Abstract
Background
Candida infections are common and often fatal in infants and neonates. Anidulafungin has excellent activity against Candida sp, but unknown pharmacokinetics and safety in infants and neonates.
Objective
Determine the pharmacokinetics and safety of anidulafungin in infants and neonates at risk for invasive candidiasis.
Methods
Intravenous anidulafungin (1.5 mg/kg/day maintenance dose) was administered to 15 infants and neonates over 3 to 5 days. Plasma samples were obtained following the first and third to fifth dosesPharmacokinetic parameters were determined by non-compartmental analysis. Safety was assessed using National Cancer Institute common toxicity criteria.
Results
Drug exposure was similar between neonates and infants: median area under the curve (range) was 75 (30–109) μg*h/mL and 98 (55–278) μg*h/mL (P=0.12), respectively. No drug-related serious adverse events were observed.
Conclusions
Neonates and infants receiving 1.5 mg/kg/day have similar anidulafungin exposures compared to children receiving similar weight-based dosing and adult patients receiving 100 mg/day.
Keywords: antifungal agents, prematurity, infection, candidiasis, echinocandins
Introduction
Candida species are the fourth most common pathogen recovered from the bloodstream of hospitalized patients and are a leading cause of infectious mortality in critically ill infants and neonates(1). The incidence of candidemia in premature infants is approximately 10%(1, 2) with an attributable mortality of 20–30%(1, 3). In addition to high rates of mortality, candidemia frequently results in severe morbidities including poor long-term neurodevelopmental outcomes(4). Candida species invade virtually all tissues, but a unique aspect of candidiasis in young infants is central nervous system (CNS) invasion. Hematogenous Candida meningoencephalitis (HCME) is common in candidemic infants; approximately 20% have culture confirmed CNS involvement(4). However, HCME cannot be reliably excluded using currently available diagnostic technology; the infection is characterized by involvement of brain parenchyma, which is missed during cerebrospinal fluid culture evaluation. HCME frequently results in seizures, abscesses, neurodevelopmental delays, and death(2, 5).
Anidulafungin, an echinocandin antifungal agent, has excellent activity against Candida species in vitro(6), has potent activity in treatment of experimental disseminated candidiasis(7), as well as oropharyngeal and esophageal candidiasis(8). These laboratory findings were predictive of subsequent clinical trials in adult patients. The use of this drug is also attractive for critically ill infants and neonates due to its favorable safety profile in older patients and prolonged half life that allows for once-daily dosing(9). Notably, anidulafungin lacks significant hepatic metabolism(10, 11) and is not renally cleared; it undergoes chemical degradation in the blood(10). Therefore, dose adjustments are not required in patients with liver or renal insufficiency (9, 11). Anidulafungin is FDA-approved for adults at dosages ranging from 50 to 100 mg/day for the treatment of esophageal candidiasis, invasive candidiasis, and candidemia. Only one study has evaluated the safety and pharmacokinetics (PK) of anidulafungin in children at high risk of invasive fungal infections(12). This study showed that pediatric subjects receiving intravenous anidulafungin (1.5 mg/kg/day) had concentration profiles and drug exposures similar to those of adults receiving 100 mg/day. The present study was conducted to assess the safety and PK of intravenous anidulafungin administered to infants and neonates at risk for candidemia and invasive candidiasis.
Results
Patients
Between January 2008 and January, 2010, 15 subjects [8 neonates (<30 postnatal days), 7 infants (≥30 postnatal days)] at risk of candidemia and invasive candidiasis were enrolled in the study, received at least one dose of study drug, and were included in the safety analysis (Table 1). In the infant cohort, 43% (3/7) were female, 86% (6/7) were white, and 71% (5/7) were receiving mechanical ventilation. Of the 8 neonates, 75% (6/8) were born prematurely, 25% (2/8) were female, 63% (5/8) were white (1 white Hispanic), and 50% (4/8) were receiving mechanical ventilation. Two neonates were supported by extracorporeal membrane oxygenation (ECMO).
