Abstract
Maldevelopment of outflow tract and aortic arch arteries is among the most common forms of human congenital heart diseases. Both Bmp4 and Tbx1 are known to play critical roles during cardiovascular development. Expression of these two genes partially overlaps in pharyngeal arch areas in mouse embryos. In this study, we applied a conditional gene inactivation approach to test the hypothesis that Bmp4 expressed from the Tbx1 expression domain plays a critical role for normal development of outflow tract and pharyngeal arch arteries. We showed that inactivation of Bmp4 from Tbx1-expressing cells leads to the spectrum of deformities resembling the cardiovascular defects observed in human DiGeorge syndrome patients. Inactivation of Bmp4 from the Tbx1 expression domain did not cause patterning defects, but affected remodeling of outflow tract and pharyngeal arch arteries. Our further examination revealed that Bmp4 is required for normal recruitment/differentiation of smooth muscle cells surrounding the PAA4 and survival of outflow tract cushion mesenchymal cells.
Key Words: Bmp4, Outflow tract, Pharyngeal arch artery, Morphogenesis
Introduction
Congenital heart diseases occur in as many as 1% of newborns, and are the leading cause of infant morbidity and mortality [Hoffman, 1995; Hoffman and Kaplan, 2002]. Maldevelopment of the cardiac outflow tract and great vessels are the most often observed forms of congenital heart diseases in human patients [Hoffman, 1995; Hoffman and Kaplan, 2002]. The genetic, molecular and cellular mechanisms underlying proper development of the outflow tract and aortic arch arteries have been extensively studied, and yet remain elusive.
The embryonic outflow tract is derived from the second heart field and is initially formed as a single tube connecting the primitive right ventricle with symmetric pharyngeal arch arteries [Eisenberg and Markwald, 2004; Buckingham et al., 2005; Kelly, 2005; Black, 2007; Dyer and Kirby, 2009; Nakajima, 2010]. Pharyngeal arch arteries reside in a set of temporal embryonic apparatus termed pharyngeal arches, and are surrounded by mesenchymal cells derived from both paraxial mesodermal cells and neural crest cells (NCCs). During midgestation, a septum is formed within the single tube of the outflow tract to divide it into pulmonary and aortic outlets, which are connected with the right and left ventricles, respectively. In coordination with outflow tract septation, the original symmetric pharyngeal arch arteries are remodeled into the mature asymmetric aortic arch arteries [Graham, 2001; Hiruma et al., 2002; Graham, 2003; Yamagishi and Srivastava, 2003].
DiGeorge syndrome (DS) is the most common chromosome microdeletion syndrome in humans, affecting 1:4,000 live births [Lindsay and Baldini, 1998]. About 75% of DS patients are born with cardiac malformations mainly affecting the outflow tract and aortic arch arteries [Epstein, 2001; Grossfeld, 2003; Yamagishi and Srivastava, 2003; Baldini, 2004; Scambler, 2010]. Most DS patients have an about 3-Mb deletion in the 22q11.2 region, containing about 30 genes including TBX1, which encodes a T box transcription factor [Ryan and Chin, 2003; Yamagishi and Srivastava, 2003; Baldini, 2004; Plageman and Yutzey, 2005; Scambler, 2010]. Direct evidence linking TBX1 to DS first came from mouse genetic studies showing that heterozygosity of Tbx1 causes aortic arch defects affecting 4th pharyngeal arch artery derivatives similar to DS, while homozygosity of Tbx1 causes most cardiovascular defects (including outflow tract and aortic arch defects) and pharyngeal arch defects (including hypoplasia or aplasia of pharyngeal glands, craniofacial dysmorphism and ear defects) seen in DS patients [Jerome and Papaioannou, 2001; Lindsay et al., 2001; Merscher et al., 2001]. The discovery of mutations in TBX1 from patients with DS phenotypes but without chromosomal deletion provides the conclusive evidence supporting the critical role of Tbx1 in the pathogenesis of DS [Yagi et al., 2003]. During midgestation of mouse embryos, Tbx1 is expressed in pharyngeal endodermal and mesodermal cells, and acts in both cell autonomous and nonautonomous manners to regulate outflow tract and pharyngeal arch artery development [Chapman et al., 1996; Jerome and Papaioannou, 2001; Lindsay et al., 2001; Merscher et al., 2001; Abu-Issa et al., 2002; Frank et al., 2002; Kochilas et al., 2002; Vitelli et al., 2002a, b; Brown et al., 2004; Hu et al., 2004; Xu et al., 2004; Zhang et al., 2005, 2006; Arnold et al., 2006a, b]. Therefore, the Tbx1 expression domain defines a group of cells with critical functions for outflow tract development and pharyngeal arch artery remodeling.
