 2007)), raising the possibility that three secretion states exist: no secretion, translocator secretion and effector secretion. In P. aeruginosa, however, translocator secretion appears to be constitutive and is even detectable in minimal media (Cisz et al., 2008). Relying on a low level of constitutive translocator export to allow assembly of the translocation pore upon cell contact and subsequent triggering of effector secretion seems to be the simpler model, but more work will have to be done to fully understand how needle-tip proteins control secretion.
We were able to demonstrate that PcrG is not secreted and therefore must control effector secretion in the cytoplasm. This observation fits well with the most recent data regarding LcrG function, which also argue that LcrG controls effector secretion at the cytoplasmic face of the apparatus (Reina et al., 2008). Whether PcrG controls effector secretion by stabilizing the effector secretion ‘off’ state of the secretion apparatus, or whether it controls effector secretion by directly blocking access of effectors to the T3SS is unclear. Interestingly, unlike the situation in Yersinia sp., our data demonstrate that effector secretion control by PcrG does not require the ability to interact with PcrV. Both a mutant of PcrV (L262D) that has lost the ability to interact with PcrG and a reciprocal mutant of PcrG (A16R) that had lost the ability to interact with PcrV could still prevent premature effector secretion. Moreover, using MBP fusions of truncated versions of PcrG we were able to demonstrate that the regulatory activity and the PcrV-binding activity of PcrG can be separated. The ability to control effector export is tied to the C-terminal part of the protein. While the interaction between PcrG and PcrV is not required for effector secretion control, it is required for efficient secretion of PcrV, which suggests that PcrG serves as export chaperone for PcrV. A similar LcrV secretion defect had also been noticed in the context of an LcrG mutant (A16R) that had lost the ability to bind to LcrV (Matson & Nilles, 2001).
Reconciling control of effector secretion by PcrV with the mode of regulation exhibited by its nearest neighbor, LcrV, is more challenging. In some instances, a slight deregulation of effector secretion has been observed, even in an lcrV null mutant (Davis & Mecsas, 2007, Lee et al., 2000). However, in most instances, it would appear that deletion of lcrV down-regulates secretion, even when expression has been artificially up-regulated by deleting the negative regulator gene lcrQ (Broms et al., 2007, Fields et al., 1999, Pettersson et al., 1999, Sarker et al., 1998). It may well be that LcrV performs multiple functions, such as control of T3SS gene expression and effector secretion control and that the regulatory function of LcrV has to be taken out of the equation before its role in effector secretion control can be fully appreciated.
Taken together, we have presented data that PcrV controls effector secretion in P. aeruginosa by assembling at the needle-tip of the type III secretion system. This control is likely mediated allosterically by stabilizing the effector secretion ‘off’ conformation of the apparatus. PcrG, which facilitates PcrV export, does not have to bind to PcrV to control effector export and likely does so independently through an interaction with the type III secretion system at the cytoplasmic face of the apparatus.
Experimental Procedures
Media and culture conditions
All E. coli strains were routinely grown at 37°C in LB medium containing 10g/l NaCl. P. aeruginosa was grown at 37°C in a modified LB medium (LB-MC) containing 200mM NaCl, 0.5mM CaCl2 and 10mM MgCl2. Strains and plasmids used in this study are listed in Table 1.
Table 1.
| strain # | genotype | reference |
|---|---|---|
| BL21(DE3) Codon+ - RP | E. coli B F– ompT hsdS(rB– mB–) dcm+ Tetr gal λ (DE3) endA Hte [argU proL Camr] | Stratagene |
| KDZif1∆Z | two-hybrid analysis strain lacking rpoZ (encoding ω) and harboring a test promoter-lacZ fusion to detect Zif-dependent two-hybrid interactions | (Vallet-Gely et al., 2005) |
| RP1831 | PAO1F, wild type P. aeruginosa PAO1 | (Bleves et al., 2005) |
| RP1868 | PAO1F ∆exoS::GFP-lacZ | this study |
| RP3082 | PAO1F ∆pcrG2 ∆exoS::GFP-lacZ | this study |
| RP2645 | PAO1F ∆pcrV2 ∆exoS::GFP-lacZ | this study |
| RP3335 | PAO1F ∆pcrGV2 ∆exoS::GFP-lacZ | this study |
| RP3666 | PAO1F pcrG(A16R) ∆exoS::GFP-lacZ | this study |
| RP3466 | PAO1F pcrV(L262D) ∆exoS::GFP-lacZ | this study |