Table 1.
Subject demographics.
| Subject ID | Gestational age at birth (weeks) | Birth weight (grams) | Postnatal age (days) | Postmenstrual age (weeks) | Anasarca | Extracorporeal membrane oxygenation |
|---|---|---|---|---|---|---|
| Neonates | ||||||
| 1 | 39 | 3730 | 2 | 39 | - | X |
| 2 | 26 | 770 | 7 | 27 | - | - |
| 3 | 26 | 980 | 7 | 27 | - | - |
| 4 | 27 | 1180 | 11 | 29 | - | - |
| 5 | 27 | 1060 | 13 | 29 | - | - |
| 6 | 27 | 915 | 17 | 29 | - | - |
| 7 | 36 | 2345 | 24 | 39 | X | X |
| 8 | 39 | 3653 | 28 | 43 | - | - |
| Median | 27 | 1120 | 12 | 29 | ||
| Range | 26–39 | 770–3730 | 2–28 | 27–43 | ||
| Infants | ||||||
| 9 | 35 | 2080 | 50 | 42 | - | - |
| 10 | 37 | - | 68 | 47 | - | - |
| 11 | 40 | 3700 | 104 | 55 | - | - |
| 12 | 37 | 2100 | 105 | 52 | - | - |
| 13 | 39 | 3969 | 169 | 63 | - | - |
| 14 | 24 | 660 | 197 | 52 | X | - |
| 15 | 38 | 3856 | 451 | 102 | X | - |
| Median | 37 | 2900 | 105 | 52 | ||
| Range | 24–40 | 660–3969 | 50–451 | 42–102 | ||
| Total median | 36 | 2090 | 28 | 42 | ||
| Total range | 24–40 | 660–3969 | 2–451 | 27–102 |
Subject #12 died soon after administration of first dose of study drug
Pharmacokinetics
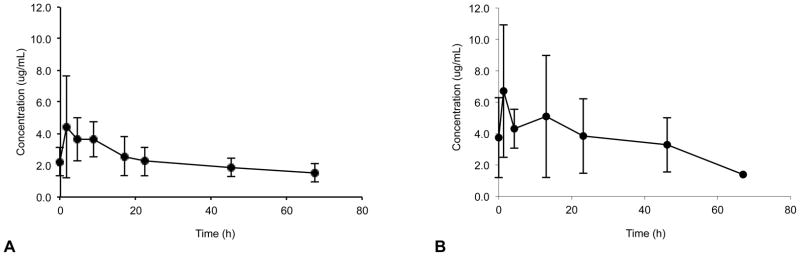
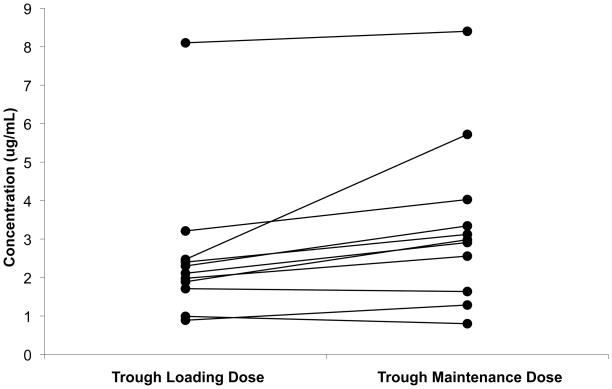
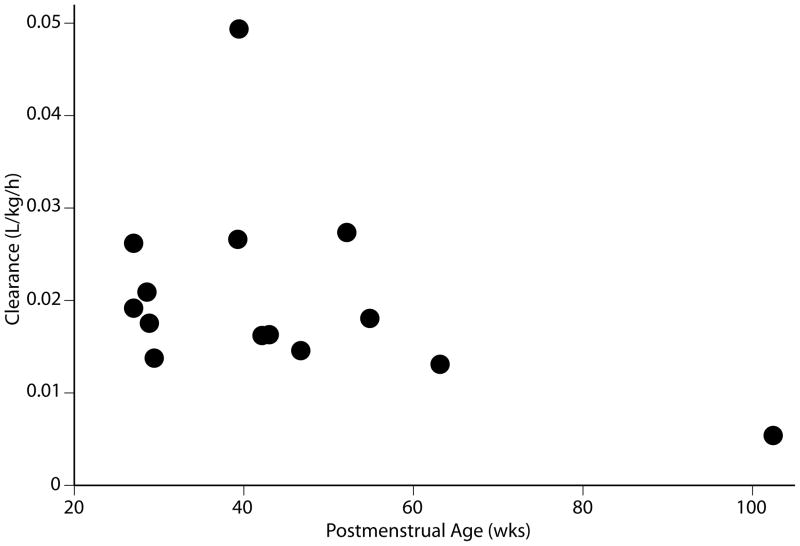
Data from 119 plasma concentrations obtained from 14 subjects were included in the PK analysis; one subject (#12) died soon after administration of first dose of study drug and was excluded from the PK analysis. Anidulafungin plasma concentration-time profiles after multiple dosing (3rd to 5th dose) are presented for each age group (Figure 1). The PK sampling times did not deviate from the allowed windows, except for the last sampling window (72–120 h) where the majority of subjects who had a sample obtained in this window were sampled at 67 hours. The