Bmp4 belongs to the bone morphogenic protein (BMP) family of secreted cytokines. Bmp4, like other BMP ligands, exerts its activity by binding to the type 1 and 2 receptor complex on the surface of target cells. The ligand-receptor complex then activates downstream signaling cascades through the canonical Smad pathway and noncanonical kinase pathways [Datto and Wang, 2000; Shi and Massagué, 2003; de Caestecker, 2004; ten Dijke and Hill, 2004; Feng and Derynck, 2005; Massague et al., 2005; Moustakas and Heldin, 2005; Massagué and Gomis, 2006]. Previous studies have demonstrated that Bmp4 plays an essential role during outflow tract and pharyngeal arch artery development [Jiao et al., 2003; Liu et al., 2005; McCulley et al., 2008]. Of particular significance, Bmp4 is highly expressed in the pharyngeal mesenchyme, overlapping with Tbx1 expression in this region [Vitelli et al., 2002a; Jiao et al., 2003; Liu et al., 2004; Xu et al., 2004]. In this study, we use a conditional gene inactivation approach to address whether Bmp4 expressed from the Tbx1 expression domain is an essential source for normal cardiovascular development.
Materials and Methods
Mouse Maintenance and Genotyping
All procedures using mice were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Tbx1-Cre, Bmp4loxP-lacZ and Bmp4tm1 (Bmp4 null allele) mouse lines have been previously described [Fujiwara et al., 2002; Kulessa and Hogan, 2002; Jiao et al., 2003; Brown et al., 2004]. Tbx1-Cre mice were crossed with Bmptm1/+ mice to generate Tbx1-Cre;Bmp4tm1/+ male mice, which were then crossed with Bmp4loxP-lacZ/loxP-lacZ female mice to produce Tbx1-Cre;Bmptm1/loxP-lacZ mutant embryos. Mouse genotypes were determined by PCR analysis using Cre and Bmp4 primers.
Histology, in situ Hybridization, X-Gal and Ink Injection
For histological analysis, all samples were fixed with 4% paraformaldehyde (PFA) and processed into paraffin-embedded sections using routine procedures. Procedures for wholemount in situ hybridization, X-gal staining and Indian ink injection were the same as described in Nie et al. [2008]. The plasmids for generation of in situ probes against AP-2 and Crabp1 were originally described in Feng and Williams [2003] and Dolle et al. [1990], respectively.
Apoptosis and Immunohistochemistry Assays
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using DeadEnd Colorimetric TUNEL System (Promega) following the manufacturer's protocol. An anti-phosphorylated histone H3 polyclonal antibody (1:1,000, cat. No. 06-570, Upstate) and an anti-smooth muscle actin (SMA) monoclonal antibody (1:1,000, clone 1A4, Sigma) were used for immunohistochemical analysis. Signals were visualized using the Envision + system (DakoCytomation), and sections were counterstained with hematoxylin to visualize nuclei.
