Abstract
Congenital heart malformations are the most common of all congenital human birth anomalies. During the past decade, research with zebrafish, chick and mouse models have elucidated many fundamental genetic pathways that govern early cardiac patterning and differentiation. This review highlights the roles of the Bmp signaling pathway in cardiogenesis and how defective Bmp signals can disrupt the intricate steps of cardiac formation and cause congenital heart defects.
Keywords: cardiac development, Bmp signaling, congenital heart disease
Overview of Bmp signaling
The Bone morphogenetic proteins (Bmp) are members of the evolutionarily conserved transforming growth factor-β (Tgf-β) superfamily that signals via a heterodimeric complex composed of type I and type II receptors. Bmps were originally discovered by their ability to induce the formation of bone and cartilage, but additional studies have implicated their important roles in multiple aspects of embryogenesis (Hogan, 1996a; Hogan, 1996b). In the canonical Bmp signaling pathway, Bmp ligands bind to the type II receptor, such as Bmpr2, and then activate a type I receptor, such as Bmpr1a, to phosphorylate the Bmp receptor regulated Smads (R-Smad) signal transducers, Smad1, Smad5 or Smad8 (Derynck and Zhang, 2003; Shi and Massague, 2003). Following release from the receptor complex, phosphorylated R-Smad associates with the common Smad4 to form a trimeric complex composed of two R-Smads and Smad4, which can then induce transcription of downstream genes (Fig.1). In addition, Bmp signaling is also mediated via non-Smad signaling pathways such as a MAPK-signaling pathway (Aubin et al., 2004; Nohe et al., 2004; Xu et al., 2008).
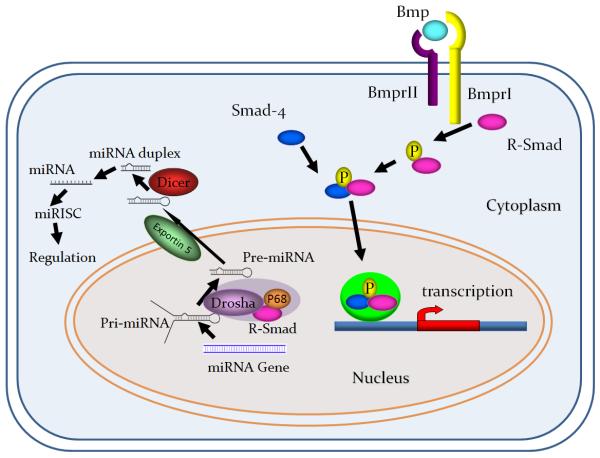
Figure 1. Summary of Bmp-signaling pathway.
In the canonical pathway, Bmp ligands bind to type II receptors then activate type I receptors. Activated type I receptors phosphorylate R-Smads (Smad 1,5,8) and following release from the type I receptors, phosphorylated R-Smads associate with the common Smad4 to form complex, which can then initiate transcription of target genes. Recent findings indicate that Bmp signals regulate gene expression through pathways mediated by microRNAs. Bmp signaling can regulate miRNA expression via the canonical pathway, and in addition, Smad1/5 can bind to a sequence specific site in the primary (pri)-miRNA to recruit the Drosha complex and promote the processing of the pri-miRNA to the precursor (pre)-miRNA (Davis et al., 2008; Davis et al., 2010; Li et al., 2010).
Recent findings further indicate that Bmp signals could regulate gene expression through microRNA (miRNA)-mediated pathways (Davis et al., 2008). In some contexts, Bmp signaling regulates miRNA expression via the canonical effector pathway (Li et al., 2008). In addition, Smad1/5 forms a protein-protein interaction with the Drosha complex to regulate processing of the primary (pri-) miRNA to the precursor (pre-) miRNA (Fig.1). This interaction between Smad and Drosha complex is both Smad4 independent and C-terminal phosphorylation independent (Davis et al., 2008). Furthermore, very recent work has identified an RNA Smad-binding element (R-SBE) within the stem sequence of a subset of pri-miRNAs which binds to the MH1 domain of Smad1/5 and is necessary and sufficient for Drosha cleavage (Davis et al., 2010). Together these new findings indicate that Smad1/5 is a multifunctional molecule that in addition to transcriptional regulation also functions as part of an enzymatic complex to promote miRNA processing.
Overview of Bmp ligand and receptor germ line loss of function studies in mice
Mice with functional disruption (knockout) of Bmp2 and 4 are nonviable. Bmp2 knockout mice die at 7.5~10.5 days post coitum (dpc) and have defects in amnion/chorion and cardiac development (Zhang and Bradley, 1996). Bmp4 knockout mice die at 6.5~9.5dpc and show defective mesodermal differentiation (Winnier et al., 1995). Both Bmp5 and Bmp6 knockout mice are viable and fertile with no gross cardiac abnormalities (Kingsley et al., 1992; Solloway et al., 1998). Bmp7 deficient mice die shortly after birth and display severe defects of kidney and eye development, as well as minor skeletal defects (Dudley et al., 1995; Luo et al., 1995). Since Bmp5, Bmp6 and Bmp7 are in the same Bmp family subgroup and individual knockout mice failed to uncover a role in cardiogenesis, functional redundancy may underlie the observed phenotypes of individual knockouts. Indeed, the double knockout mice have more severe phenotypes than the individual knockouts. Bmp5 and Bmp7 double knockout mice are embryonic lethal at 10.5dpc with defective cardiac cushion formation, and show other defects within co-expressing tissues (Solloway and Robertson, 1999). Bmp5 and Bmp6 double mutant animals have slightly exacerbated sternal defects compared to the Bmp6 knockout mice (Solloway et al., 1998). Bmp6 and Bmp7 double knockout mice die at 10.5 ~15.5dpc due to cardiac defects (Kim et al., 2001).
