Abstract
Purpose:
To explore the preparation and characterization of a novel nanosized magnetic liposome containing the PEI-As2O3/Mn0.5Zn0.5Fe2O4 complex.
Methods:
Mn0.5Zn0.5Fe2O4 and As2O3/Mn0.5Zn0.5Fe2O4 nanoparticles were prepared by chemical coprecipitation and loaded with PEI. The PEI- As2O3/Mn0.5Zn0.5Fe2O4 complex was characterized using transmission electron and scanning electron microscopy, X-ray diffraction, energy dispersive spectrometry, and Fourier transform infrared spectroscopy. Cell transfection experiments were performed to evaluate the transfect efficiency. Magnetic nanoliposomes were prepared by rotatory evaporation and their shape, diameter, and thermodynamic characteristics were observed.
Results:
Mn0.5Zn0.5Fe2O4 and PEI-As2O3/Mn0.5Zn0.5Fe2O4 nanoparticles were spherical, with an average diameter of 20–40 nm. PEI-As2O3/Mn0.5Zn0.5Fe2O4 was an appropriate carrier for the delivery of a foreign gene to HepG2 cells. Energy dispersive spectrometry results confirmed the presence of the elements nitrogen and arsenic. Nanoliposomes of approximately 100 nm were observed under a transmission electron microscope. Upon exposure to an alternating magnetic field, they also had good magnetic responsiveness, even though Mn0.5Zn0.5Fe2O4was modified by PEI and encased in liposomes. Temperatures increased to 37°C–54°C depending on different concentrations of PEI-As2O3/Mn0.5Zn0.5Fe2O4and remained stable thereafter.
Conclusion:
Our results suggest that PEI-As2O3/Mn0.5Zn0.5Fe2O4 magnetic nanoliposomes are an excellent biomaterial, which has multiple benefits in tumor thermotherapy, gene therapy, and chemotherapy.
Keywords: nanoliposomes, magnetic fluid hyperthermia, As2O3, DNA
Introduction
Completion of the human genome project has greatly increased the discovery of functional and disease-related genes. Gene therapy has become one of the most active research areas in biomedicine in the 21st century. One of the main advantages of gene therapy over traditional therapy is the potential to target the expression of therapeutic genes in desired cells or tissues. To achieve targeted gene expression, we developed a novel nonviral gene delivery system.
Arsenic trioxide (As2O3) has been adopted from traditional Chinese medicine and used successfully to treat refractory or relapsed acute promyelocytic leukemia.1 In recent years, some researchers have found that As2O3 was not only effective in leukemia treatment, but also effective in inhibiting several animal and human solid cancers, such as neuroblastoma, liver, and gastric cancers.2–4 However, there is a lack of drug specificity with the intravenous or oral administration of As2O3, resulting in the requirement of large doses to achieve high local concentrations. This can cause nonspecific toxicity and other adverse side effects. Moreover, As2O3 has been generally considered to be an extremely effective environmental cocarcinogen for some human malignancies, especially for renal, skin, and lung cancer.5–7 Therefore, we investigated an alternative means of administering As2O3 to enhance its curative effects and reduce toxicity.
Clinically, the use of thermotherapy, such as heating certain organs or tissues to temperatures between 41°C and 46°C, also known as hyperthermia, has been long-used in the treatment of tumors. With the development of nanotechnology, magnetic nanoparticles have been used for tumor hyperthermia, to absorb energy from an applied high frequency alternating magnetic field (AMF). This treatment is known as magnetic fluid hyperthermia (MFH).8 MFH is more effective in the uniform heating of deeply-situated tumors, with relatively good targeting. Generally, in this technique, magnetic fluids are dispersed into the target tissue, which are then heated through the application of an external AMF.9
In this paper, we aimed to develop a novel nanosized magnetic liposome containing the PEI-As2O3/Mn0.5Zn0.5Fe2O4 (PEI-As2O3/MZF) complex. Herein we also show multiple antitumor functions, including thermotherapy of magnetic MZF nanoparticles, gene therapy of PEI-modified complexes, and chemotherapy using As2O3. With further research, it may develop into a new approach for the treatment of tumors.
Experimental details
Main reagents and cell line
As2O3 (Sigma Aldrich, St Louis, MO); PEI (Sigma Aldrich); phosphatidyl choline (Lipoid LLC, Newark, NY); cholesterol (Sigma Aldrich); human liver cancer HepG2 cell lines were provided by the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Science, Chinese Academy of Sciences, China.
