Abstract
The purpose of this study was to assess the induced apoptosis of self-assembled iron oxide magnetic nanoparticles (MNPs) co-loaded with daunorubicin (DNR) and 5-bromotetrandrin (Br Tet) (DNR/Br Tet-MNPs), acting as a drug depot system for the sustained release of the loaded DNR and BrTet, in the drug resistant human leukemia K562/A02 cells and further to explore potential mechanisms. After being incubated for 48 hours, K562/A02 cells were treated with DNR/Br Tet-MNPs or DNR and Br Tet in solution (DNR/Br Tet-Sol). Morphologic characteristics of K562/A02 cells were observed under a fluorescence microscope; cell apoptosis and intracellular accumulation of DNR were analyzed by FACS Calibur flow cytometry. Furthermore, reverse transcriptase polymerase chain reaction (RT-PCR) and Western blotting analyses were performed to study the apoptosis associated gene transcription and protein expression, respectively. Typical apoptotic characteristics, including chromatin condensation and fragmentation of nuclei, were observed and a high rate of apoptosis was detected in K562/A02 cells treated with DNR/Br Tet-MNPs and DNR/Br Tet-Sol. Detection of relative fluorescence intensity of intracellular DNR demonstrated that intracellular DNR was higher in K562/A02 cells treated with DNR/Br Tet-MNPs than that of DNR/Br Tet-Sol. Further study demonstrated that both DNR/Br Tet-MNPs and DNR/Br Tet-Sol reduced the gene transcriptions and protein expressions of bcl-2 and survivin and enhanced that of bax and caspase 3. It is concluded that self-assembled DNR/Br Tet-MNPs, as one of the potential antitumor agents for hematologic malignancies, may effectively induce apoptosis of K562/A02 cells through elevating the ratio of bax/bcl-2, activating caspase 3, and inactivating survivin.
Keywords: magnetic iron oxide nanoparticles, daunorubicin, 5-bromotetrandrin, target therapy, multidrug resistance, apoptosis, K562/A02 cells
Introduction
Currently, serious toxicity and poor antitumor efficacy resulting from the induction of drug resistance in hematological malignancies and other tumors are common hindrances to successful systemic chemotherapy.1 Combined use of reversal agents and antitumor agents to increase the antitumor effects, reduce dose-limiting side effects of chemotherapy agents, and reverse multidrug resistance (MDR) has shown potential achievements.2 However, there is still a problem of how to ensure that two different agents with different pharmacokinetics and tissue disposition execute their effects simultaneously at the same organ. What is exciting is that a nanoparticle drug delivery system has shown potential in its application to carry drugs to target sites effectively.3–5 Moreover, it potentially avoids damaging healthy cells when cancer cells are killed. Nowadays, nanosized iron oxide magnetic particles, mostly magnetite (Fe3O4) and maghemite (γ-Fe2O3), are widely used in biomedicine owing to their notable properties, such as super-paramagnetism and biocompatibility.6 Previous research has shown that magnetic nanoparticles (MNPs) of Fe3O4 combined with daunorubicin (DNR) and 5-bromotetrandrine (Br Tet) displayed a significant cytotoxicity effect on drug resistant leukemia K562/A02 cells.7,8 However, the polymerization of MNPs with DNR and Br Tet was by way of mechanical absorption, which meant that the binding of these three agents might not be so compact and that lots of drugs would be released before they reached the target site. To solve this problem, we developed a novel DNR/Br Tet-MNPs formulation, magnetic iron oxide nanoparticles co-loaded with DNR and Br Tet. We used oleic acid-coated iron oxide nanoparticles as drug carriers, which were further modified by pluronic F-127. DNR and Br Tet were then permeated into the OA shell. It possessed high drug loading capacity and acted as a drug depot for the sustained release of drugs up to 25 days. To study its potential effect on hematologic malignancies, we evaluated the induced apoptosis of self-assembled DNR/Br Tet-MNPs formulation in human leukemia K562/A02 cells and further exploited the potential mechanisms.