PK parameters after multiple dosing are shown in Table 2. After the loading dose and subsequent multiple doses, plasma anidulafungin trough concentrations were higher than the minimum inhibitory concentration for most Candida sp (> 2 μg/mL) (Figure 2). Neonates demonstrated similar weight adjusted CLss and multiple-dose anidulafungin exposures when compared to infants [median weight adjusted CLss 0.020 L/kg/h (range; 0.013–0.049) vs. 0.015 L/kg/h (0.005–0.027) and median AUCss 74.9 μg*h/mL (30.4–108.9) vs. 97.7 μg*h/mL (54.8–278.0), p=0.12] (Table 2). The lowest anidulafungin exposures in neonates were observed in those 2 subjects supported by ECMO. The median AUCss in neonates excluding the ones supported by ECMO was 81.9 μg*h/mL (57.3–108.9). In addition, one neonate with an AUCss of 71.7 ug*h/mL did not have a peak sample drawn after multiple dosing; the first sample included in the AUCss calculation was obtained 18 hours post dose. The median AUCss (range) in neonates excluding those supported by ECMO and 1 neonate with incomplete sampling was 85.5 μg*h/mL (57.3–108.9). The highest anidulafungin exposure (AUCss 278 μg*h/mL) was observed in an infant with direct hyperbilirubinemia. No trend was observed in weight-adjusted anidulafungin clearance (CLss) with changes in postmenstrual age (Figure 3).
Figure 1.
(a) Mean (SD) steady-state anidulafungin plasma concentration-time profiles in neonates after receiving maintenance dosages of 1.5 mg/kg/day (day 3–5); 8, 2, 6, 3, 5, 5, 5, and 4 samples per data point for time points 1, 2, 3, 4, 5, 6, 7, and 8, respectively. (b) Mean steady-state anidulafungin plasma concentration-time profiles in infants after receiving maintenance dosages of 1.5 mg/kg/day (day 3–5); 6, 5, 5, 6, 3, and 1 samples per data point for time points 1, 2, 3, 4, 5, and 6, respectively.
Table 2.