| RP3468 | PAO1F pcrV(F279R) ∆exoS::GFP-lacZ | this study |
| RP3898 | PAO1F pcrG(i3VG) ∆exoS::GFP-lacZ | this study |
| RP3900 | PAO1F pcrG(i3VG, A16R) ∆exoS::GFP-lacZ | this study |
| RP4348 | PAO1F pcrG(i3VG) pcrV(L262D) ∆exoS::GFP-lacZ | this study |
| RP4349 | PAO1F pcrG(i3VG) pcrV(F279R) ∆exoS::GFP-lacZ | this study |
| RP3929 | PAO1F ∆exsE ∆fleQ pcrG(i3VG) | this study |
| RP3770 | PAO1F ∆exsE ∆fleQ ∆pcrV2 | this study |
| plasmid | relevant features | reference |
| pPSV37 | colE1 origin, gentR, PA origin, oriT, lacUV5 promoter, lacIq, stops in every reading frame preceding the MCS and T7 terminator following the MCS (relative to the lacUV5 promoter) | this study |
| pEXG2 | Allelic exchange vector, colE1 origin, oriT, gentamycin resistance, sacB | (Rietsch et al., 2005) |
| pDuet1 | Co-expression vector with T7 origin, bla resistance | Novagen |
| pACTR-AP-Zif | pACYC origin, tetR, plasmid allowing for the creation of N-terminal fusions to Zif, the zinc-finger DNA binding domain of the murine Zif268 protein, under control of a lac promoter | (Vallet-Gely et al., 2005) |
| pBRω | colE1 origin, bla, plasmid allowing for the creation of N-terminal fusions to the ω subunit of RNA polymerase under control of a lac promoter | (Vallet-Gely et al., 2005) |
| pZifVG | pACTR-AP-Zif modified to include a linker encoding the VSV-G tag between the fusion partner and the Zif moiety (see Experimental Procedures) | this study |
| pMal | pPSV37 encoding a signal-sequenceless malE gene (codons 27–396) lacking a stop codon followed by a polylinker to create MBP fusions | this study |
| pP37-pcrG | pcrG under control of the lacUV5 promoter in pPSV37 | this study |
| pP35-pcrV | pcrV under control of the lacUV5 promoter in pPSV35 | this study |
| pP37-pcrV | pcrV under control of the lacUV5 promoter in pPSV37 | this study |
| pP37-pcrGV | pcrGV under control of the lacUV5 promoter in pPSV37 | this study |
| pP35-pcrV(∆3–21) | pcrV lacking codons 3–21 under control of the lacUV5 promoter | this study |
| pP35-exoS(1–21)-pcrV(22–294) | codons 1–21 of exoS fused to codons 22–294 of pcrV, expressed under the control of the lacUV5 promoter | this study |
| pEXG2-∆exoS::GFP-lacZ | allelic exchange vector which deletes exoS and inserts translationally coupled versions of GFP and lacZ in its place | (Rietsch et al., 2004) |
| pEXG2-∆pcrG2 | allelic exchange vector designed to delete codons 6–88 of pcrG | this study |
| pEXG2-pcrG(ATG1-) | allelic exchange vector to change the annotated ATG of pcrG to ATC | this sudy |
| pEXG2-pcrG(ATG2-) | allelic exchange vector to change the second ATG (codon 4 of the annotated sequence) to GGG | this study |
| pEXG2-pcrG(i3VG) | allelic exchange vector designed to replace the chromosomal copy of pcrG with a version of the open reading frame in which codons 55–57 have been replaced by 3 tandem repeats of the VSV-G tag | this study |
| pEXG2-pcrG(i3VG, A16R) | allelic exchange vector designed to replace the chromosomal copy of pcrG(A16R) with a version of the open reading frame in which codons 55–57 have been replaced by 3 tandem repeats of the VSV-G tag | this study |
| pEXG2-∆pcrGV2 | allelic exchange vector designed to delete pcrG and pcrV starting at codon 6 of pcrG and ending at codon 180 of pcrV | this study |
| pEXG2-pcrV(L262D) | allelic exchange vector designed to introduce the L262->D mutation into pcrV | this study |
| pEXG2-pcrV(F279R) | allelic exchange vector designed to introduce the F279->R mutation into pcrV | this study |
| pEXG2-pcrG(A16R) | allelic exchange vector designed to introduce the A16->R mutation into pcrG | this study |
| pACZif-mvaU | two-hybrid plasmid encoding a MvaU-Zif fusion protein | (Vallet-Gely et al., 2005) |
| pBRω-mvaT | two-hybrid plasmid encoding a MvaT-ω fusion protein | (Vallet-Gely et al., 2005) |
| pAcZif-pcrG | two-hybrid plasmid encoding a PcrG-Zif fusion protein | this study |
| pBRω-pcrV | two-hybrid plasmid encoding a PcrV-ω fusion protein | this study |
| pBRω-pcrV(L262D) | two-hybrid plasmid encoding a PcrV(L262D)-ω fusion protein | this study |
| pBRω-pcrV(F279R) | two-hybrid plasmid encoding a PcrV(F279R)-ω fusion protein | this study |
| pZifVG-pcrG | two-hybrid plasmid encoding a PcrG-VSV-G tag-Zif fusion protein | this study |
| pZifVG-pcrG(A16R) | two-hybrid plasmid encoding a PcrG(A16R)-VSV-G tag-Zif fusion protein | this study |
| pDuet-pcrG | T7 promoter expression vector for the expression of an amino-terminally His-tagged version of PcrG | this study |