Results
Efficient Inactivation of Bmp4 in the Pharyngeal Region by Tbx1-Cre
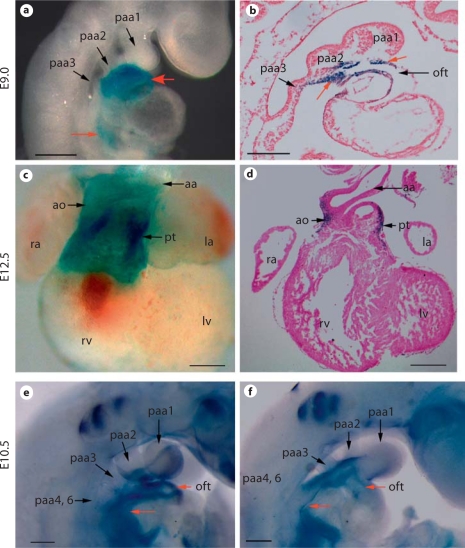
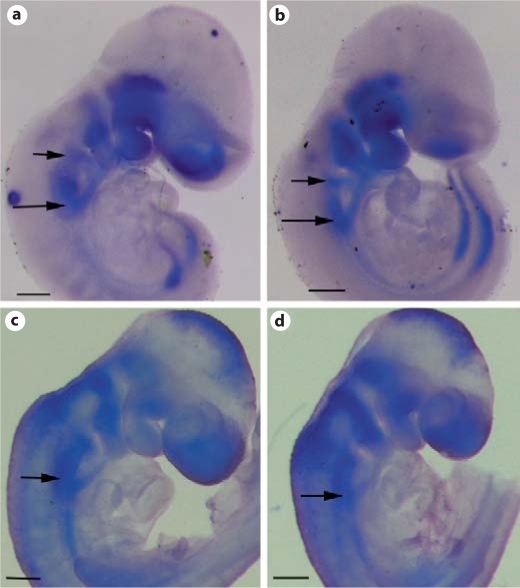
The Tbx1-Cre transgene has been shown previously to faithfully label Tbx1 expressing cells in pharyngeal mesodermal and endodermal cells [Brown et al., 2004]. To determine whether Tbx1-Cre can efficiently inactivate Bmp4, we crossed Tbx1-Cre mice with Bmp4loxP-lacZ/loxP-lacZ mice to obtain Tbx1-Cre;Bmp4loxP-lacZ/+ embryos, and performed X-gal staining on these embryos. As demonstrated in previous studies [Kulessa and Hogan, 2002; Jiao et al., 2003], Cre-mediated recombination in the Bmp4loxP-lacZallele will lead to expression of lacZ under the control of endogenous Bmp4 regulatory elements. Therefore, the lacZ-positive cells are derivatives of Tbx1-expressing cells that express endogenous Bmp4. As early as E9.0, lacZ-positive cells could be detected in the outflow tract myocardial cells and pharyngeal mesenchymal cells from both wholemount and section studies (fig. 1a, b). We also observed weak signals in cells adjacent to the venous pole of embryonic hearts from wholemount staining (fig. 1a). These results confirmed that Bmp4 is expressed in Tbx1 expression derivatives and demonstrated that Tbx1-Cre can mediate recombination at the Bmp4 locus at E9.0 when cells from the anterior heart field actively join the outflow tract through the arterial pole. It was shown previously that Tbx1-Cre can inactivate target genes in the pharyngeal endoderm [Brown et al., 2004], where expression of Bmp4 is also detected [Liu et al., 2005; McCulley et al., 2008]. However, we did not observe lacZ-positive endodermal cells in the pharyngeal region. This result suggests that the group of cells expressing Bmp4 do not overlap with those expressing Cre in the endoderm. At E12.5, lacZ-positive cells were mainly detected in the cardiomyocytes at the roots of the aorta and pulmonary trunk (fig. 1c, d).
Fig. 1.
Effective inactivation of Bmp4 by Tbx1-Cre. a–d Tbx1-Cre male mice were crossed with Bmp4loxP-lacZ/loxP-lacZ female mice to obtain Tbx1-Cre;Bmp4loxP-lacZ/+ embryos at different stages. Cre-mediated recombination on the Bmp4loxP-lacZ allele will lead to lacZ knocked into the Bmp4 locus, and therefore expression of the lacZ reporter will be under the control of endogenous Bmp4 regulatory elements. An embryo at E9.0 was wholemount stained with X-gal (a), and was sagittally sectioned (b). A heart isolated from an E12.5 embryo was stained with X-gal (c) and further sectioned (d). The red arrows indicate examples of positively stained cells. e, f Tbx1-Cre;Bmp4tm1/+ male mice were crossed with Bmp4loxP-lacZ/loxP-lacZ female mice to get mutant embryos (Tbx1-Cre;Bmp4tm1/loxP-lacZ) and their littermate controls at E10.5. Wholemount in situ hybridization analysis was performed using a probe corresponding to the exon 4 of Bmp4, which is expected to be removed upon Cre-mediated recombination. The red arrows indicate the region where the signal was dramatically reduced in the mutant embryo (f) compared to the control (e). Scale bar = 200 μm.
To directly test the reduction of Bmp4 at the mRNA level, we crossed Tbx1-Cre;Bmp4tm1/+ mice with Bmp4loxP-lacZ/loxP-lacZ mice to acquire mutant embryos (Tbx1-Cre;Bmp4tm1/loxP-lacZ). Bmp4tm1 is a null allele of Bmp4 [Fujiwara et al., 2002]. Wholemount in situ hybridization was performed using a probe corresponding to the exon 4 of Bmp4. Exon 4 is flanked with two LoxP sites in the Bmp4loxP-lacZ allele and is expected to be deleted upon Cre-mediated recombination [Kulessa and Hogan, 2002]. As shown in figure 1e, f, expression of Bmp4 was nearly eliminated from the developing outflow tract and was visibly reduced from the pharyngeal mesenchyme in mutant embryos compared with litter mate control embryos. Therefore, results from both reporter and in situ hybridization assays collectively indicate that Tbx1-Cre can efficiently inactivate expression of Bmp4 from the pharyngeal mesenchymal and outflow tract myocardial cells.