Materials and methods
Experimental agents
Iron chloride hexagydrate (FeCl3 · 6H2O) and iron sulfate heptahydrate (FeSO4 · 7H2O) were from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China). Ammonium hydroxide (NH4 · OH) and oleic acid (OA) were from Shanghai Lingfeng Chemical Reagent Co, Ltd (Shanghai, China). Pluronic® F-127 was from Sigma-Aldrich (San Diego, CA). Other reagents included Daunorubicin hydrochloride (Huifengda Chemical Co, Jinan, China), 5-bromotetrandrin (Kanghong Phamaceuticals Inc, Chengdu, China), RPMI 1640 medium (Gibco, Gaithersburg, MD), newborn bovine serum (Sijiqing, Hangzhou, China), Adriamycin (Hisun Phamaceutical Co, Zhejiang, China), and 4,6-diamidino-2- phenylindole (DAPI) staining solution (Beyotime Institute of Biotechnology, Jiangsu, China). RT-PCR kit was from Takara Biotechnology (Dalian, China). Monoclonal antibodies of bax, bcl-2, cleaved caspase 3, survivin, and GAPDH were from Santa Cruz Biotechnology (Santa Cruz, CA).
Preparation of DNR/Br Tet-MNPs
Aqueous dispersion of MNPs, OA-coated and pluronic-stabilized iron-oxide nanoparticles, were prepared as previously described.9 The hydrophobic DNR or Br Tet solution were prepared by adding triethylamine into DNR hydrochloride or Br Tet hydrochloride methanolic solution,10 and a combination of DNR and Br Tet (1:1 w/w) were added dropwise into the above mentioned aqueous dispersion of MNPs and stirred vigorously on a magnetic stirring plate overnight to make the drug participate into OA layer.11 The total drug loading capacity was 8.25%, and about 19% of DNR and 16% of Br Tet were released in the first 2 days. Interestingly, the drug loaded MNPs were able to release drugs persistently up to 25 days.
Cell culture
Human chronic myeloid leukemia resistant cell line, adriamycin-selected K562/A02, was received as a gift from the Institute of Hematology, Chinese Academy of Medical Sciences. Cells were cultured in RPMI 1640 medium with 10% newborn bovine serum, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified 5% CO2 atmosphere. To maintain MDR phenotype, K562/A02 cells were cultured in the medium containing 1 μg/mL adriamycin at least 1 week before experiments.
Morphologic characteristics of apoptosis by DAPI stain assay
After being cultured as above, cells were collected and smeared. Once fixed with methanol for 15 minutes, cells were stained with fluorochrome dye DAPI and then observed under a fluorescence microscope (IX51; Olympus, Tokyo, Japan) with a peak excitation wavelength of 340 nm.
Apoptosis assay by flow cytometry
4 × 105 K562/A02 cells were exposed to DNR alone in solution (DNR-Sol, 0.5 μg/mL), Br Tet alone in solution (Br Tet-Sol, 0.5 μg/mL), combined DNR and Br Tet in solution (DNR/Br Tet-Sol, total drug 1 μg/mL, DNR:Br Tet = 1:1 w/w), and DNR alone in MNPs (DNR-MNPs), Br Tet alone in MNPs (Br Tet-MNPs), or combined DNR and Br Tet in MNPs (DNR/Br Tet-MNPs) for 48 hours. Following two washes with phosphate-buffered saline (pH = 7.4), cells were suspended with 500 μL of binding buffer and labeled with 5 μL of Annexin V-FITC for 15 minutes at room temperature in the dark. Thereafter, cell apoptosis was determined by flow cytometry (FCM) using CellQuest software (Becton Dickinson, Franklin Lakes, NJ) at excitation/emission wavelengths of 488/575 nm in 1 hour.
Intracellular accumulation of DNR by flow cytometry assay
Intracellular accumulation of DNR was directly detected by FCM.7 Briefly, the K562/A02 cells were treated as previously described. After being cultured for 48 hours, cells were washed three times with phosphate-buffered saline and then suspended with 500 μL of phosphate-buffered saline. Thereafter, each sample was measured by FCM at excitation/emission wavelengths of 488/575 nm. The relative fluorescence intensity (RFI) of DNR was calculated as FItreated group/FIcontrol group.