Methods
Preparation of MZF and PEI-As2O3/MZF nanoparticles
MZF nanoparticles were prepared using chemical coprecipitation. They were examined by transmission electron microscopy (TEM) (H-600; Hitachi, Tokyo, Japan) and scanning electron microscopy (SEM) (JSM-6360LV; Jeol, Tokyo, Japan). The element composition and content of the nanoparticles were analyzed by X-ray diffraction (XRD) and energy dispersive spectrometry (EDS) (Vantage; Thermo Noran, Middleton, WI). MZF was subsequently modified by As2O3 and adsorbed by PEI, according to a previously-described method. The adsorption efficiency of PEI was demonstrated by Fourier transform infrared spectroscopy (FTIR) (560; Thermo Nicolet, Madison, WI) and EDS.
Cell transfection
Prior to transfection, pGFP plasmid DNA and PEI-As2O3/MZF nanoparticles were diluted separately in serum- and supplement-free medium. Subsequently, 6 μg plasmid DNA per well was complexed with various amounts of PEI-As2O3/MZF-NPs (10 mg/mL), corresponding to a nitrogen/phosphorus (N/P) ratio of particles to DNA in a final volume of 400 μL serum- and supplement-free medium. The complexes were then incubated at room temperature for 30 minutes. As a reference,10 PEI (complexed with DNA at an N/P ratio of 7) or commercially-available transfection reagents, such as Lipofectamine™ (Invitrogen, Carlsbad, CA) were used in accordance with the manufacturer’s instructions.
HepG-2 cells were seeded in six well plates, at an initial density of 2 × 105 cells/well in 2 mL growth medium. After incubation for 18 hours, when cells had reached 80% confluency, the medium was replaced with 1.6 mL serum-free media and 400 μL PEI-As2O3/MZF-NPs/pGFP complexes, followed by a further incubation for 4 hours. The medium was then exchanged for fresh medium containing serum and incubated further. Cells were observed using inverted fluorescence microscopy at 24 hours and 48 hours post-transfection, to evaluate transfection efficiency.
Preparation of nanoliposomes, containing PEI-As2O3/MZF and heating tests
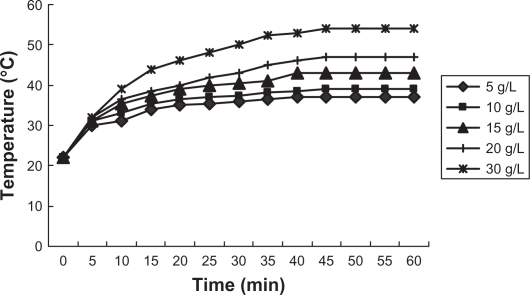
Magnetic nanoliposomes were obtained by the combined methods of rotatory film and high-pressure homogenization. A heat test was performed to detect the thermodynamic characteristics of the magnetic nanoliposomes. Various doses of nanoliposomes were decentralized in 0.9% sodium chloride, with concentrations of PEI-As2O3/MZF as follows: 5, 10, 15, 20, 30 g/L. The output electric current was 300A and the fluid was heated for 1 hour with temperature measurements every 5 minutes.
Results and discussion
Characterization of MZF and PEI-As2O3/MZF nanoparticles
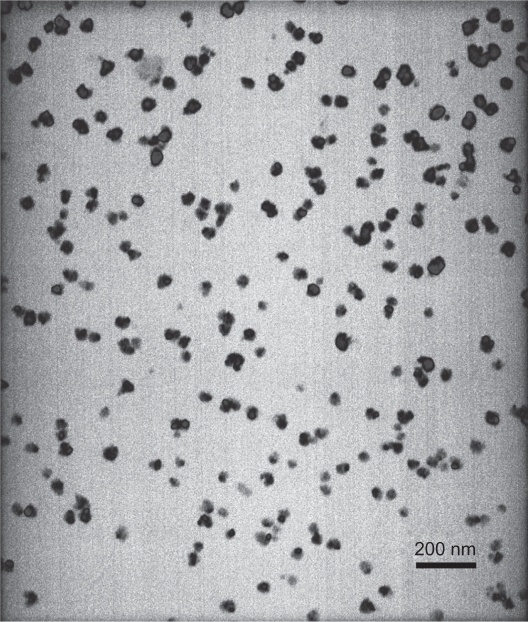
MZF nanoparticles were evaluated by TEM, XRD, and EDS. Using TEM, MZF particles appeared round or elliptical in shape, with good dispersion and were 20–30 nm in diameter (Figure 1). These nanoparticles were identified as Mn0.5Zn0.5Fe2O4 by XRD and EDS.
Figure 1.