Bmpr1a knockout mice die at 9.5dpc due to a lack of mesoderm formation and morphological defects are first detected at 7.5dpc, which suggests the critical role of Bmpr1a in mesoderm induction during gastrulation (Mishina et al., 1995). Bmpr1b is not expressed during early cardiac development and knockout mice are viable but exhibit defects in the appendicular skeleton (Yi et al., 2000). Bmpr2 is required for mouse gastrulation and its knockout is early embryonic lethal (Beppu et al., 2000).
Germ line loss of function studies have also uncovered roles for Smad1 and Smad5 in embryogenesis. Smad1 knockout mice die at 10.5dpc due to failure to form umbilical-placenta connections (Tremblay et al., 2001). Smad5 knockout mice die at 10.5~11.5dpc due to defects in angiogenesis and cardiac looping that are secondary to aberrant left-right asymmetry signaling (Chang et al., 2000; Yang et al., 1999). Smad8 knockout mice are viable and fertile, but have defective pulmonary vascular remodeling (Huang et al., 2009). A nonsense mutation of Smad8 resulting in a truncated protein in human patients is associated with pulmonary arterial hypertension (Shintani et al., 2009). Smad4 knockout mice die before 7.5dpc and have growth retardation, failure of gastrulation and abnormal visceral endoderm development (Sirard et al., 1998).
Bmp signaling function in cardiogenic mesoderm specification
During gastrulation, heart precursor cells are generated within bilateral fields in the anterior lateral plate mesoderm (Fig.2) and the cardiogenic regions receive inductive cues that promote cardiac differentiation. Insight into the function of Bmp signaling in specification of cardiogenic mesoderm was initially found through studies of Dpp, which is a Drosophila orthologue of vertebrate Bmp2. Dpp is required for heart-inducing activity during gastrulation and mesoderm migration. Dpp deficient embryos failed to form progenitor cells of the dorsal vessel, the Drosophila cardiac organ, while mutant embryos with ectopic Dpp expression resulted in ectopic formation of dorsal vessel cells (Frasch, 1995; Yin and Frasch, 1998).
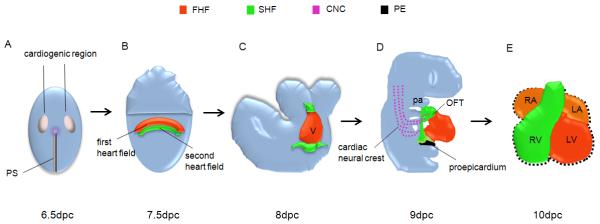
Figure 2. Summary of cardiac development.
(A) Cardiac progenitor stage. About 6.5dpc, myocardial progenitor cells, which originate in the primitive streak (PS), start to migrate to the anterior bilateral fields of the embryo. (B) Cardiac crescent stage. About 7.5dpc, myocardial progenitor cells form the cardiac crescent and the first heart field (FHF) starts to give rise to differentiated myocardial cells. The second heart field (SHF) is located medially to the cardiac crescent at this stage. Shown in red: FHF location and contribution; Shown in green: SHF location and contribution (the same color coding as here for the lineage contributions to the heart at later stages in C to E). (C) Linear heart tube stage. About 8dpc, the cardiac crescent fuses at the midline of the embryo and forms a linear cardiac tube. SHF cells start extending anterior and dorsal at 8-8.5dpc to add to the heart tube. (D) Heart looping stage. The linear heart tube subsequently undergoes looping at about 8.5dpc. About 9dpc, the cardiac neural crest (CNC) cells (shown in purple) start to migrate into the outflow tract (OFT) and contribute to the developing heart tube. Proepicardium (PE) (shown in dark) located at the cardiac venous pole also start to emigrate onto the looping heart tube to form the epicardium. (E) Chamber formation stage. About 10dpc, the heart starts to form well-defined four chambers and chamber septation is completed by 12.5dpc (Buckingham et al., 2005).
pa, pharyngeal arch; LA, left atrium; LV, left ventricle; OFT, outflow tract; PS, primitive streak; RA, right atrium; RV, right ventricle.
Data from zebrafish, chick and mouse models also provided solid evidence that Bmp signals have essential roles in cardiac induction. Bmp2 is a cardiac specification factor that elicits ectopic expression of the early cardiac markers Nkx2.5 and Gata4 when it is ectopically expressed (Andree et al., 1998; Brand, 2003; Jamali et al., 2001; Liberatore et al., 2002; Lien et al., 2002; Reiter et al., 2001; Schlange et al., 2000; Schultheiss et al., 1997; van Wijk et al., 2007). Nkx2.5 has an evolutionarily conserved Smad binding site in its enhancer and maintenance of Nkx2.5 expression requires regulation by Bmp signaling in Xenopus, chick, and mouse embryonic hearts (Andree et al., 1998; Jamali et al., 2001; Liberatore et al., 2002; Lien et al., 2002; Reiter et al., 2001; Schlange et al., 2000; Schultheiss et al., 1997; van Wijk et al., 2007). Analysis of zebrafish mutant for the type I serine/threonine kinase receptor Alk8 (Lost-a-fin), also known as Acvr1, which is required for Bmp 2, 4 and 7 signaling, indicated that Bmp activity is required for cardiac progenitor specification (Marques and Yelon, 2009). Moreover, data from studies using Bmp inhibitors including Noggin, truncated versions of Bmp receptors, and inhibitory Smad6 further confirmed the heart-inducing activity of Bmp signaling (Schlange et al., 2000; Schultheiss et al., 1997; Shi et al., 2000).