Reverse transcriptase polymerase chain reaction (RT-PCR) assay
The K562/A02 cells were treated as previously described for 48 hours. Total RNA extraction was conducted with TRIzol and RT-PCR was performed according to manufacturer’s instructions. Briefly, total RNA (1 μg) of samples were added to RT buffer, 25 mM of MgCl2, 10 mM dNTPs, Random 9 mers (50 pmol/μL), RNase inhibitor (40 U/μL), and AMV reverse transcriptase (5 U/μL) to provide a final total volume of 20 μL. The conditions of RT were 30°C for 10 minutes, 42°C for 30 minutes, 99°C for 5 minutes, and 5°C for 5 minutes. The newly synthesized cDNA was then amplified by PCR and the conditions were 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 1 minute. There were pre-denaturing at 94°C for 2 minutes and final extension at 72°C for 7 minutes. The designed PCR primers to amplify products within target and control sequences were listed in Table 1. The PCR products were visualized on a 1.5% agarose gels by ethidium bromide staining. For densitometric analysis, band intensity was assessed using ScnImage software (Scion Corporation, Frederick, MD). After band intensity was adjusted by GAPDH intensity, data were calculated as the mean ± standard deviation of at least three experiments.
Table 1.
Summary of primer sequences for bcl-2, bax, caspase 3, survivin, and GAPDH
| Gene | Primer for RT-PCR | Band size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| bcl-2 | Forward 5′-TGTGGCCTTCTTTGAGTTCG-3′ | 281 | 56 |
| Reverse 5′-TCACTTGTGGCCCAGATAGG-3′ | |||
| bax | Forward 5′-TTTGCTTCAGGGTTTCATCC-3′ | 246 | 52 |
| Reverse 5′-CAGTTGAAGTTGCCGTCAGA-3′ | |||
| caspase 3 | Forward 5′-TTGATGCGTGATGTTTCTA-3′ | 223 | 51 |
| Reverse 5′-CAATGCCACAGTCCAGTTC-3′ | |||
| survivin | Forward 5′-CTCAAGGACCACCGCATCTC-3′ | 392 | 55 |
| Reverse 5′-CCTCAATCCATGGCAGCCAG-3′ | |||
| GAPDH | Forward 5′-GCCACATCGCTCAGACAC-3′ | 614 | 53 |
| Reverse 5′-CATCACGCCACAGTTTCC-3′ |
Abbreviation: RT-PCR, reverse transcriptase polymerase chain reaction.
Western blotting assay
Western blotting was performed as previously described.7 Briefly, total protein was isolated from the harvested cells in 25 mM Tris-HCl (pH = 7.4), 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 5 mM EGTA, 10 mM NaF, 1 mM PMSF, 0.5% NP-40, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM pepstatin for 15 minutes at 4°C and then centrifuged at 10,000 rpm for 5 minutes. Samples containing 25 μg of protein were fractionated by 10% SDS-PAGE. The protein was transferred onto nitrocellulose filter (Bio-Rad, Hercules, CA) by electroblotting. Non-specific binding sites were blocked for 1 hour with 5% non-fat milk. Mouse monoclonal anti-human bax (1:200), bcl-2 (1:200), cleaved caspase 3 (1:200), survivin, or GAPDH (1:400) antibodies were incubated to the membrane overnight at 4°C. Then a secondary horseradish peroxidase-labeled rabbit-mouse IgG (1:5000) was added and incubated for 1 hour at room temperature. The blots were visualized by densitometry scans (ECL system, Amersham, UK) according to manufacturer’s instruction.
Statistical analysis
Accordingly, all the data were expressed as mean ± standard deviation. Statistical analyses comparing data between groups were performed using one-way analysis of variance (ANOVA) by means of Statistical Package for Social Science (version 13.0; SPSS Inc, Chicago, IL). A value of P < 0.05 denoted statistical significance.