Multiple-dose pharmacokinetic parameters of anidulafungin by age group.
| Subject | CLss (L/kg/h) | Vss (L/kg) | AUCss (μg*h/mL) | Cmaxss (μg/mL) | C24-ss (μg/mL) | Cmax LD (μg/mL) | C24 LD (μg/mL) |
|---|---|---|---|---|---|---|---|
| Neonates | |||||||
| 1 | 0.025 | 4.4 | 56.4 | 3.1 | 1.3 | 2.9 | 0.9 |
| 2 | 0.026 | 2.2 | 57.3 | 3.7 | 1.3 | - | - |
| 3 | 0.019 | 1.1 | 78.3 | 4.3 | 2.6 | - | - |
| 4 | 0.021 | 1.8 | 71.7 | 3.3 | 2.9 | 4 | 2.1 |
| 5 | 0.018 | 2.4 | 85.5 | 4.0 | 2.6 | - | - |
| 6 | 0.014 | 1.0 | 109.0 | 6.7 | 4.0 | 5.9 | 3.2 |
| 7 | 0.049 | 1.6 | 30.4 | 2.2 | 0.8 | 1.6 | 0.9 |
| 8 | 0.016 | 0.5 | 92.0 | 5.4 | 2.5 | 4.9 | 1.9 |
| Median | 0.020 | 1.7 | 74.9 | 3.9 | 2.6 | 4 | 1.9 |
| Range | 0.013–0.049 | 0.5–4.4 | 30.4–108.9 | 2.2–6.7 | 0.8–4.0 | 1.6–5.9 | 0.9–3.2 |
| Infants | |||||||
| 9 | 0.016 | 1.0 | 92.5 | 4.1 | 3.3 | 7.1 | 2.3 |
| 10 | 0.015 | 2.8 | 102.9 | 5.8 | 3.1 | 5 | 2.4 |
| 11 | 0.018 | 0.9 | 83.1 | 4.7 | 2.9 | 6.8 | 1.9 |
| 13 | 0.013 | 0.6 | 114.5 | 7.2 | 5.7 | 7.7 | 2.5 |
| 14 | 0.027 | 1.2 | 54.8 | 3.6 | 1.6 | 4.3 | 1.7 |
| 15 | 0.005 | 0.2 | 278.0 | 14.9 | 8.4 | 15.9 | 8.1 |
| Median | 0.015 | 0.9 | 97.7 | 5.2 | 3.2 | 6.9 | 2.3 |
| Range | 0.005–0.027 | 0.2–2.8 | 54.8–278.0 | 3.6–14.9 | 1.6–8.4 | 4.3–15.9 | 1.7–8.1 |
| Total median | 0.017 | 1.1 | 84.3 | 4.2 | 2.7 | 5.0 | 2.1 |
| Total range | 0.005–0.049 | 0.2–4.4 | 30.3–278.0 | 2.2–14.9 | 0.8–8.4 | 1.6–15.9 | 0.9–8.1 |
AUCss: Area under the curve at steady-state (0–24 h); CLss: steady-state clearance; Vss: steady-state volume of distribution; Cmax: maximum concentration; C24-ss: trough (24 h) concentration after a maintenance dose (predicted); LD: loading dose; C24 LD: trough (24 h) concentration after the loading dose. Cmax for subject #4 drawn 18 hours post dose.
Figure 2.
Anidulafungin concentrations after loading and maintenance (SS) doses (n=11, 3 subjects did not have trough samples collected after the loading dose). Predicted trough concentrations used for maintenance doses.
Figure 3.
Weight-normalized anidulafungin steady-state clearance versus postmenstrual age.
Safety
Eight out of 15 subjects (53%) experienced at least one adverse event; most of these events were mild or moderate in severity (Table 3). All but 2 adverse events were considered by the investigator to be unrelated to anidulafungin. The most commonly reported adverse event was worsening hyperbilirubinemia. These 2 hyperbilirubinemia adverse events were considered unlikely related to anidulafungin and observed in the infant group. One subject developed worsening hyperbilirubinemia (baseline 4.7 mg/dL, direct 2.8 mg/dL) up to 11.5 mg/dL (direct 7.4 mg/dL) leading to discontinuation of anidulafungin with resolution of hyperbilirubinemia back to baseline. This subject, however, had 5 similar direct hyperbilirubinemia episodes prior to administration of study drug. The other subject in this group who developed worsening hyperbilirubinemia (baseline 9.1 mg/dL, direct 6.1 mg/dL) had a similar episode 2 months prior to start of study drug. The maximum bilirubin level for this subject during study drug administration was 10.4 mg/dL (direct 6.8 mg/dL) and it peaked at 27.8 mg/dL (direct 17.9 mg/dL) 9 days after discontinuation of study drug.