Bmp signaling function in second heart field (SHF)
Two major cardiac progenitor pools, the primary/first heart field (PHF/FHF) and the anterior/second heart field (AHF/SHF), contribute to the heart and give rise to cardiomyocyte, smooth muscle and endothelial cells, the major cardiac lineages (Fig.2) (for detailed review see (Abu-Issa and Kirby, 2008; Buckingham et al., 2005; Dyer and Kirby, 2009)). The FHF mainly contributes to atrial and left ventricular (LV) myocardium. The SHF, located dorsomedial to cardiac crescent at 7.5dpc, starts extending anterior and dorsal at 8.0-8.5dpc to add to form the outflow tract (OFT) and right ventricular (RV) myocardium and endocardium. The SHF also contributes to the inflow tract and smooth muscle walls of the intra-pericardial aortic and pulmonary trunks (Fig.2) (Abu-Issa and Kirby, 2008; Buckingham et al., 2005; Cai et al., 2003; Meilhac et al., 2004a; Meilhac et al., 2004b; Waldo et al., 2005; Zaffran et al., 2004). The FHF and SHF express both distinct and overlapping molecular markers: the Hand1 and Tbx5 transcriptional regulators mark FHF while Fgf10 and the Lim-homeobox gene Isl1 mark SHF, and the Nkx2.5 homeobox gene is expressed in both FHF and SHF (Bruneau et al., 2001; Cai et al., 2003; Kelly et al., 2001; Schwartz and Olson, 1999; Srivastava and Olson, 1997; Takeuchi et al., 2003; Yuan and Schoenwolf, 2000).
Bmp signaling plays a critical role in SHF specification, regulation of proliferation, and induction of myocardial differentiation. Nkx2.5 null embryos have up-regulated Bmp2 expression and activity that is associated with expanded SHF specification (Prall et al., 2007). Nkx2.5 mutants also had drastically reduced cardiac progenitor proliferation that could be rescued by Smad1 loss of function indicating Bmp signaling limits progenitor cell growth (Prall et al., 2007). After progenitor cell specification and expansion, Bmp signaling is still required for SHF differentiation. Based on data from chick embryos, Bmp2 induced SHF explants to differentiate into myocardium (Waldo et al., 2001). Very recent work using mouse embryos indicated that Bmp signaling regulates the miR-17-92 cluster as a mechanism to promote myocardial differentiation (Wang et al., 2010). The oncogenic miRNA miR-17-92 cluster, also known as oncomir-1, has essential functions not only in tumor formation but also in normal embryonic development, and has specifically been shown to be required for lung, heart and immune system development (Hayashita et al., 2005; He et al., 2005; Lu et al., 2007; Ventura et al., 2008; Xiao et al., 2008). The miRNA 17-92 cluster encodes six miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1) that are processed from a common primary transcript, Bmp signaling directly regulates transcription of the miRNA-17-92 cluster via Smad binding sites in the 5′ flanking region of miRNA-17-92. In normal embryos, the Bmp-miRNA-17-92 regulatory pathway down-regulates cardiac progenitor genes such as Isl1 and Tbx1 to enhance myocardial differentiation, while in Bmp mutant embryos, cardiac progenitor genes failed to be down-regulated and myocardial differentiation was defective (Wang et al., 2010).
Bmp signaling events during chamber and cushion tissue morphogenesis
Cardiac looping brings the future chamber forming areas into an alignment with the atrioventricular canal separating the atrial and ventricular regions. As cardiac remodeling occurs chamber septation is completed by 12.5dpc, forming the distinct left and right ventricles as well as the left and right atria. Chamber formation involves septation of the atrial and ventricular chambers and septation of the primary heart tube into left and right components. The myocardially-derived septums of the inter-atrial and inter-ventricular regions fuse with the endocardial cushion-derived atrioventricular septum, resulting in complete separation of the chambers (for detailed review see (Harvey, 2002)).
Clinically, heterozygous deletion of BMP2 within 20p12.3, has been found to predispose human patients to Wolff-Parkinson-White syndrome (WPW), a pre-excitation syndrome that is often asymptomatic, but in some presents as tachycardia as a result of abnormal connection between the atria and ventricles (Lalani et al., 2009). While germline deletion of Bmp2 in mouse models leads to embryonic lethality, some mutants fail to form a heart, while some formed hearts in the exocoelomic cavity (Zhang and Bradley, 1996). Conditional deletion of Bmp4 in cardiomyocyte in mice results in ventricular septal defects (VSD), atrioventricular canal defect (AVCD) and double outlet right ventricle (DORV) (Jiao et al., 2003). Bmp2 and Bmp4 compound heterozygous mice embryos also have VSD (Goldman et al., 2009; Uchimura et al., 2009). Additionally, Bmp6 and Bmp7 deficient mice display defective chamber septation and hypoplastic ventricles with reduced trabeculations (Kim et al., 2001). Likewise, compound Bmp5 and Bmp7 mutant mice have defective chamber septation with myocardial and pericardial abnormalities (Solloway and Robertson, 1999). Interestingly, as chamber formation begins around 9.0dpc, Bmp10 expression can first be detected in the ventricular myocardium, when its expression is restricted to the trabeculated part of the common ventricular chamber and the bulbus cordis of the developing heart, and after 12.5dpc can be detected in the atrial wall (Neuhaus et al., 1999). Mice deficient for this novel member of the Tgf-β family die around 9.0dpc due to incomplete ventricular development including profound hypoplastic ventricular walls and absence of ventricular trabeculae, and abnormal cushion proliferation in both the OFT and atrioventricular canal (AVC) (Chen et al., 2004). Endocardial Notch1 expression has been shown to be required for myocardial Bmp10 expression during ventricular chamber formation (Grego-Bessa et al., 2007).