Results
Morphological characteristics of K562/A02 cells
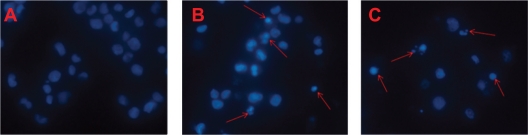
The morphological characteristics of K562/A02 cells were detected by DAPI staining assay (Figure 1). The nuclei of K562/A02 cells in control group were stained widespread with steady and low intensity blue fluorescence, suggesting the chromatins were equably distributed in nuclei. However, those treated with combination of DNR and Br Tet in MNPs or in solution for 48 hours showed typical features of apoptosis, such as chromatin condensation, nucleolus pyknosis, and nuclear fragmentation.
Figure 1.
The morphological characteristics of apoptosis in K562/A02 cells incubated for 48 hours. (DAPI staining, ×100). A) Control; B) DNR/Br Tet-Sol; C) DNR/Br Tet-MNPs. Arrows indicate cells with apoptotic nuclear condensation and fragmentation.
Abbreviations: DNR/Br Tet-Sol, daunorubicin and 5-bromotetrandrin in solution; DNR/Br Tet-MNPs, daunorubicin and 5-bromotetrandrin in magnetic nanoparticles.
Apoptosis assay by flow cytometery
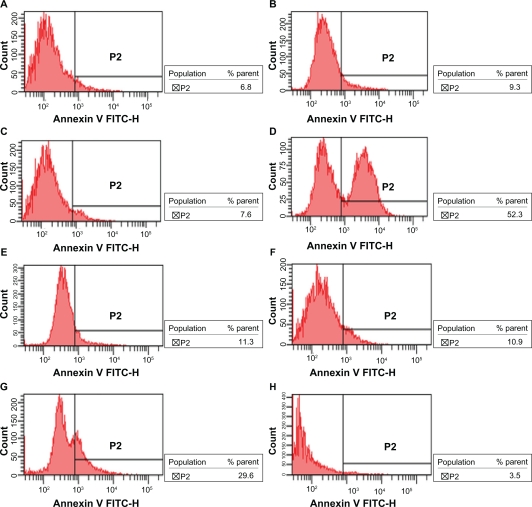
After incubation for 48 hours, the apoptotic rates of K562/A02 cells treated with medium, DNR-Sol, Br Tet-Sol, DNR/Br Tet-Sol, DNR-MNPs, Br Tet- MNPs, DNR/Br Tet-MNPs, and MNPs were 4.94% ± 1.89%, 6.53% ± 4.05%, 6.16% ± 1.99%, 44.02% ± 9.09%, 8.84% ± 6.45%, 6.26% ± 4.39%, 24.60% ± 4.42%, and 3.85% ± 1.90%, respectively. Compared to control group, the apoptotic rates in DNR/Br Tet-MNPs group and DNR/Br Tet-Sol group significantly increased (P < 0.05), and in the DNR/Br Tet-Sol group was higher than in the DNR/Br Tet-MNPs group (P < 0.05) (Figure 2).
Figure 2.
The apoptotic rates of K562/A02 cells incubated for 48 hours by flow cytometry assay. A) Control; B) DNR-Sol; C) Br Tet-Sol; D) DNR/Br Tet-Sol; E) DNRMNPs; F) Br Tet- MNPs; G) DNR/Br Tet- MNPs; H) MNPs.
Abbreviations: DNR-Sol, daunorubicin in solution; Br Tet-Sol, 5-bromotetrandrin in solution; DNR/Br Tet-Sol, daunorubicin and 5-bromotetrandrin in solution; DNR-MNPs, daunorubicin in magnetic nanoparticles; Br Tet-MNPs, 5-bromotetrandrin in magnetic nanoparticles; DNR/Br Tet-MNPs, daunorubicin and 5-bromotetrandrin in magnetic nanoparticles; MNPs, magnetic nanoparticles.