| pDuet-pcrV | T7 promoter expression vector for the expression of an untagged version of PcrV | this study |
| pDuet-pcrGV | T7 promoter expression vector for the concomitant expression of an amino-terminally His-tagged version of PcrG and an untagged version of PcrV | this study |
| pDuet-pcrG(A16R) pcrV | T7 promoter expression vector for the concomitant expression of an amino-terminally His-tagged version of PcrG(A16R) and an untagged version of PcrV | this study |
| pDuet-pcrG pcrV(L262D) | T7 promoter expression vector for the concomitant expression of an amino-terminally His-tagged version of PcrG and an untagged version of PcrV(L262D) | this study |
| pDuet-pcrG pcrV(F279R) | T7 promoter expression vector for the concomitant expression of an amino-terminally His-tagged version of PcrG and an untagged version of PcrV(F279R) | this study |
| pMal-pcrG | plasmid encoding an MBP-PcrG fusion protein under control of a lacUV5 promoter | this study |
| pMal-pcrG(2–40) | plasmid encoding an MBP-PcrG(aa 2–40) fusion protein under control of a lacUV5 promoter | this study |
| pMal-pcrG(41–95) | plasmid encoding an MBP-PcrG(aa 41–95) fusion protein under control of a lacUV5 promoter | this study |
Plasmid construction
Primers used in this study are listed in Table S1. Plasmid pPSV37 was created in two steps from plasmid pPSV35 (Rietsch et al., 2005). First a T7 terminator was inserted after the multiple cloning site by digesting pPSV35 using enzymes HindIII and PvuI and ligating the cut vector with a linker created by annealing primers T7ter1 and T7ter2. The resultant construct was then digested with EcoRI and ligated with a linker containing stop codons in every reading frame leading up to the polylinker (created by annealing the primers stops1 and stops2) resulting in plasmid pPSV37. Plasmid pZifVG was created by digesting plasmid pACTR-AP-Zif with NotI and XhoI and ligating the cut vector with a linker created by annealing primers ZifVG-sense and ZifVG-AS. Plasmid pMal was created by amplifying a signal-sequenceless version of malE in which the stop codon had been omitted to allow the creation of C-terminal fusions to MBP. To this end, malE was amplified using primers malE-5R and malE-3K and cloned into plasmid pPSV37 as an EcoRI/KpnI fragment.
Inserts cloned into the above vectors were amplified using primers listed in Table S1. Constructs designed to introduce mutations on the chromosome (cloned into the allelic exchange vector pEXG2) were created using splicing by overlap extension (SOE) PCR (Warrens et al., 1997). Internal primers (defining the mutation) and external primers (defining the ends of the two flanking regions amplified, as well as the restriction sites used to cloned the spliced cross-over PCR product) are listed in Table S1.
β-galactosidase assay
Cells were permeabilized with chloroform/SDS, and β-galactosidase activity was assayed as described previously (Miller, 1992)
RECC assay
P. aeruginosa was diluted 1:300 from overnight cultures and grown to an OD600 of ~ 0.4 in LB-MC medium supplemented with the calcium chelator EGTA (5mM). At this point, 2 × 1.5ml of each culture were spun down in microcentrifuge tubes, the supernatants were removed and one cell pellet was resuspended in 2 ml prewarmed LB-MC medium with calcium, the other in 2ml pre-warmed medium with EGTA. The cultures were incubated for an additional 25 minutes, at which point the cultures were placed on ice. Cells from 1 ml of each culture were pelleted by centrifugation and 0.5ml of the supernatant were transferred to a fresh microcentrifuge tube, wherein supernatant proteins were precipitated by the addition of trichloroacetic acid to a final concentration of 10%. The remainder of the culture was used to determine the OD600. Cell pellets and supernatant proteins were resuspended in 1 × SDS sample buffer to correspond to an OD600 of 10. The samples were then boiled for 10’ before separating the proteins on SDS-PAGE. Proteins were transferred to PVDF membrane and probed with specific antisera. Primary antibodies were detected using horse-raddish peroxidase conjugated secondary antibodies and a chemiluminescent detection reagent (SuperSignal West Pico (Pierce)). Exposure times for cell and supernatant fractions varied. Antibodies to ExoT and PcrV were generated in rabbits using a commercial service (Covance). The PcrV antiserum was further purified using an affinity purification protocol. Antibodies directed against RpoA (Neoclone) and the VSV-G tag (Rockland) were obtained commercially.