Moreover, analysis of Bmp receptors further determined that Bmp signaling has essential roles in development of chamber myocardium. Data from zebrafish mutant for Alk8 indicated that Bmp activity is required for chamber formation, regulation of overall heart size, and atrial and ventricular fate (Marques and Yelon, 2009). Further, conditional deletion of Bmpr2 in the developing heart in mice results in cardiac defects including DORV, VSD, and AV cushion defects (Beppu et al., 2009).
By using Smad4 conditional loss of function alleles in conjunction with several different Cre lines, Smad4 deletion in cardiomyocytes resulted in mice with severe cardiac defects including ventricular myocardium defects and VSD (Azhar et al., 2010; Qi et al., 2007; Song et al., 2007; Wang et al., 2005). Despite variations in the timing and efficiency of the myocardial specific Smad4 inactivation, these mouse models mutant for the common Smad4 further demonstrated the essential roles of Bmp signaling in myocardium.
During myocardial patterning, Bmp signaling is required for precise regulation of many myocardial target genes. Myocardial patterning within the AVC is tightly regulated by Bmp signaling in the AV myocardium where Bmp2 induces Tbx2 transcription, which has precise expression patterns in chamber formation (Ma et al., 2005). Tbx2 induction is required to inhibit chamber-specific genes such as Natriuretic precursor peptide type A (Nppa), Connexin (Cx) 40, Cx43, and Chisel in AVC myocardium, suggesting a role for Bmp2 in myocardial differentiation (Christoffels et al., 2004). Bmp2 directly regulates Tbx2 through canonical transcriptional pathway (Shirai et al., 2009). In addition, an inhibitory protein-protein interaction between Smad1 and Tbx20, a Tbx2 repressor, in AVC myocardium, is permissive for Tbx2 transcription in AVC myocardium (Singh et al., 2009). Interestingly, there is further evidence in Xenopus that Bmp signaling also regulates Tbx20 transcription via a canonical Smad1/4 pathway suggesting a complex regulatory network involving Bmp-regulated Tbx expression in AVC myocardium (Mandel et al., 2010).
Defects in cardiac cushion derived structures, including cardiac valves and associated structures, are the most common subtype of cardiovascular malformations and account for 25% to 30% of defects (Loffredo, 2000). The mouse heart tube at 8.5dpc is composed of an outer myocardial layer and an inner monolayer of specialized endothelium, the endocardium. The two layers are separated by a thick extracellular matrix named cardiac jelly, which is secreted by myocardial cells. At about 9.5dpc, a subset of endocardial cells in the atrioventricular (AV) canal and OFT regions undergo epithelial-mesenchymal transition (EMT) in response to regionalized myocardial signals. These cells delaminate from endocardium and invade the cardiac jelly to form the endocardial cushions, which will contribute to cardiac valve development and heart chamber formation. EMT, essential in numerous developmental processes, can be induced by a number of signal pathways such as Tgf-β and Notch, and transcription effectors such as Snail, Twist, and FoxC2 (Mani et al., 2007; Perez-Pomares and Munoz-Chapuli, 2002; Thiery and Sleeman, 2006; Timmerman et al., 2004; Yang et al., 2004) .
Data from mouse embryos showed that Bmp2 is important for enhancing cardiac jelly formation, endocardial EMT, and patterning the AV myocardium (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006; Sugi et al., 2004). Bmp2 is required for myocardial expression of Has2, which is a crucial component of the cardiac jelly matrix and required for endocardial EMT (Camenisch et al., 2000; Ma et al., 2005). In addition, in the endocardium, Bmp2 signals via Bmpr1a to promote expression of Twist1, which was a reported inducer of EMT (Ma et al., 2005; Yang et al., 2004). Data from chick models showed that Bmp2 protein is expressed in the endocardial cushions of the OFT and AV, as well as adjacent myocardial layers (Keyes et al., 2003). Other data indicate that Bmp2 is important for the formation of endocardial cushion tissue and acts synergistically with Tgf-β3 to regulate EMT (Boyer et al., 1999; Nakajima et al., 2000; Yamagishi et al., 1999).
Bmp4 has also been implicated in AV cushion morphogenesis. Bmp2 and Bmp4, encoding highly related peptides with 92% identity in the C-terminal mature ligand domain, have overlapping functions in heart development (Goldman et al., 2009; Uchimura et al., 2009). Inactivation of Bmp4 in the heart using a cardiac troponin T (cTnT) Cre transgene, a cardiomyocyte-specific Cre, and a hypomorphic Bmp4 conditional allele (Kulessa and Hogan, 2002), indicated that Bmp4 regulates proliferation of atrioventricular cushion mesenchyme (Jiao et al., 2003). In Bmp6 and Bmp7 deficient mice, formation of the OFT endocardial cushions was markedly delayed, providing further evidence for the central role of Bmps in cushion development (Kim et al., 2001).
Bmp receptors are expressed in both OFT and AV cushions of the chick embryo (Keyes et al., 2003). Conditional deletion of the Alk3/ Bmpr1a in AV myocardium resulted in abnormal cushion formation, which indicated a feedback loop with Tgf-β2 in cushion development (Gaussin et al., 2002). Endocardial deletion of Bmpr1a resulted in failed cushion EMT, which indicated a direct requirement for Bmp signaling in cushion forming endocardium (Ma et al., 2005). Moreover, studies with inhibitors of Bmp signaling further demonstrated the essential roles of Bmp signaling in cushion morphogenesis. Targeted mutation of Smad6 resulted in hyperplasia of the cardiac valves and OFT septation defects, which suggests the role of Bmp signaling in promoting endocardial cushion transformation (Galvin et al., 2000). Mouse explant experiments indicated that when Noggin was added to AV endothelial cells co-cultured with associated myocardium, it blocked endothelial cells undergoing EMT (Sugi et al., 2004).