Enhancement of intracellular DNR accumulation with nanoparticle delivery
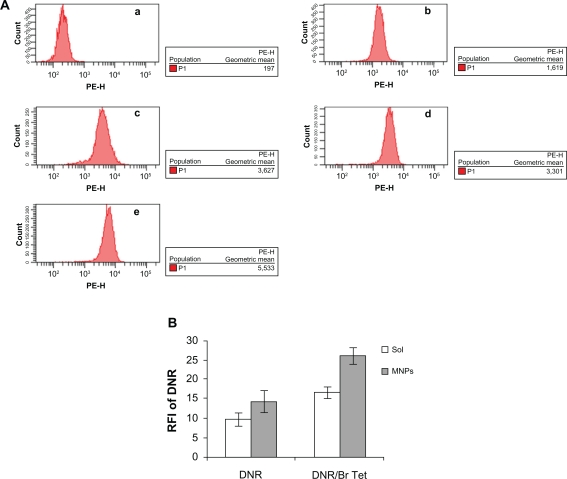
The RFI of DNR in K562/A02 cells incubated with DNR-Sol, DNR/Br Tet-Sol, DNR-MNPs, and DNR/Br Tet-MNPs were 9.84 ± 1.73, 16.75 ± 1.47, 14.36 ± 2.98, and 25.97 ± 2.28, respectively. Compared to either DNR-Sol or DNR-MNPs, both DNR/Br Tet-Sol and DNR/Br Tet-MNPs significantly enhanced the intracellular DNR accumulation, respectively (P < 0.05). What’s more, the relative fluorescence intensity of intracellular DNR demonstrated that intracellular DNR was higher in K562/A02 cells treated with DNR/Br Tet-MNPs than that of DNR/Br Tet-Sol (P < 0.05) (Figure 3).
Figure 3.
Intracellular accumulation of DNR in K562/A02 cells incubated for 48 hours by flow cytometry assay. A) Intracellular fluorescence intensity associated with DNR in treated cells. a) Control, b) DNR-Sol, c) DNR/Br Tet-Sol, d) DNR-MNPs, e) DNR/Br Tet-MNPs; B) RFI of DNR in treated cells.
Abbreviations: DNR-Sol, daunorubicin in solution; DNR/Br Tet-Sol, daunorubicin and 5-bromotetrandrin in solution; DNR-MNPs, daunorubicin in magnetic nanoparticles; DNR/Br Tet-MNPs, daunorubicin and 5-bromotetrandrin in magnetic nanoparticles; FI, fluorescence intensity; RFI, relative fluorescence intensity.
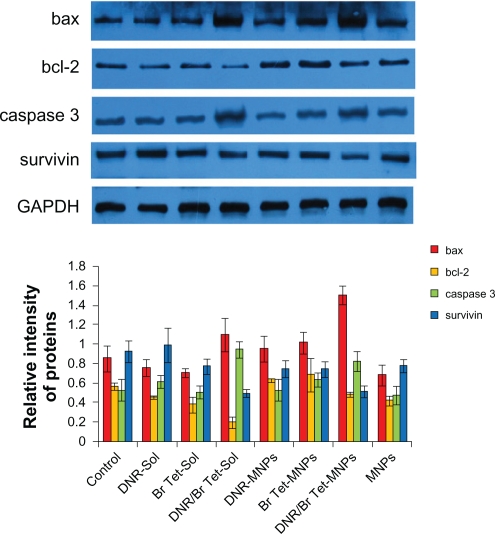
Effects of DNR/Br Tet-MNPs on apoptosis-associated genes and proteins
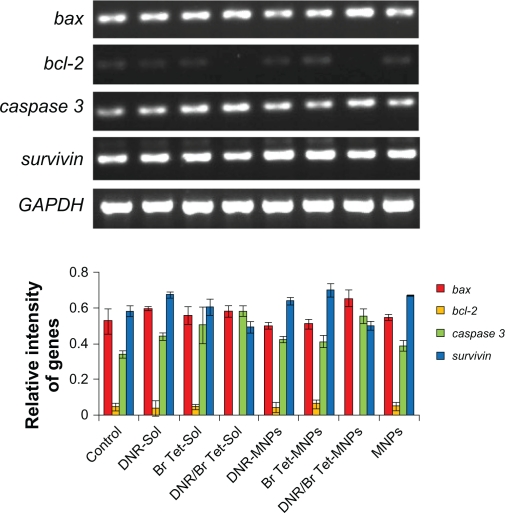
When treated with DNR/Br Tet-MNPs or DNR/Br Tet-Sol for 48 hours, the levels of bcl-2 and survivin mRNA in K562/A02 cells were both significantly down-regulated, while those of bax and caspase 3 mRNA were both up-regulated (Figure 4). Interestingly, the effects were further demonstrated by the reduction of bcl-2 and survivin proteins, and the increase of bax and caspase 3 proteins in K562/A02 cells (Figure 5), suggesting that the relative ratio of bax/bcl-2 in these two groups increased significantly (P < 0.05) and in DNR/Br Tet-MNPs was slightly lower than that of DNR/Br Tet-Sol. Meanwhile, the up-regulated levels of caspase 3 mRNA and protein in DNR/Br Tet-MNPs group were a little lower than those in DNR/Br Tet-Sol group (P < 0.05), while the down-regulated levels of survivin mRNA and protein in the former were lower than those in the latter (P < 0.05). Unluckily, the above-mentioned apoptosis-related gene mRNA or protein were not obviously altered in other groups when compared to control group (P > 0.05).
Figure 4.
Transcription of apoptosis-associated genes by RT-PCR analysis.
Note: Data are presented as means ± standard deviation (n = 3).