Table 3.
Adverse events by age group.
| AE category | Neonates | Infants |
|---|---|---|
| Blood/bone marrow (anemia) | 0 | 1 |
| Cardiovascular (hypotension) | 1 | 0 |
| Constitutional symptoms (fever) | 0 | 1 |
| Endocrine (adrenal insufficiency) | 1 | 0 |
| Gastrointestinal (abnormal KUB) | 1 | 0 |
| General disorders (death) | 1 | 1 |
| Hepatobiliary disorders | 0 | 4 |
| Worsening ALT elevation | 0 | 1 |
| Worsening AST elevation | 0 | 1 |
| Worsening hyperbilirubinemia | 0 | 2 |
| Infection | 1 | 0 |
| Pulmonary (pulmonary edema) | 1 | 0 |
| Renal/genitourinary (oliguria, uremia) | 2 | 0 |
| Total | 8 | 7 |
| Total subjects with adverse events | 5 | 3 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Among infants, the median recorded aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin values did not exceed values higher than 3 times the upper limit of normal. One critically ill subject with complex congenital heart disease (hypoplastic left heart syndrome) in this group experienced a serious adverse event; the subject died soon after administration of the first dose of study drug. This event was considered by the investigator to be unlikely related to study drug. Withdrawal of life-sustaining therapies was requested by the patient’s family for this patient due to poor cardiac output and chronic respiratory failure that was unresponsive to maximal medical therapy.
In the neonate cohort, 5 (63%) subjects experienced adverse events; all events were considered by the investigators to be unrelated to anidulafungin and no subjects experienced adverse events that led to discontinuation of anidulafungin. The median recorded AST, ALT, and total bilirubin values for neonates did not exceed values higher than 3 times the upper limit of normal.. One neonate experienced a serious adverse event. This subject died after discontinuation of ECMO support as directed by the medical team and family and was not attributed to study drug.
Changes from baseline hematology values were attributed to changes in underlying disease or therapy, not study drug. None of the subjects enrolled developed candidemia.
Discussion
This is the first study to evaluate the PK and safety of weight-based anidulafungin in neonates and infants. The overall anidulafungin exposure in infants and neonates is similar (within 15%) to that of older children and adults (AUCss 100 μg*h/mL). This finding is consistent with the unique clearance and metabolic profiles of the drug. Because anidulafungin undergoes non-enzymatic degradation in the blood, is not renally cleared nor metabolized by the liver(10), and children 2–17 years of age show anidulafungin exposures similar to adults(12), clearance differences are not expected across patient populations including infants and neonates.
Penetration of echinocandins into the cerebrospinal fluid (CSF) is low in normal hosts with an intact blood brain barrier. However, laboratory animal studies demonstrate that HCME can be successfully treated with echinocandins in animal models(7, 13). Although the concentrations of echinocandins in CSF remain low in these animals, the concentrations in infected brain tissue are sufficiently elevated to significantly reduce the residual fungal burden of Candida albicans. These data are substantiated from individual case reports of successful treatment of Candida meningoencephalitis(14). Nevertheless, the dosage and antifungal drug exposure necessary to achieve this effect for individual echinocandins are not well understood, which suggests that the appropriate anidulafungin dose to treat HCME in neonates and infants remains to be determined.
The anidulafungin exposure in the neonates may have been biased by one subject who had incomplete sampling after multiple dosing (no peak concentration) and two subjects who received anidulafungin while supported by ECMO. The ECMO circuit is known to alter the PK of several drugs and could potentially increase the volume of distribution, drug clearance, or both, resulting in decreased drug exposure(15–17). This is particularly important for drugs with high protein binding such as anidulafungin, which may adhere to the ECMO circuit tubing system and oxygenator(17). This suggests that patients supported by ECMO may require anidulafungin doses 2 to 3 fold higher to achieve drug concentrations similar to patients not receiving ECMO support and this deserves further study.