TEM image of Mn0.5Zn0.5Fe2O4 nanoparticles.
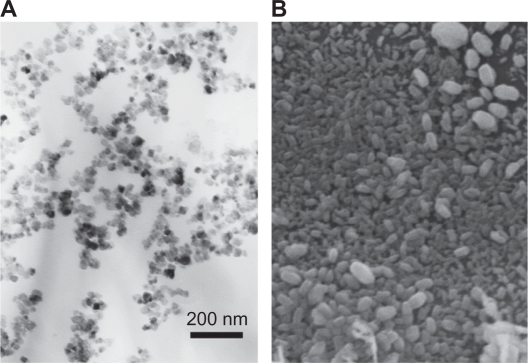
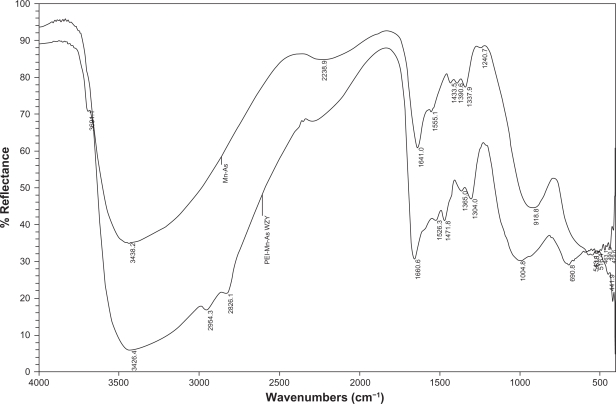
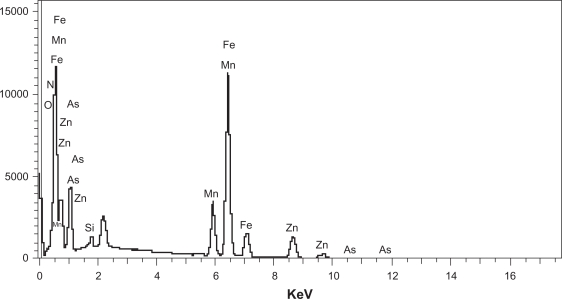
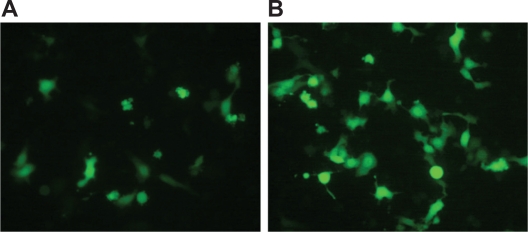
Figures 2A and 2B show PEI-As2O3/MZF nanoparticles under TEM and SEM. The nanoparticles were brown spherical particles, with diameters ranging from 30 to 40 nm. Furthermore, they suspended stably in water, with good diffusibility. FTIR showed specific peaks at 1,471 cm−1, 2,826 cm−1, and 2,954 cm−1, as compared to As2O3/MZF complexes (Figure 3). The EDS results verified the presence of the elements manganese, zinc, iron, arsenic, and nitrogen (Figure 4). Further cell transfection experiments suggested that PEI-As2O3/MZF nanoparticles were efficient carriers for gene transfection. The observed green cellular fluorescence was due to pGFP expression, which was confirmed by inverted fluorescence microscopy 24 hours post-transfection. The expression of GFP was much clearer at 48 hours post-transfection (Figure 5). This high transfer efficiency was due to the inherent proton absorption properties of PEI.11 However, the use of PEI as a gene carrier is limited by cytotoxicity and nonspecific interactions with serum proteins. In our study, we used nanoliposomes to engulf the PEI-As2O3/MZF complexes and reduce their toxic side effects, which has not been previously reported.
Figure 2.
PEI-As2O3/MZF as seen by TEM (A) and SEM (B).
Abbreviations: PEI, polyethyleneimine;TEM, transmission electron microscopy; SEM, scanning electron microscopy.
Figure 3.
FTIR of PEI-As2O3/MZF: the top curve is As2O3/MZF and the bottom is PEI-As2O3/MZF.
Abbreviations: FTIR, Fourier transform infrared spectroscopy; PEI, polyethyleneimine.
Figure 4.
EDS results of PEI-As2O3/MZF.
Abbreviations: EDS, energy dispersive spectrometry.
Figure 5.
HepG2 cells observed under fluorescence microscopy (×400) 24 hours after transfection (A) and 48 hours after transfection (B).