Abbreviations: DNR-Sol, daunorubicin in solution; DNR/Br Tet-Sol, daunorubicin and 5-bromotetrandrin in solution; DNR-MNPs, daunorubicin in magnetic nanoparticles; DNR/Br Tet-MNPs, daunorubicin and 5-bromotetrandrin in magnetic nanoparticles; MNPs, magnetic nanoparticles; RT-PCR, reverse transcriptase polymerase chain reaction.
Figure 5.
Expression of apoptosis-associated proteins by Western blotting analysis.
Note: Data are presented as means ± standard deviation (n = 3).
Abbreviations: DNR-Sol, daunorubicin in solution; DNR/Br Tet-Sol, daunorubicin and 5-bromotetrandrin in solution; DNR-MNPs, daunorubicin in magnetic nanoparticles; DNR/Br Tet-MNPs, daunorubicin and 5-bromotetrandrin in magnetic nanoparticles; MNPs, magnetic nanoparticles.
Discussion
Owing to the MDR of tumor cells to conventional antitumor drugs, systemic chemotherapy in treatment of hematological malignancies is impeded.12 The mechanisms in the development of MDR are complicated, including overexpression of transporter proteins such as P-gp, intracellular detoxification by increased levels of glutathione, altered DNA repair, and failure to undergo apoptosis.13 Various reversal agents have been studied to overcome MDR, but the results are not satisfactory due to their dose-limiting toxicity.14,15 Previous studies demonstrate that Br Tet is an effective reversal agent of MDR in vitro and in vivo by inhibiting the overexpression of P-gp and increasing intracellular accumulation of anticancer drugs.16,17 Some reports displayed that one of the important mechanisms of tumorigenesis and resistance to anticancer drugs was blockade of the apoptosis-inducing pathway.18,19 To reverse MDR and minimize serious undesired side effects of systemic chemotherapy, we have developed a promising DNR/Br Tet-MNPs formulation, and in this study, we focus on its effects on the induction of apoptosis in drug resistant human leukemia K562/A02 cells.
The morphology of K562/A02 cells treated with DNR/Br Tet-MNPs shows chromatin condensation, nucleolus pyknosis, and nuclear fragmentation, which are typical characteristics of apoptosis. The similar morphological changes are also observed in cells treated with DNR/Br Tet-Sol. Flow cytometric analysis shows that the cell apoptotic rate of K562/A02 cells treated with either DNR/Br Tet-MNPs or DNR/Br Tet-Sol is significantly different when compared to the control group, and the effect of DNR/Br Tet-MNPs is weaker than that of DNR/Br Tet-Sol, which may be related to the sustained release property of DNR/Br Tet-MNPs in the first 2 days of in vitro drug release study. But there is no obvious difference of cell apoptotic rates in cells treated with either DNR or Br Tet alone or combination with MNPs.