The beta-elimination half-life of anidulafungin was calculated in 10 of 14 subjects who were sampled at least 48 hours after the last dose of study drug and appeared to be longer in neonates than in infants, although it was not statistically significant [(median 78 h (40–219) vs. 33 h (30–173), respectively, p=0.13]. The half life of anidulafungin in subjects enrolled in this study appears to be longer when compared to other pediatric patients (median of 33–78 vs. 20–24 h), which suggests that a prolonged dosing interval (>24 h) may be adequate in infants and neonates. In the present study, half life values should be interpreted with caution because sampling times after the last dose were not uniform across subjects, and, thus, the half life may not truly represent the drug’s terminal elimination phase. Overall, drug accumulation after multiple anidulafungin doses was not apparent when comparing trough concentrations after the loading dose and those obtained after multiple doses. This suggests that steady state was roughly achieved with the loading dose.
There were few adverse events and no serious drug-related adverse events in this study. Two infants experienced worsening of baseline elevated bilirubin levels, which has been rarely described in adult patients receiving anidulafungin(18). However, the biliary effects were not thought to be related to the study drug given that these subjects had previous hyperbilirubinemia episodes prior to the start of study drug. Interestingly, the subject with the highest drug exposure was the one with the highest degree of direct hyperbilirubinemia. Although it is not plausible to make any causal associations with one subject, this finding raises the question of the role of hepatic transporters in the clearance of anidulafungin. It has been previously shown that anidulafungin is not metabolized by the cytochrome P450 enzymatic system(10); however, the role of hepatic transporters in the clearance of the drug has not been evaluated. Because a fraction of the drug is excreted in the feces, involvement of hepatic transporters in drug clearance seems reasonable and should be investigated further. It is also plausible that drug interactions with co-administered medications could have accounted for the observed hyperbilirubinemia. To elucidate whether anidulafungin is associated with hepatobiliary side effects, larger trials of anidulafungin in the pediatric population are required.
The study is limited by use of a single site for enrollment, the number and heterogeneity of participants, and use of scavenged samples for PK analysis of one subject. The latter, however, is unlikely to affect the PK assessments given that samples spent <1 hour in the clinical laboratory before processing and freezing(10).
In this small and heterogenous cohort, anidulafungin 1.5 mg/kg/day preceded by a 3 mg/kg loading dose demonstrates overall drug exposures in infants and neonates similar to older children receiving the same weight-adjusted dosage and adult patients receiving 100 mg/day. Higher doses may be required in specific patient populations such as neonates or infants supported by ECMO, but this needs to be further evaluated. Anidulafungin at this dose was safe in this small study of infants and neonates and should be considered in future clinical trials designed to study the prevention and treatment of invasive fungal infections in infants and neonates.
Methods
Study design
This was a single center, open-label study conducted to determine the safety and PK of intravenous multiple-dose anidulafungin administered to infants and neonates at risk for serious systemic infections.
Subjects
We enrolled infants and neonates admitted to the neonatal and pediatric intensive care units at Duke University Medical Center who were 2 days to 2 years of age and had a suspected serious systemic infection evaluated by a blood culture within 48 h of study entry. Subjects were divided into 2 age cohorts: neonates (2–30 days) and infants (>30 days –2 years). Exclusion criteria included previous participation in the study, a history of anaphylaxis attributed to an echinocandin, and previous exposure to an echinocandin in the month prior to study. The Duke University institutional review board approved the study. Prior to the start of the study, written, informed consent was obtained from parents or legal guardians.