FACS analysis
Bacteria were diluted 1:300 into fresh LB-MC medium supplemented with 5mM EGTA to chelate calcium. The cultures were then grown at 37°C to mid-log phase, whereupon 1 ml of culture was removed to a microcentrifuge tube (at this point 50ng/ml PcrV were added to one of the ΔpcrV control samples and incubated at room temperature for 10’). The cells were pelleted by centrifugation (4’ 4000 rpm), washed once in PBST (PBS with 0.1% triton, 10mM MgCl2, 0.5mM CaCl2) and resuspended in 500 µl of the same buffer. At this point 500µl 4% paraformaldehyde, were added, mixed by inversion and incubated for 20 minutes at room temperature. The remaining crosslinker was quenched by the addition of 50µl 1M Tris.Cl (pH 7.5) and the cell suspension was again mixed by inversion, incubated for five minutes at room temperature, and cells were pelleted (3’ 13000 rpm), washed 1 × with PBST and 1 × with PBS (containing Mg2+ and Ca2+, as indicated for PBST) and resuspended in PBS with 2% goat serum and 2% BSA. Blocking was performed for 30’ on ice at which point the bacteria were pelleted and resuspended in 100µl of the blocking solution containing an affinity purified rabbit anti-PcrV antibody (1:500 dilution). The primary antibody incubation was performed at room temperature for 1 hour, at which point 1 ml of PBS was added to each sample, the cells were pelleted and washed 2× with PBS before being resuspended in 250µl of the secondary antibody solution (anti-rabbit-APC conjugated antibody (Invitrogen) diluted 1:2500 in blocking solution). The tubes were then wrapped in aluminum foil and rocked at 4°C for 1 hour. The bacteria were then pelleted, washed 1× with PBS and resuspended in 50µl of PBS. For each sample, 10µl of the labeled bacteria were diluted into 1ml of PBS in a 5ml polystyrene tube (BD Falcon) and analyzed by flow Cytometry (Becton Dickinson LSR II, a 4 laser, 42 parameter (12 color) bench-top flow cytometer.) at the Case Comprehensive Cancer Center Flow Cytometry Core.
Immunoprecipitation
Bacteria were diluted 1:300 into fresh LB-MC medium and subsequently grown at 37°C for ~ 3hours, at which point bacteria from 5ml of culture were pelleted and resuspended in IPP150 buffer (10 mM Tris pH 7.5, 150 mM NaCl, 1 mM PMSF) at an OD600 of 5. The cell suspensions were kept on ice and sonicated four times for 30 seconds (power level 4, Sonicator Cell Disruptor (W200R) Heat System Ultrasonics Inc.). At this point, cell debris was pelleted at 4°C (13200 rpm, 10’) and the supernatant was transferred to a new microcentrifuge tube. For each sample, 45µl of the lysate was removed and combined with 15µl of 4× SDS sample buffer (input control). The remainder of each lysate was precleared for 15 minutes by the addition of 20µl of IPP150-washed protein A/G agarose beads (Santa Cruz Biotechnology). The beads were removed by centrifugation (8000 rpm, 3’) and the supernatant was transferred to a new microcentrifuge tube. 3µl rabbit anti-VSV-G antiserum (Sigma) were added to each tube and the lysates were incubated on a rocker at 4°C for 45 minutes. Next, 30µl of washed protein A/G agarose beads were added to each tube and the samples were rocked for an additional 45 minutes at 4°C. The beads were then pelleted, washed 3× with wash buffer (10 mM Tris pH 7.5, 50 mM NaCl, 1% Triton X-100, 1 mg/ml BSA) and resuspended in 60µl 1× SDS sample buffer. The samples were incubated at 55°C for 10 minutes to dissociate the bound proteins, vortexed, the beads were pelleted, and the supernatant was removed (elution fraction).
Fractionation
P. aeruginosa PAO1F ΔexsE ΔfleQ pcrG(i3VG) harboring pPSV18 (ampR – source of beta-lactamase) was grown overnight in high salt LB (no antibiotics). Sphaeroblast formation was carried out based on the technique of Cheng et al. (Cheng et al., 1971). The overnight culture was diluted 1:300 into fresh high salt LB (12 ml)and grown at 37C to an OD600 of ~0.3–0.4. At this point, 1 ml of culture was removed, the cells were pelleted and the supernatant removed. The cell pellet was resuspended in 100µl 1× SDS sample buffer. Supernatant protein was collected by TCA precipitation (10% final TCA concentration) and also resuspended in 100 µl 1× SDS sample buffer. The bacteria from 10 ml of the remaining culture were pelleted and resuspend in 0.9 ml PEB buffer (20% sucrose, 30 mM Tris pH 8.0) to which 100 µl of a 10mg/ml lysozyme solution in PEB buffer were added and 5 µl of 400 mM EDTA, pH 8.0. The suspension was incubated at room temperature for 20 minutes, at which point the bacteria were pelleted (5 minutes at 5000×g). 75µl of the supernatant fraction (periplasm) were removed and mixed with 25µl of 4× sample buffer. The remaining supernatant was removed and discarded. The cell pellet was then resuspended in 1 ml of cold PBS (with 1 mM PMSF). Sphaeroblast formation was confirmed by microscopy. The cells were then lysed by sonication (4× 30 second interval, power level 4, Sonicator Cell Disruptor (W200R) Heat System Ultrasonics Inc.). The tube was kept on ice to prevent overheating of the sample. Lysis of the cells was controlled by microscopic examination of the sample. After sonication, unlysed cells were removed by centrifugation (10 minutes at 13,200 rpm at 4°C). The supernatant was then moved to ultracentrifuge tubes and spun for 1 hour at 4°C (45,000 rpm). 75µl of the supernatant fraction (cytoplasm) were removed and mixed with 25µl of 4× sample buffer. The pellet (membrane fraction) was resuspended in 1ml 1× SDS sample buffer. PcrG was detected by western blot using a commercial rabbit anti-VSV-G antibody (Sigma). The fractionation was controlled by detecting OprH (membrane fraction), β-lactamase (periplasm), RpoA (cytoplasm) and PcrV (secreted). The antibody against β-lactamase was a kind gift from Dr. Robert Bonomo (Case Western Reserve University/VA hospital). The antibody against OprH was a kind gift from Dr. Robert E. Hancock (University of British Columbia).