Fluorospectrophotometric assay shows that the intracellular DNR in DNR-MNPs group is higher than that in DNR-Sol group, which may be related to the ability of K562/A02 cells to take up nanoparticles with loaded DNR and thus reduce excretion by P-gp. Compared to DNR alone, the combination of DNR and Br Tet makes intracellular DNR increase prominently. Moreover, compared to DNR-MNPs, DNR and Br Tet co-loaded in MNPs significantly increase the intra-cellular DNR. Meanwhile, we find that more accumulation of DNR in cells treated with DNR/Br Tet-MNPs than that with DNR/Br Tet-Sol. We consider there are three reasons to explain these phenomena: 1) the inhibition of released Br Tet from MNPs makes the cells reduce the excretion of DNR; 2) a large proportion of DNR is still stored in MNPs for the property of sustained release, which is the reason why the rate of apoptosis in the DNR/Br Tet-MNPs group is lower than DNR/Br Tet-Sol; and 3) the underlying mechanism of nanoparticles entering cells is endocytotic and/or pinocytotic activity rather than a consequence of simple passive permeation.
In order to explore the potential mechanisms of our DNR/Br Tet-MNPs formulation, we have observed the alternation of some apoptosis-associated gene transcription and protein expression. A large amount of evidence has shown that the sensitivity of cells to apoptotic stimuli is determined by the relative ratio of pro-apoptotic and anti-apoptotic members of the bcl-2 family, which is the mitochondrial-related death switch.20 Bcl-2 is believed to promote tumorigenesis and prevent cells undergoing apoptosis, while bax is well known to initiate cell death by disrupting the integrity of the mitochondrial membrane and promoting release of cytochrome c from mitochondria, resulting in caspase 9/caspase 3 activation and DNA fragmentation.21,22 Caspase 3, a key protease and activated during the early stages of apoptosis, is synthesized initially as an inactive pro-enzyme in cells undergoing apoptosis and then hydrolyzed by itself and/or cleaved by other proteases.23 In our study, the increased ratios of bax/bcl-2 are found in both DNR/Br Tet-MNPs and DNR/Br Tet-Sol. In addition, DNR/Br Tet-MNPs obviously raise the level of caspase 3 mRNA and protein, inferring their facilitative effect on caspase 3 activation. Survivin is a member of the inhibitor of apoptosis protein family and expresses specifically during embryogenesis and in tumor cells and suppresses cell death signaling.24 Our DNR/Br Tet-MNPs formulation also displays the significant down-regulation of survivin mRNA and protein in K562/A02 cells. Similarly, the effects of DNR/Br Tet-MNPs are less than that of DNR/Br Tet-Sol as a result of the sustained release property. Collectively, DNR/Br Tet-MNPs have shown to dramatically elevate bax/bcl-2 ratio, enhance caspase 3 activities, and further stimulate the initiation of mitochondrial apoptosis signaling, and inhibit the expression of survivin.
Conclusion
The current in vitro study indicates that self-assembled DNR/Br Tet-MNPs formulation induces apoptosis in drug resistant K562/A02 cells by elevating the ratio of bax/bcl-2, activating caspase 3, and inhibiting survivin. These findings further support that DNR/Br Tet-MNPs formulation may be a potential application as an antitumor agent in the treatment of hematologic malignancies.