Properties of nanoliposomes containing PEI-As2O3/MZF
We prepared magnetic nanoliposomes containing PEI-As2O3/MZF complexes, with an average diameter of approximately 100 nm. The encapsulation ratio of As2O3 was analyzed by fluorescence spectrophotometry and determined to be 75.33%. An in vitro heating test was performed to detect the thermodynamic characteristics of the nanoliposomes. The temperature of the nanoliposomes exposed to AMF rose rapidly within the first 5 minutes and slowly continued to increase from 5–40 minutes, remaining stable thereafter. The temperature rose from 37°C to 54°C, depending on various doses of PEI-As2O3/MZF (Figure 6). The results showed that nanoliposomes had good absorption properties in high-frequency alternating electromagnetic fields, due to the presence of magnetic nanoparticles. The modified PEI, As2O3, and lipid membrane did not influence the magnetic responsiveness of MZF. Therefore, we can select a suitable temperature range (42°C–46°C) for tumor hyperthermia by adjusting the concentration of PEI-As2O3/MZF.
Figure 6.
Heating test result of magnetic nanoliposomes.
Conclusion
Nanotechnology has been increasingly applied in the medical field, with potential treatments for cancer. In our study, we prepared a new kind of magnetic nanoliposome containing PEI-As2O3/MZF complexes. It has multiple antitumor applications, including thermotherapy using magnetic MZF nanoparticles, gene therapy with PEI-modified complexes, and chemotherapy using As2O3. In future studies, we will apply this novel material to tumor therapy.
Acknowledgments
This work was supported by the Chinese National 863 Plan (No: 2007 AA03Z356), and the Chinese National Natural Science Foundation (No: 30770584).
Footnotes
Disclosure
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Jing Y, Xia L, Waxman S. Targeted removal of PML-RARalpha protein is required prior to inhibition of histone deacetylase for overcoming all-trans retinoic acid differentiation resistance in acute promyelocytic leukemia. Blood. 2002;100(3):1008–1013. doi: 10.1182/blood.v100.3.1008. [DOI] [PubMed] [Google Scholar]
- 2.Luo L, Qiao H, Meng F, et al. Arsenic trioxide synergizes with B7H3-mediated immunotherapy to eradicate hepatocellular carcinomas. Int J Cancer. 2006;118(7):1823–1830. doi: 10.1002/ijc.21557. [DOI] [PubMed] [Google Scholar]
- 3.Florea AM, Splettstoesser F, Busselberg D. Arsenic trioxide (As2O3) induced calcium signals and cytotoxicity in two human cell lines: SY-5Y neuroblastoma and 293 embryonic kidney (HEK) Toxicol Appl Pharmacol. 2007;220(3):292–301. doi: 10.1016/j.taap.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Xiao YF, Wu DD, Liu SX, Chen X, Ren LF. Effect of arsenic trioxide on vascular endothelial cell proliferation and expression of vascular endothelial growth factor receptors Flt-1 and KDR in gastric cancer in nude mice. World J Gastroenterol. 2007;13(48):6498–6505. doi: 10.3748/wjg.v13.i48.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Feng T, Chen H, et al. Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic Clin Pharmacol Toxicol. 2008;102(5):419–425. doi: 10.1111/j.1742-7843.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Piao F, Liu S, et al. Preventive effects of taurine and vitamin C on renal DNA damage of mice exposed to arsenic. J Occup Health. 2009;51(2):169–172. doi: 10.1539/joh.l8038. [DOI] [PubMed] [Google Scholar]
- 7.Patrick L. Toxic metals and antioxidants: Part II. The role of anti-oxidants in arsenic and cadmium toxicity. Altern Med Rev. 2003;8(2):106–128. [PubMed] [Google Scholar]
- 8.Jordan A, Wust P, Scholz R, et al. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int J Hyperthermia. 1996;12(6):705–722. doi: 10.3109/02656739609027678. [DOI] [PubMed] [Google Scholar]
- 9.Tasci TO, Vargel I, Arat A, Guzel E, Korkusuz P, Atalar E. Focused RF hyperthermia using magnetic fluids. Med Phys. 2009;36(5):1906–1912. doi: 10.1118/1.3106343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang QS, Zhang DS, Cong XM, Wan ML, Jin LQ. Using thermal energy produced by irradiation of Mn-Zn ferrite magnetic nanoparticles (MZF-NPs) for heat-inducible gene expression. Biomaterials. 2008;29(17):2673–2679. doi: 10.1016/j.biomaterials.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Pathak A, Vyas SP, Gupta KC. Nano-vectors for efficient liver specific gene transfer. Int J Nanomedicine. 2008;3(1):31–49. [PMC free article] [PubMed] [Google Scholar]