Bmp2, which is critical for the induction of cushion EMT, also has roles during later stages in cushion and valve morphogenesis. Bmp2 has persistent expression in cushion mesenchyme at 13.5-16dpc during valve remodeling and in the valve tissue of adult mice (Sugi et al., 2004). Very recent studies with mouse models suggest the interaction between Notch1 in the endocardium and myocardial Bmp2 coordinately regulates endocardial EMT. In this model Bmp2 promotes endocardial cell invasiveness within the valve forming region between the chambers (Luna-Zurita et al., 2010). Data from mouse model also indicated that an Fgf- Bmp signaling axis is required for regulation of OFT valve remodeling, mainly through promoting the differentiation of cranial neural crest cells in OFT cushion (Zhang et al., 2010).
Bmp signaling in the outflow tract: the second heart field (SHF) and cranial neural crest (CNC)
Unseptated initially, the OFT divides into the pulmonary trunk (PT) and aorta, which is critical for separation of postnatal pulmonary and systemic circulation. Malformations in human OFT development include double outlet right ventricle (DORV) where both of the great arteries completely or partially connect to the right ventricle and transposition of the great arteries (TGA), which results in reversed ventriculoarterial connections. Other malformations in OFT development including persistent truncus arteriosus (PTA) where truncus arteriosus never properly divides into the pulmonary artery and aorta, patent ductus arteriosus (PDA) wherein a neonate’s ductus arteriosus fails to close after birth. Congenital OFT malformations are found in 4 per 10,000 live births and are commonly lethal (Webb, 2003), therefore insight into the genetic pathways regulating OFT development is critical for developmental biology and clinical medicine.
The OFT myocardium receives a cellular input from SHF (Fig.2) and defective development of the SHF may cause malformations in OFT development including DORV, TGA, PTA or overriding aorta (the aorta straddles the interventricular septum instead of positioned over the left ventricle). Bmp4 is expressed in SHF and within the OFT myocardium itself, suggesting a role in OFT morphogenesis (Abdelwahid et al., 2001). In mouse conditional deletion studies, inactivation of Bmp4 in SHF, OFT endocardium, pharyngeal endoderm, and OFT myocardium using Nkx2.5 Cre resulted in defects in OFT septation with aorto-pulmonary (AP) window and also defects in vascular smooth muscle recruitment to forming vessels (Liu et al., 2004). It has also been reported that feedback repression of Bmp2/Smad1 signaling by Nkx2.5 regulates OFT morphogenesis (Prall et al., 2007). The Nkx2.5 mutants have dramatically narrowed and shortened OFT, while specific inactivation of Smad1 in anterior mesoderm using Mesp1 Cre, results in increased OFT length. Importantly, Smad1 deletion in Nkx2.5 mutants partially rescued the OFT anomalies in Nkx2.5 mutants, potentially because the suppression of BMP2/Smad1 signaling by Nkx2-5 is permissive for SHF proliferation that drives OFT morphogenesis.
In addition, the developing OFT receives a cellular contribution from the cardiac neural crest (CNC) (Fig.2). The CNC is a migratory cell population that originates from the dorsal neural tube in the hindbrain region at the level of rhombomeres 6, 7, and 8. The CNC precursors migrate to the caudal 3rd, 4th and 6th pharyngeal arches and a subpopulation of these neural crest–derived cells in these pharyngeal arches continue to migrate into the outflow tract of the developing heart and contribute extensively to cardiac OFT cushion formation (Kirby et al., 1983; Kirby and Waldo, 1995). The CNC-derived aorticopulmonary septum is required for septation of the single outflow vessel into two trunks while CNC cells in the OFT cushions and truncal valves appear to make little contribution to the resulting structures postnatally (Webb et al., 2003). Ablation of neural crest in chick embryos resulted in failure of cardiac cushion formation and OFT defects including PTA and anomalies of aortic arches (Kirby et al., 1983; Kirby and Waldo, 1995). In mouse embryos, lineage tracing experiments have validated the notion that the CNC contributes to OFT cushions and the aorto-pulmonary septum (Jiang et al., 2000). Important roles of Bmp proteins, particularly Bmp2 and Bmp4, in induction, maintenance, migration and differentiation of neural crest cells have been indicated in different model organisms (Aybar and Mayor, 2002; Christiansen et al., 2000; Knecht and Bronner-Fraser, 2002).
Analysis of Bmp receptors provides further insight into functions of Bmp signaling in CNC cells during OFT morphogenesis. Conditional inactivation of Bmpr1 in cardiac neural crest, using the Wnt1Cre transgenic driver to direct Cre activity, led to severe OFT defects such as shortened OFT and PTA (Kaartinen et al., 2004; Stottmann et al., 2004). Additionally, analysis of a hypomorphic allele of the ubiquitously expressed Bmpr2, containing a partial ectodomain deletion, revealed defective proximal OFT septation in mutant mouse embryos (Delot et al., 2003). The hypomorphic mutants undergo normal gastrulation but die at midgestation with major OFT phenotype PTA. Moreover, specific inactivation of common Smad4 in CNC resulted in increased cell death and reduced contribution of CNC cells to the developing OFT (Jia et al., 2007; Nie et al., 2008). Smad4 mutants had decreased expression of genes that are critical for neural crest cells development, such as Msx1 and Msx2, and displayed severe OFT defects including defective OFT elongation and positioning, cushion hypoplasia and PTA. Lastly, over-expression of Noggin in chick embryos resulted in OFT abnormalities such as PTA and DORV. Data from these Bmp inhibitor studies suggested that Bmp signaling is required for both migration of CNC cells into the developing OFT and proliferation of OFT mesenchyme (Allen et al., 2001)
Bmp signaling related to epicardium development and differentiation
The proepicardium (PE) is a transient extracardiac mesothelial rudiment located at the cardiac venous pole and emigrates onto the looping heart tube to form the epicardium, the epithelial outer lining of the heart (Fig.2). The PE derived from the mesodermal lining of the prospective pericardial cavity, expresses specific marker genes Tbx18, Wt1, and Cfc. PE contributes multiple cell types to the heart, giving rise to cardiac fibroblasts and coronary smooth muscle cells, also perhaps contributing to the cardiac myocyte lineage (Cai et al., 2008; Christoffels et al., 2009; Ishii et al., 2010; Ishii et al., 2009; Kruithof et al., 2006; Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996; Perez-Pomares et al., 2002; van Wijk et al., 2009; Winter and Gittenberger-de Groot, 2007; Zhou et al., 2008).