Acknowledgments
This work was financially supported by National Key Basic Research Program 973 of China (Nr 2010CB732404), National High Technology Research and Development Program 863 of China (Nr 2007AA022007), and National Nature Science Foundation of People’s Republic China (Nr 3074006230872970). We would like to thank Dr Yu Zhang (National Laboratory of Molecular and Biomolecular Electronics, Southeast University) for providing technical assistance.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen H, Bi W, Cao B, et al. A novel podophyllotoxin derivative (YB-1EPN) induces apoptosis and down-regulates express of P-glycoprotein in multidrug resistance cell line KBV200. Eur J Pharmacol. 2010;627:69–74. doi: 10.1016/j.ejphar.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 2.Miettinena S, Grenmand S, Ylikomia T. Inhibition of P-glycoprotein-mediated docetaxel efflux sensitizes ovarian cancer cells to concomitant docetaxel and SN-38 exposure. Anticancer Drugs. 2009;20:67–76. doi: 10.1097/CAD.0b013e328329977f. [DOI] [PubMed] [Google Scholar]
- 3.Alexiou C, Schmid RJ, Jurgons R, et al. Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J. 2006;35:446–450. doi: 10.1007/s00249-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 4.Nobs L, Buchegger F, Curny R, et al. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci. 2004;93:1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- 5.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 6.Dias AM, Hussain A, Marcos AS, et al. A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol Adv. 2011;9:142–155. doi: 10.1016/j.biotechadv.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Wu WW, Chen BA, et al. Effect of magnetic nanoparticles of Fe3O4 and 5-bromotetrandrine on reversal of multidrug resistance in K562/A02 leukemic cells. Int J Nanomedicine. 2009;4:209–216. doi: 10.2147/ijn.s7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen BA, Cheng J, Shen MF, et al. Magnetic nanoparticles of Fe3O4 and 5-bromotetrandrin interact synergistically to induce apoptosis by daunorubicin in leukemia cells. Int J Nanomedicine. 2009;4:65–71. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Chen BA, Cheng J, et al. Synthesis and antitumor efficacy of daunorubicin-loaded magnetic nanoparticles in vitro. Int J Nanomedicine. 2010;6:203–211. doi: 10.2147/IJN.S16165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohori F, Sakai K, Aoyagi T, et al. Control of adriamycin cytotoxic activity using thermally responsive polymeric micelles composed of poly (N- sopropylacrylamide-co-N, N- imethylacrylamide)-bpoly(D L-lactide) Colloids Surf B Biointerfaces. 1999;16:195–205. [Google Scholar]
- 11.Jain TK, Richey J, Strand M, et al. Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials. 2008;29:4012–4021. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;3:467–473. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- 13.García MG, Alaniz L, Lopes EC, et al. Inhibition of NF-κB activity by BAY 11-7082 increases apoptosis in multidrug resistant leukemic T-cell lines. Leuk Res. 2005;29:1425–1434. doi: 10.1016/j.leukres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 16.Jin J, Wang FP, Wei H, et al. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrine. J Lipid Res. 2006;47:51–58. doi: 10.1007/s00280-004-0868-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang JQ, Chen BA, Cheng J, et al. Comparison of reversal effects of 5-bromotetrandrine and tetrandrine on P-glycoprotein-dependent resistance to adriamycin in human leukemia cell line K562/A02. Ai Zheng. 2008;27:491–495. [PubMed] [Google Scholar]
- 18.Tsuruo T, Naito M, Tomida A, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 20.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 21.Rodriguez-Nieto S, Zhivotovsky B. Role of alterations in the apoptotic machinery in sensitivity of cancer cells to treatment. Curr Pharm Des. 2006;12:4411–4425. doi: 10.2174/138161206779010495. [DOI] [PubMed] [Google Scholar]
- 22.Condorelli G, Morisco C, Stassi G, et al. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation. 1999;99:3071–3078. doi: 10.1161/01.cir.99.23.3071. [DOI] [PubMed] [Google Scholar]
- 23.Ko CH, Shen SC, Hsu CS, et al. Mitochondrial-dependent, reactive oxygen species-independent apoptosis by myricetin: roles of protein kinase C, cytochrome c, and caspase cascade. Biochem Pharmacol. 2005;69:913–927. doi: 10.1016/j.bcp.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Suzukia A, Ito T, Kawano H, et al. Survivin initiates ptrocaspase 3/p21complex formation as result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene. 2000;19:1346–1353. doi: 10.1038/sj.onc.1203429. [DOI] [PubMed] [Google Scholar]