Overexpression in E.coli and Ni-chromatography
Combinations of PcrG, PcrV and mutants of either protein were expressed in E. coli BL21(DE3) Codon+ -RP (Stratagene) using the pDuet1 co-overexpression plasmid (Novagen). Bacteria were grown overnight in 2× YT broth with 30µg/ml chloramphenicol and 60µg/ml carbenicillin. The overnight culture was then diluted 1:300 into fresh 2×YT and grown for 2–2.5 hours, at which point expression was induced by the addition of IPTG (100µM final). The incubation was continued for an additional 25 minutes, at which point the cultures were placed on ice. For each strain, bacteria from 5 ml of culture were pelleted and resuspended cold Equilibrium buffer (50 mM Na2PO4 300 mM NaCl, 5 mM imidazole, 1 mM PMSF pH 7.0). The OD600 was normalized to 5. The bacteria were then lysed by sonication (power level 4 with 30 second interval for 4 times). Cellular debris was pelleted (13,200 rpm for 10 minutes at 4°C) and discarded. 45 µl of the supernatant were removed and mixed with 15 ul of 4× SDS sample buffer (input control). Equilibrium buffer washed TALON beads (50 ul of original volume of bead suspension) were added to the remaining supernatant and the suspension was rocked for 1 hour at 4° C. The beads were then pelleted (8,000 rpm for 3 min) and washed 4× with Equilibrium buffer. Bound proteins were eluted by adding 200 µl Elution buffer (50 mM Na2PO4 300 mM NaCl, 300 mM imidazole, 1 mM PMSF pH 7.0) to the beads and pelleting the beads. This process was repeated once and the two elution fractions were combined. PcrV and PcrG were detected by Western blot.
MBP-fusion purification
P. aeruginosa PAO1F ΔpcrG2 ΔexoS::GL3 was transformed with pMal, pMal-pcrG, pMal-pcrG(2–40) or pMal-pcrG(41–95). Overnight cultures of each strain were diluted 1:300 into fresh LB-MC with 50µM IPTG and grown to an OD600 of ~ 1 and placed on ice for 10 minutes. At this point, the OD600 of the cultures was normalized and 4.5 ml of cells were pelleted and resuspended in 500µl of wash buffer (20 mM Tris (pH 7.5), 0.2M NaCl, 10mM β-mercaptoethanol, 1mM EDTA, 2mM PMSF). The cells were lysed by sonication and cellular debris was removed by centrifugation (10 minutes, 14000 rpm, at 4°C). 45µl of the supernatant were removed to a fresh tube and mixed with 15µl of 4× SDS sample buffer (input). 400µl of the remaining supernatant were removed to a separate tube and mixed with 50µl of amylose resin (NEB) washed 1× with wash buffer. The suspension was rotated for 1 hour at 4°C, at which point the beads were pelleted and washed 3× with wash buffer. Bound protein was eluted by washing the beads 4× with 100µl elution buffer (wash buffer + 10mM maltose) and combining the elution fractions. 45µl of the eluate was combined with 15µl of 4× SDS sample buffer (eluate). Samples were separated by SDS-PAGE. PcrV and RpoA were detected by western blot.
Supplementary Material
Acknowledgements
We would like to thank Dr. Simon Dove and Dr. Joseph Mougous for critical reading of the manuscript. We also gratefully acknowledge Dr. Robert E. Hancock for the gift of the anti-OprH antiserum, Dr. Robert Bonomo for the gift of the anti-β-lactamase antibody, as well as Dr. Simon Dove for the components of the Zif-omega two hybrid system. This research was supported by the Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland (P30 CA43703). A.G.S. was supported by the Pulmonary Host Defense training grant from the National Institutes of Health (T32 HL083823). This work was also supported by an American Cancer Society Research Scholar Grant (RSG-09-198-01-MPC) to A.R. as well as a Pilot and Feasibility grant awarded to A.R. through a Cystic Fibrosis Foundation program project grant (R447-CR07).