Although the signals that control the epicardium morphogenetic events are still largely unknown, the essential roles of Bmp signaling during different events of epicardium morphogenesis have been indicated in different animal model studies. In the chick, a distinct level of Bmp signaling is required for PE identity and expression of PE-specific marker genes (Schlueter et al., 2006). PE and the inflow myocardium separate from the same precursor under the regulation of proper crosstalk between Bmp and Fgf signals, but a misbalance between them could cause a developmental arrest of the epicardium and enhancement of myocardium formation (van Wijk et al., 2009). Data from zebrafish also indicate that Bmp signaling in conjunction with Tbx5 is essential for PE specification (Liu and Stainier, 2010). In chick, myocardium-derived Bmp signals promote the PE protrusion toward and the attachment to the looping heart tube, and ectopic expression of Bmp signals resulted in ectopic attachment of PE (Ishii et al., 2010). In contrast, expression of Bmp antagonist, Noggin, resulted in diminished PE attachment to the heart tube (Ishii et al., 2010).
V. Concluding remarks
Although much has been learned Bmp signaling in cardiac development there are many areas for fruitful investigation. The role of Bmp signaling in miRNA processing is an important area for future studies. Further insight into what Bmp-mediated cardiac functions are due to defective miRNA activity is needed. Currently, it is unclear how generally important this mechanism will be in the heart. It will also be important to determine how many miRNAs are regulated by the canonical Bmp pathway and to integrate this information into a larger model for heart development.
Recent experiments have uncovered the importance of feedback loops in cardiac development (Prall et al., 2007). This mechanism is undoubtedly of broad importance in many different aspects of cardiac development and will need to be intensively investigated in order to solidify our understanding of heart development. Lastly, the integration of Bmp signaling and other important signaling pathways, such as Wnt and Notch signaling, will need to be understood with much more clarity.
References
- Abdelwahid E, Rice D, Pelliniemi LJ, Jokinen E. Overlapping and differential localization of Bmp-2, Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001;305:67–78. doi: 10.1007/s004410100399. [DOI] [PubMed] [Google Scholar]
- Abu-Issa R, Kirby ML. Patterning of the heart field in the chick. Dev Biol. 2008;319:223–33. doi: 10.1016/j.ydbio.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SP, Bogardi JP, Barlow AJ, Mir SA, Qayyum SR, Verbeek FJ, Anderson RH, Francis-West PH, Brown NA, Richardson MK. Misexpression of noggin leads to septal defects in the outflow tract of the chick heart. Dev Biol. 2001;235:98–109. doi: 10.1006/dbio.2001.0291. [DOI] [PubMed] [Google Scholar]
- Andree B, Duprez D, Vorbusch B, Arnold HH, Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech Dev. 1998;70:119–31. doi: 10.1016/s0925-4773(97)00186-x. [DOI] [PubMed] [Google Scholar]
- Aubin J, Davy A, Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–94. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev. 2002;12:452–8. doi: 10.1016/s0959-437x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Azhar M, Wang PY, Frugier T, Koishi K, Deng C, Noakes PG, McLennan IS. Myocardial deletion of Smad4 using a novel alpha skeletal muscle actin Cre recombinase transgenic mouse causes misalignment of the cardiac outflow tract. Int J Biol Sci. 2010;6:546–55. doi: 10.7150/ijbs.6.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–58. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Beppu H, Malhotra R, Beppu Y, Lepore JJ, Parmacek MS, Bloch KD. BMP type II receptor regulates positioning of outflow tract and remodeling of atrioventricular cushion during cardiogenesis. Dev Biol. 2009;331:167–75. doi: 10.1016/j.ydbio.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–45. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–21. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–35. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr., Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Zwijsen A, Vogel H, Huylebroeck D, Matzuk MM. Smad5 is essential for left-right asymmetry in mice. Dev Biol. 2000;219:71–8. doi: 10.1006/dbio.1999.9594. [DOI] [PubMed] [Google Scholar]
- Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–31. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen JH, Coles EG, Wilkinson DG. Molecular control of neural crest formation, migration and differentiation. Curr Opin Cell Biol. 2000;12:719–24. doi: 10.1016/s0955-0674(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–9. doi: 10.1038/nature07916. discussion E9-10. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Hoogaars WM, Tessari A, Clout DE, Moorman AF, Campione M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev Dyn. 2004;229:763–70. doi: 10.1002/dvdy.10487. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell. 2010;39:373–84. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–20. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–44. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–7. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr., et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–4. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–83. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DC, Donley N, Christian JL. Genetic interaction between Bmp2 and Bmp4 reveals shared functions during multiple aspects of mouse organogenesis. Mech Dev. 2009;126:117–27. doi: 10.1016/j.mod.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, et al. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–29. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RP. Patterning the vertebrate heart. Nat Rev Genet. 2002;3:544–56. doi: 10.1038/nrg843. [DOI] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–32. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996a;6:432–8. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996b;10:1580–94. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Huang Z, Wang D, Ihida-Stansbury K, Jones PL, Martin JF. Defective pulmonary vascular remodeling in Smad8 mutant mice. Hum Mol Genet. 2009;18:2791–801. doi: 10.1093/hmg/ddp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Garriock RJ, Navetta AM, Coughlin LE, Mikawa T. BMP signals promote proepicardial protrusion necessary for recruitment of coronary vessel and epicardial progenitors to the heart. Dev Cell. 2010;19:307–16. doi: 10.1016/j.devcel.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Langberg J, Rosborough K, Mikawa T. Endothelial cell lineages of the heart. Cell Tissue Res. 2009;335:67–73. doi: 10.1007/s00441-008-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali M, Karamboulas C, Rogerson PJ, Skerjanc IS. BMP signaling regulates Nkx2-5 activity during cardiomyogenesis. FEBS Lett. 2001;509:126–30. doi: 10.1016/s0014-5793(01)03151-9. [DOI] [PubMed] [Google Scholar]