PK assessments
Anidulafungin was administered intravenously as a loading dose of 3 mg/kg infused over 60 min on day 1 and daily maintenance dosages of 1.5 mg/kg infused over 60 min without exceeding an infusion rate of 1.1 mg/min. Two anidulafungin presentations were used in the study. Infants received an intravenous alcohol (20%) based presentation whereas neonates received an alcohol-free, water for injection presentation. Both anidulafungin presentations are approved commercially. Anidulafungin was administered to each subject once daily. The maximum duration of study drug administration was 5 days. PK sampling occurred on day 1 and on days 3–5. On day 1, venous blood sampling (200 μl) occurred at the following time points/intervals: up to 2 h before the start of the infusion, immediately after the completion of the infusion, and 20–24 h after the start of the infusion. On days 3–5, venous blood sampling (approximately 200 μl) occurred at the following time points/intervals: up to 2 h before the start of the infusion, immediately after the completion of the infusion, and 3–6, 8–12, and 18–24 h after the start of the infusion. After the last dose of study drug additional PK sampling occurred at 24–48 and 72–120 h after the start of the infusion. Sampling windows rather than sampling time points were designed to provide flexibility in sample collection due to the critical condition of the subjects enrolled in the study as well as limitations related to venous access and number of heel sticks/venipunctures drawn specifically for PK sampling. Sample collection tubes were immediately placed on ice and, within 30 min, were centrifuged (1,500 × g for 10 min) at 4°C. Plasma was stored in transfer tubes at −80°C until analyzed. Plasma samples from one subject (#13) were scavenged from the clinical laboratory, but were frozen (−80°C) immediately after processing. Plasma samples were assayed for anidulafungin by use of a validated liquid chromatography-tandem mass spectrometry method (PPD, Richmond, VA). The method was validated in the linear range from 0.05 to 20.0 μg/ml with an overall precision of over 95.9% and accuracy of 96.8% to 104.2%.
Noncompartmental methods (WinNonlin v5.0; Pharsight Corp., Mountain View, CA) were used to calculate steady-state PK parameters from plasma concentration data obtained during doses 3–5. PK measures were maximum observed plasma concentration (Cmax), area under the plasma concentration-time curve (AUC) at steady state (AUCss) calculated by the linear trapezoidal rule, clearance (CLss) calculated by dividing dose by AUCss, volume of distribution at steady state (Vss) calculated by multiplying the dose by the ratio of the area under the first moment curve to the square of AUC, and terminal elimination half-life (t1/2) calculated from a linear regression of the log-linear portion of the log concentration-time curve. Trough concentrations (C24) of anidulafungin after multiple dosing were predicted using the elimination rate constant generated from the linear regression model. The relationship between anidulafungin clearance (CLss) and postmenstrual age was explored by a scatter plot.
Safety
Safety of anidulafungin was assessed by monitoring the frequency, intensity, and relationship to study drug of adverse events throughout the study. These included any new clinical problem or disease diagnosed during the study and up to 72 h after the last dose of study drug. Results of clinical laboratory tests (hematology, serum chemistry, liver transaminases, and bilirubin) performed within 72 h prior to administration of study drug and at least once during study drug administration were recorded. If a clinical or laboratory adverse event developed during the study and was attributed to study drug, it was followed daily until resolution. Adverse events (AEs) were graded according to the National Cancer Institute’s Common Terminology Criteria (CTC) and reported as mild (CTC grade 1), moderate (CTC grade 2), or severe (CTC grades 3 or 4). Causality (unrelated and possibly, probably and definitely related) of AEs and study drug was determined by the principal investigator. Microbiological data was also obtained as part of the study. All cultures from sterile body sites collected during the study were also recorded.
Statistical analyses
The PK parameters and safety data of anidulafungin were summarized using descriptive statistics. Data from all subjects who received at least one dose of anidulafungin were analyzed for safety. Data from all subjects who received anidulafungin and who provided blood samples were included in summaries of the PK profiles. Safety and PK parameters were summarized by age cohort. The Kruskal-Wallis rank test was used to compare PK parameters between age groups and the Wilcoxon matched-pairs signed-ranks test was used to compare mean trough concentrations after the loading dose and after multiple dosing. STATA 10 (College Station, TX) was used to perform the statistical analysis. A p-value of <0.05 was considered statistically significant.