References
- Bergman T, Hakansson S, Forsberg A, Norlander L, Macellaro A, Backman A, Bolin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleves S, Soscia C, Nogueira-Orlandi P, Lazdunski A, Filloux A. Quorum sensing negatively controls type III secretion regulon expression in Pseudomonas aeruginosa PAO1. J Bacteriol. 2005;187:3898–3902. doi: 10.1128/JB.187.11.3898-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker AJ, Deane JE, Veenendaal AK, Roversi P, Hodgkinson JL, Johnson S, Lea SM. What's the point of the type III secretion system needle? Proc Natl Acad Sci U S A. 2008;105:6507–6513. doi: 10.1073/pnas.0708344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteaux A, Sory MP, Biskri L, Parsot C, Allaoui A. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06537.x. [DOI] [PubMed] [Google Scholar]
- Broms JE, Forslund AL, Forsberg A, Francis MS. PcrH of Pseudomonas aeruginosa is essential for secretion and assembly of the type III translocon. J Infect Dis. 2003;188:1909–1921. doi: 10.1086/379898. [DOI] [PubMed] [Google Scholar]
- Broms JE, Francis MS, Forsberg A. Diminished LcrV secretion attenuates Yersinia pseudotuberculosis virulence. J Bacteriol. 2007 doi: 10.1128/JB.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Mueller CA, Muller SA, Philippsen A, Sorg I, Engel A, Cornelis GR. Function and molecular architecture of the Yersinia injectisome tip complex. Mol Microbiol. 2007;65:1311–1320. doi: 10.1111/j.1365-2958.2007.05871.x. [DOI] [PubMed] [Google Scholar]
- Brutinel ED, Vakulskas CA, Brady KM, Yahr TL. Characterization of ExsA and of ExsA-dependent promoters required for expression of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol. 2008;68:657–671. doi: 10.1111/j.1365-2958.2008.06179.x. [DOI] [PubMed] [Google Scholar]
- Brutinel ED, Vakulskas CA, Yahr TL. Functional domains of ExsA, the transcriptional activator of the Pseudomonas aeruginosa type III secretion system. J Bacteriol. 2009;191:3811–3821. doi: 10.1128/JB.00002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KJ, Ingram JM, Costerton JW. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971;107:325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LW, Schneewind O. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol. 2000;182:3183–3190. doi: 10.1128/jb.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisz M, Lee PC, Rietsch A. ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J Bacteriol. 2008;190:2726–2738. doi: 10.1128/JB.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes FS, Daniell S, Kenjale R, Saurya S, Picking WL, Picking WD, Booy F, Lea SM, Blocker A. Helical packing of needles from functionally altered Shigella type III secretion systems. J Mol Biol. 2005;354:206–211. doi: 10.1016/j.jmb.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Dacheux D, Goure J, Chabert J, Usson Y, Attree I. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol Microbiol. 2001;40:76–85. doi: 10.1046/j.1365-2958.2001.02368.x. [DOI] [PubMed] [Google Scholar]
- Davis AJ, Mecsas J. Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells. J Bacteriol. 2007;189:83–97. doi: 10.1128/JB.01396-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JB, Ferracci F, Plano GV. Translocation of YopE and YopN into eukaryotic cells by Yersinia pestis yopN, tyeA, sycN, yscB and lcrG deletion mutants measured using a phosphorylatable peptide tag and phosphospecific antibodies. Mol Microbiol. 2003;47:807–823. doi: 10.1046/j.1365-2958.2003.03343.x. [DOI] [PubMed] [Google Scholar]
- Day JB, Plano GV. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol Microbiol. 1998;30:777–788. doi: 10.1046/j.1365-2958.1998.01110.x. [DOI] [PubMed] [Google Scholar]
- Deane JE, Roversi P, Cordes FS, Johnson S, Kenjale R, Daniell S, Booy F, Picking WD, Picking WL, Blocker AJ, Lea SM. Molecular model of a type III secretion system needle: Implications for host-cell sensing. Proc Natl Acad Sci U S A. 2006;103:12529–12533. doi: 10.1073/pnas.0602689103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBord KL, Lee VT, Schneewind O. Roles of LcrG and LcrV during type III targeting of effector Yops by Yersinia enterocolitica. J Bacteriol. 2001;183:4588–4598. doi: 10.1128/JB.183.15.4588-4598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J, Mounier J, Sansonetti P, Tran Van Nhieu G. Secretion of type III effectors into host cells in real time. Nat Methods. 2005;2:959–965. doi: 10.1038/nmeth804. [DOI] [PubMed] [Google Scholar]
- Espina M, Olive AJ, Kenjale R, Moore DS, Ausar SF, Kaminski RW, Oaks EV, Middaugh CR, Picking WD, Picking WL. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect Immun. 2006;74:4391–4400. doi: 10.1128/IAI.00440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracci F, Schubot FD, Waugh DS, Plano GV. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol Microbiol. 2005;57:970–987. doi: 10.1111/j.1365-2958.2005.04738.x. [DOI] [PubMed] [Google Scholar]