- Jia Q, McDill BW, Li SZ, Deng C, Chang CP, Chen F. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev Biol. 2007;311:172–84. doi: 10.1016/j.ydbio.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–16. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–7. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–90. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Keyes WM, Logan C, Parker E, Sanders EJ. Expression and function of bone morphogenetic proteins in the development of the embryonic endocardial cushions. Anat Embryol (Berl) 2003;207:135–47. doi: 10.1007/s00429-003-0337-2. [DOI] [PubMed] [Google Scholar]
- Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–66. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–61. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL. Neural crest and cardiovascular patterning. Circ Res. 1995;77:211–5. doi: 10.1161/01.res.77.2.211. [DOI] [PubMed] [Google Scholar]
- Knecht AK, Bronner-Fraser M. Induction of the neural crest: a multigene process. Nat Rev Genet. 2002;3:453–61. doi: 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev Biol. 2006;295:507–22. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Kulessa H, Hogan BL. Generation of a loxP flanked bmp4loxP-lacZ allele marked by conditional lacZ expression. Genesis. 2002;32:66–8. doi: 10.1002/gene.10032.abs. [DOI] [PubMed] [Google Scholar]
- Lalani SR, Thakuria JV, Cox GF, Wang X, Bi W, Bray MS, Shaw C, Cheung SW, Chinault AC, Boggs BA, et al. 20p12.3 microdeletion predisposes to Wolff-Parkinson-White syndrome with variable neurocognitive deficits. J Med Genet. 2009;46:168–75. doi: 10.1136/jmg.2008.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Pashmforoush M, Sucov HM. Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev Cell. 2010;18:480–5. doi: 10.1016/j.devcel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–11. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore CM, Searcy-Schrick RD, Vincent EB, Yutzey KE. Nkx-2.5 gene induction in mice is mediated by a Smad consensus regulatory region. Dev Biol. 2002;244:243–56. doi: 10.1006/dbio.2002.0604. [DOI] [PubMed] [Google Scholar]
- Lien CL, McAnally J, Richardson JA, Olson EN. Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev Biol. 2002;244:257–66. doi: 10.1006/dbio.2002.0603. [DOI] [PubMed] [Google Scholar]
- Liu J, Stainier DY. Tbx5 and Bmp signaling are essential for proepicardium specification in zebrafish. Circ Res. 2010;106:1818–28. doi: 10.1161/CIRCRESAHA.110.217950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci U S A. 2004;101:4489–94. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo CA. Epidemiology of cardiovascular malformations: prevalence and risk factors. Am J Med Genet. 2000;97:319–25. doi: 10.1002/1096-8628(200024)97:4<319::aid-ajmg1283>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–53. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Zurita L, Prados B, Grego-Bessa J, Luxan G, del Monte G, Benguria A, Adams RH, Perez-Pomares JM, de la Pompa JL. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest. 2010;120:3493–507. doi: 10.1172/JCI42666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–11. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Mandel EM, Kaltenbrun E, Callis TE, Zeng XX, Marques SR, Yelon D, Wang DZ, Conlon FL. The BMP pathway acts to directly regulate Tbx20 in the developing heart. Development. 2010;137:1919–29. doi: 10.1242/dev.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104:10069–74. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques SR, Yelon D. Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality. Dev Biol. 2009;328:472–82. doi: 10.1016/j.ydbio.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004a;6:685–98. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kerszberg M, Moss JE, Buckingham ME. Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J Cell Biol. 2004b;164:97–109. doi: 10.1083/jcb.200309160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992;89:9504–8. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–32. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Gilbert DJ, Copeland NG, Jenkins NA, Ueno N, Behringer RR. Genomic organization and chromosomal location of the mouse type I BMP-2/4 receptor. Biochem Biophys Res Commun. 1995;206:310–7. doi: 10.1006/bbrc.1995.1043. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–27. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Neuhaus H, Rosen V, Thies RS. Heart specific expression of mouse BMP-10 a novel member of the TGF-beta superfamily. Mech Dev. 1999;80:181–4. doi: 10.1016/s0925-4773(98)00221-4. [DOI] [PubMed] [Google Scholar]
- Nie X, Deng CX, Wang Q, Jiao K. Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Dev Biol. 2008;316:417–30. doi: 10.1016/j.ydbio.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–9. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–13. [PubMed] [Google Scholar]
- Perez-Pomares JM, Munoz-Chapuli R. Epithelial-mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in Metazoans. Anat Rec. 2002;268:343–51. doi: 10.1002/ar.10165. [DOI] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–59. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Yang G, Yang L, Lan Y, Weng T, Wang J, Wu Z, Xu J, Gao X, Yang X. Essential role of Smad4 in maintaining cardiomyocyte proliferation during murine embryonic heart development. Dev Biol. 2007;311:136–46. doi: 10.1016/j.ydbio.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Verkade H, Stainier DY. Bmp2b and Oep promote early myocardial differentiation through their regulation of gata5. Dev Biol. 2001;234:330–8. doi: 10.1006/dbio.2001.0259. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–8. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlange T, Andree B, Arnold HH, Brand T. BMP2 is required for early heart development during a distinct time period. Mech Dev. 2000;91:259–70. doi: 10.1016/s0925-4773(99)00311-1. [DOI] [PubMed] [Google Scholar]