Acknowledgments
We would like to acknowledge the staff and research team in the neonatal and pediatric critical care units at Duke University for their outstanding disposition, support, and dedication to this study.
Footnotes
Conflict of Interest/Disclosures
Dr. Cohen-Wolkowiez receives support from the US Government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin) and from NICHD 1K23HD064814-01; Dr. Benjamin receives support from the US Government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C), the non profit organization Thrasher Research Foundation for his work in neonatal candidiasis, and from industry for neonatal and pediatric drug development http://www.dcri.duke.edu/research/coi.jsp; Dr. Smith received support from NICHD 1K23HD060040-01 and from industry for neonatal and pediatric drug development http://www.dcri.duke.edu/research/coi.jsp. Dr Hope has received research funding from Pfizer. Dr. Walsh has received research grant support from Vestagen and has served on advisory panels for Novartis and iCo. Drs. Liu and Aram are employees of Pfizer, Inc. and are eligible to receive Pfizer stock and stock options. Dr. Kashuba receives support from the US Government for work in clinical pharmacology (R34AI087065, P30AI37260), and from industry for clinical pharmacology (Pfizer, Gilead, Abbott, Tibotec, and Merck). Dr. Capparelli supervises the UCSD Skaggs School of Pharmacy in Oncology-Clinical Pharmacology training program for which Pfizer Inc. provides financial support. Study drug was supplied by Pfizer Inc and they partially supported the research.
References
- 1.Stoll BJ, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ, Clark RH. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112:543–7. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 3.Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635–41. doi: 10.1097/01.inf.0000128781.77600.6f. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin DK, Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634–40. doi: 10.1542/peds.112.3.634. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin DK, Jr, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J Clin Microbiol. 2005;43:5425–7. doi: 10.1128/JCM.43.11.5425-5427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petraitiene R, et al. Antifungal activity of LY303366, a novel echinocandin B, in experimental disseminated candidiasis in rabbits. Antimicrob Agents Chemother. 1999;43:2148–55. doi: 10.1128/aac.43.9.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petraitis V, et al. Dosage-dependent antifungal efficacy of V-echinocandin (LY303366) against experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. Antimicrob Agents Chemother. 2001;45:471–9. doi: 10.1128/AAC.45.2.471-479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowell JA, Knebel W, Ludden T, Stogniew M, Krause D, Henkel T. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J Clin Pharmacol. 2004;44:590–8. doi: 10.1177/0091270004265644. [DOI] [PubMed] [Google Scholar]
- 10.Damle BD, Dowell JA, Walsky RL, Weber GL, Stogniew M, Inskeep PB. In vitro and in vivo studies to characterize the clearance mechanism and potential cytochrome P450 interactions of anidulafungin. Antimicrob Agents Chemother. 2009;53:1149–56. doi: 10.1128/AAC.01279-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowell JA, Stogniew M, Krause D, Damle B. Anidulafungin does not require dosage adjustment in subjects with varying degrees of hepatic or renal impairment. J Clin Pharmacol. 2007;47:461–70. doi: 10.1177/0091270006297227. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin DK, Jr, et al. Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob Agents Chemother. 2006;50:632–8. doi: 10.1128/AAC.50.2.632-638.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope WW, et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis. 2008;197:163–71. doi: 10.1086/524063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odio CM, et al. Caspofungin therapy of neonates with invasive candidiasis. Pediatric Infectious Disease Journal. 2004;23:1093–7. [PubMed] [Google Scholar]
- 15.Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996;40:1139–42. doi: 10.1128/aac.40.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1992;12:28–32. [PubMed] [Google Scholar]
- 17.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33:1018–24. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 18.Reboli AC, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472–82. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]