- Fields KA, Nilles ML, Cowan C, Straley SC. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun. 1999;67:5395–5408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A, Viitanen AM, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Frank DW, Iglewski BH. Cloning and sequence analysis of a trans-regulatory locus required for exoenzyme S synthesis in Pseudomonas aeruginosa. J Bacteriol. 1991;173:6460–6468. doi: 10.1128/jb.173.20.6460-6468.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Gébus C, Faudry E, Bohn YS, Elsen S, Attree I. Oligomerization of PcrV and LcrV, protective antigens of Pseudomonas aeruginosa and Yersinia pestis. J Biol Chem. 2008;283:23940–23949. doi: 10.1074/jbc.M803146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goure J, Pastor A, Faudry E, Chabert J, Dessen A, Attree I. The V antigen of Pseudomonas aeruginosa is required for assembly of the functional PopB/PopD translocation pore in host cell membranes. Infect Immun. 2004;72:4741–4750. doi: 10.1128/IAI.72.8.4741-4750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad MA, Nilles ML. Structure-function analysis of the C-terminal domain of LcrV from Yersinia pestis. J Bacteriol. 2007;189:6734–6739. doi: 10.1128/JB.00539-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Hauser AR, Kang PJ, Engel JN. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- Johnson S, Roversi P, Espina M, Olive A, Deane JE, Birket S, Field T, Picking WD, Blocker AJ, Galyov EE, Picking WL, Lea SM. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J Biol Chem. 2007;282:4035–4044. doi: 10.1074/jbc.M607945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet L, Agrain C, Broz P, Cornelis GR. The needle length of bacterial injectisomes is determined by a molecular ruler. Science. 2003;302:1757–1760. doi: 10.1126/science.1091422. [DOI] [PubMed] [Google Scholar]
- Kaniga K, Trollinger D, Galan JE. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995a;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniga K, Tucker S, Trollinger D, Galan JE. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995b;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenjale R, Wilson J, Zenk SF, Saurya S, Picking WL, Picking WD, Blocker A. The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J Biol Chem. 2005;280:42929–42937. doi: 10.1074/jbc.M508377200. [DOI] [PubMed] [Google Scholar]
- Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Tejero M, Galan JE. Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect Immun. 2009;77:2635–2642. doi: 10.1128/IAI.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton DG, Longstaff C, Wallace BA, Hill J, Leary SE, Titball RW, Brown KA. Interactions of the type III secretion pathway proteins LcrV and LcrG from Yersinia pestis are mediated by coiled-coil domains. J Biol Chem. 2002;277:38714–38722. doi: 10.1074/jbc.M203632200. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44:3892–3898. doi: 10.1167/iovs.02-1302. [DOI] [PubMed] [Google Scholar]
- Lee VT, Mazmanian SK, Schneewind O. A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J Bacteriol. 2001;183:4970–4978. doi: 10.1128/JB.183.17.4970-4978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VT, Smith RS, Tummler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection. Infect Immun. 2005;73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VT, Tam C, Schneewind O. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- Lloyd SA, Norman M, Rosqvist R, Wolf-Watz H. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol Microbiol. 2001;39:520–531. doi: 10.1046/j.1365-2958.2001.02271.x. [DOI] [PubMed] [Google Scholar]
- Matson JS, Nilles ML. LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J Bacteriol. 2001;183:5082–5091. doi: 10.1128/JB.183.17.5082-5091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JS, Nilles ML. Interaction of the Yersinia pestis type III regulatory proteins LcrG and LcrV occurs at a hydrophobic interface. BMC Microbiol. 2002;2:16. doi: 10.1186/1471-2180-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaw ML, Lykken GL, Singh PK, Yahr TL. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol Microbiol. 2002;46:1123–1133. doi: 10.1046/j.1365-2958.2002.03228.x. [DOI] [PubMed] [Google Scholar]
- Menard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. A short course in bacterial genetics : a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. p. 2 v. [Google Scholar]
- Miyata S, Casey M, Frank DW, Ausubel FM, Drenkard E. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect Immun. 2003;71:2404–2413. doi: 10.1128/IAI.71.5.2404-2413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Cornelis GR. The type III secretion system tip complex and translocon. Mol Microbiol. 2008;68:1085–1095. doi: 10.1111/j.1365-2958.2008.06237.x. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, Sorg I, Kuhn M, Engel A, Cornelis GR. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science. 2005;310:674–676. doi: 10.1126/science.1118476. [DOI] [PubMed] [Google Scholar]
- Nanao M, Ricard-Blum S, Di Guilmi AM, Lemaire D, Lascoux D, Chabert J, Attree I, Dessen A. Type III secretion proteins PcrV and PcrG from Pseudomonas aeruginosa form a 1:1 complex through high affinity interactions. BMC Microbiol. 2003;3:21. doi: 10.1186/1471-2180-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilles ML, Williams AW, Skrzypek E, Straley SC. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun. 2007;75:2626–2629. doi: 10.1128/IAI.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson J, Holmstrom A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg A, Wolf-Watz H. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson KE, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- Picking WL, Nishioka H, Hearn PD, Baxter MA, Harrington AT, Blocker A, Picking WD. IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect Immun. 2005;73:1432–1440. doi: 10.1128/IAI.73.3.1432-1440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SB, Cowan C, Perry RD, Straley SC. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2(+)-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S, Kessin RH, Mekalanos JJ. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc Natl Acad Sci U S A. 2002;99:3159–3164. doi: 10.1073/pnas.052704399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina LD, O'Bryant DM, Matson JS, Nilles ML. LcrG secretion is not required for blocking of Yops secretion in Yersinia pestis. BMC Microbiol. 2008;8:29. doi: 10.1186/1471-2180-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A, Vallet-Gely I, Dove SL, Mekalanos JJ. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2005;102:8006–8011. doi: 10.1073/pnas.0503005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A, Wolfgang MC, Mekalanos JJ. Effect of Metabolic Imbalance on Expression of Type III Secretion Genes in Pseudomonas aeruginosa. Infect Immun. 2004;72:1383–1390. doi: 10.1128/IAI.72.3.1383-1390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- Sani M, Botteaux A, Parsot C, Sansonetti P, Boekema EJ, Allaoui A. IpaD is localized at the tip of the Shigella flexneri type III secretion apparatus. Biochim Biophys Acta. 2007;1770:307–311. doi: 10.1016/j.bbagen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Sarker MR, Neyt C, Stainier I, Cornelis GR. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa T, Yahr TL, Ohara M, Kurahashi K, Gropper MA, Wiener-Kronish JP, Frank DW. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- Schlumberger MC, Muller AJ, Ehrbar K, Winnen B, Duss I, Stecher B, Hardt WD. Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc Natl Acad Sci U S A. 2005;102:12548–12553. doi: 10.1073/pnas.0503407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensrud KF, Adam PR, La Mar CD, Olive AJ, Lushington GH, Sudharsan R, Shelton NL, Givens RS, Picking WL, Picking WD. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J Biol Chem. 2008;283:18646–18654. doi: 10.1074/jbc.M802799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Sundberg L, Forsberg A. TyeA of Yersinia pseudotuberculosis is involved in regulation of Yop expression and is required for polarized translocation of Yop effectors. Cell Microbiol. 2003;5:187–202. doi: 10.1046/j.1462-5822.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- Sundin C, Thelaus J, Broms JE, Forsberg K. Polarisation of type III translocation by Pseudomonas aeruginosa requires PcrG, PcrV and PopN. Microb Pathog. 2004;37:313–322. doi: 10.1016/j.micpath.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Thibault J, Faudry E, Ebel C, Attree I, Elsen S. Anti-activator ExsD forms a 1:1 complex with ExsA to inhibit transcription of type III secretion operons. J Biol Chem. 2009;284:15762–15770. doi: 10.1074/jbc.M109.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruellas J, Jackson MW, Pennock JW, Plano GV. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol Microbiol. 2005;57:1719–1733. doi: 10.1111/j.1365-2958.2005.04790.x. [DOI] [PubMed] [Google Scholar]
- Troisfontaines P, Cornelis GR. Type III secretion: more systems than you think. Physiology (Bethesda) 2005;20:326–339. doi: 10.1152/physiol.00011.2005. [DOI] [PubMed] [Google Scholar]
- Urbanowski ML, Brutinel ED, Yahr TL. Translocation of ExsE into Chinese Hamster Ovary Cells is Required for Transcriptional Induction of the Pseudomonas aeruginosa Type III Secretion System. Infect Immun. 2007;75:4432–4439. doi: 10.1128/IAI.00664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski ML, Lykken GL, Yahr TL. A secreted regulatory protein couples transcription to the secretory activity of the Pseudomonas aeruginosa type III secretion system. Proc Natl Acad Sci U S A. 2005;102:9930–9935. doi: 10.1073/pnas.0504405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Gely I, Donovan KE, Fang R, Joung JK, Dove SL. Repression of phase-variable cup gene expression by H-NS-like proteins in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2005;102:11082–11087. doi: 10.1073/pnas.0502663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal AK, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol. 2007;63:1719–1730. doi: 10.1111/j.1365-2958.2007.05620.x. [DOI] [PubMed] [Google Scholar]
- Wagner S, Sorg I, Degiacomi M, Journet L, Dal Peraro M, Cornelis GR. The helical content of the YscP molecular ruler determines the length of the Yersinia injectisome. Mol Microbiol. 2009;71:692–701. doi: 10.1111/j.1365-2958.2008.06556.x. [DOI] [PubMed] [Google Scholar]
- Warrens AN, Jones MD, Lechler RI. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene. 1997;186:29–35. doi: 10.1016/s0378-1119(96)00674-9. [DOI] [PubMed] [Google Scholar]
- Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell. 2003;4:253–263. doi: 10.1016/s1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Yahr TL, Frank DW. Transcriptional organization of the trans-regulatory locus which controls exoenzyme S synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994;176:3832–3838. doi: 10.1128/jb.176.13.3832-3838.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Goranson J, Frank DW. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Olive AJ, Smith ND, Picking WD, De Guzman RN, Picking WL. Identification of the MxiH needle protein residues responsible for anchoring invasion plasmid antigen D to the type III secretion needle tip. J Biol Chem. 2007;282:32144–32151. doi: 10.1074/jbc.M703403200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.