- Schlueter J, Manner J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Dev Biol. 2006;295:546–58. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–62. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Schwartz RJ, Olson EN. Building the heart piece by piece: modularity of cis-elements regulating Nkx2-5 transcription. Development. 1999;126:4187–92. doi: 10.1242/dev.126.19.4187. [DOI] [PubMed] [Google Scholar]
- Shi Y, Katsev S, Cai C, Evans S. BMP signaling is required for heart formation in vertebrates. Dev Biol. 2000;224:226–37. doi: 10.1006/dbio.2000.9802. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shintani M, Yagi H, Nakayama T, Saji T, Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet. 2009;46:331–7. doi: 10.1136/jmg.2008.062703. [DOI] [PubMed] [Google Scholar]
- Shirai M, Imanaka-Yoshida K, Schneider MD, Schwartz RJ, Morisaki T. T-box 2, a mediator of Bmp-Smad signaling, induced hyaluronan synthase 2 and Tgfbeta2 expression and endocardial cushion formation. Proc Natl Acad Sci U S A. 2009;106:18604–9. doi: 10.1073/pnas.0900635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Horsthuis T, Farin HF, Grieskamp T, Norden J, Petry M, Wakker V, Moorman AF, Christoffels VM, Kispert A. Tbx20 interacts with smads to confine tbx2 expression to the atrioventricular canal. Circ Res. 2009;105:442–52. doi: 10.1161/CIRCRESAHA.109.196063. [DOI] [PubMed] [Google Scholar]
- Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–19. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–39. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–68. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Song L, Yan W, Chen X, Deng CX, Wang Q, Jiao K. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ Res. 2007;101:277–85. doi: 10.1161/CIRCRESAHA.107.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, Olson EN. Knowing in your heart what’s right. Trends Cell Biol. 1997;7:447–53. doi: 10.1016/S0962-8924(97)01150-1. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–18. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–18. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Ohgi M, Koshiba-Takeuchi K, Shiratori H, Sakaki I, Ogura K, Saijoh Y, Ogura T. Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development. 2003;130:5953–64. doi: 10.1242/dev.00797. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development. 2001;128:3609–21. doi: 10.1242/dev.128.18.3609. [DOI] [PubMed] [Google Scholar]
- Uchimura T, Komatsu Y, Tanaka M, McCann KL, Mishina Y. Bmp2 and Bmp4 genetically interact to support multiple aspects of mouse development including functional heart development. Genesis. 2009;47:374–84. doi: 10.1002/dvg.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–55. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- van Wijk B, van den Berg G, Abu-Issa R, Barnett P, van der Velden S, Schmidt M, Ruijter JM, Kirby ML, Moorman AF, van den Hoff MJ. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ Res. 2009;105:431–41. doi: 10.1161/CIRCRESAHA.109.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–86. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–88. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wang J, Greene SB, Bonilla-Claudio M, Tao Y, Zhang J, Bai Y, Huang Z, Black BL, Wang F, Martin JF. Bmp Signaling Regulates Myocardial Differentiation from Cardiac Progenitors Through a MicroRNA-Mediated Mechanism. Dev Cell. 2010;19:903–12. doi: 10.1016/j.devcel.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xu N, Feng X, Hou N, Zhang J, Cheng X, Chen Y, Zhang Y, Yang X. Targeted disruption of Smad4 in cardiomyocytes results in cardiac hypertrophy and heart failure. Circ Res. 2005;97:821–8. doi: 10.1161/01.RES.0000185833.42544.06. [DOI] [PubMed] [Google Scholar]
- Webb GD. Challenges in the care of adult patients with congenital heart defects. Heart. 2003;89:465–9. doi: 10.1136/heart.89.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK. Septation and separation within the outflow tract of the developing heart. J Anat. 2003;202:327–42. doi: 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Winter EM, Gittenberger-de Groot AC. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci. 2007;64:692–703. doi: 10.1007/s00018-007-6522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–14. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Jr., Deng C, Chai Y. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell. 2008;15:322–9. doi: 10.1016/j.devcel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Nakajima Y, Miyazono K, Nakamura H. Bone morphogenetic protein-2 acts synergistically with transforming growth factor-beta3 during endothelial-mesenchymal transformation in the developing chick heart. J Cell Physiol. 1999;180:35–45. doi: 10.1002/(SICI)1097-4652(199907)180:1<35::AID-JCP4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–80. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127:621–30. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- Yin Z, Frasch M. Regulation and function of tinman during dorsal mesoderm induction and heart specification in Drosophila. Dev Genet. 1998;22:187–200. doi: 10.1002/(SICI)1520-6408(1998)22:3<187::AID-DVG2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yuan S, Schoenwolf GC. Islet-1 marks the early heart rudiments and is asymmetrically expressed during early rotation of the foregut in the chick embryo. Anat Rec. 2000;260:204–7. doi: 10.1002/1097-0185(20001001)260:2<204::AID-AR90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95:261–8. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chang JY, Huang Y, Lin X, Luo Y, Schwartz RJ, Martin JF, Wang F. The FGF-BMP Signaling Axis Regulates Outflow Tract Valve Primordium Formation by Promoting Cushion Neural Crest Cell Differentiation. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.225318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]