Inactivation of Bmp4 from the Tbx1 Expression Domain Leads to a Spectrum of Cardiovascular Defects Resembling Those of the Human DS
Tbx1-Cre;Bmptm1/+ male mice were crossed with female Bmp4loxP-lacZ/loxP-lacZ mice to generate Tbx1-Cre;Bmp4tm1/loxP-lacZ mutant animals. The number of mutants found at birth was about 14% (19/137), which is lower than the expected Mendelian ratio. Newborn mutants were externally normal, but died shortly after birth presumably due to cardiovascular defects. The number of mutant embryos at E16.5 was about 24% (23/97), close to the expected Mendelian ratio, suggesting that many mutant embryos died between E16.5 and birth.
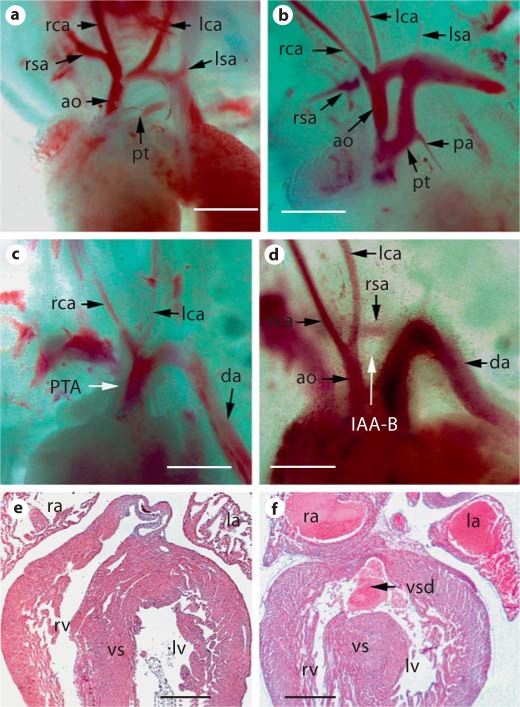
Gross and sectional examination showed that all the mutant embryos beyond E14.5 displayed outflow tract separation defect and ventricular septal defect (fig. 2, table 1). In severe cases, the outflow tract remained as a single vessel connecting to both left and right ventricles, a condition known as persistent truncus arteriosus. In less severe cases, the proximal portion of the outflow tract remained unseparated, whereas the distal portion was unaffected. Interrupted aortic arch artery type B (IAA-B), which is a characteristic vascular defect in DS patients, was frequently observed in mutant embryos. Retroesophageal right subclavian artery, another vascular defect associated with DS, was also occasionally seen in mutant embryos. In table 1, we summarized the frequency of various cardiovascular defects in embryos with Bmp4 inactivated in the Tbx1 expression domain.
Fig. 2.
Spectrum of cardiovascular defects in Tbx1-cre;Bmp4tm1/loxP-lacZ embryos. Tbx1-Cre;Bmp4tm1/+ male mice were crossed with Bmp4loxP-lacZ/loxP-lacZ female mice to obtain mutant embryos (Tbx1-Cre;Bmp4tm1/loxP-lacZ) and their littermate controls at different stages. a–d Gross examination of the outflow tract and pharyngeal arch artery regions of control (a) and mutant embryos (c, d) at E14.5. b Example of a mutant embryo with the outflow tract septation defect observed in the proximal region, but not in the distal region. c Example of a mutant embryo with complete persistent truncus arteriosus. d Embryo with IAA-B and retroesophageal right subclavian artery, which are commonly observed aortic arch artery defects in DS patients. All embryos were subsequently sectioned, revealing that they all possess a ventricular septum defect (data not shown). e, f A mutant and a control embryonic heart at E19.5 were sectioned and HE stained. A ventricular septal defect is identified in the mutant heart (f). Scale bars = 500 μm.
Table 1.
Penetrance of different phenotypes observed in mutant embryos between E14.5 and E19.5
| Phenotype | Penetrance |
|---|---|
| Persistent truncus arteriosus | 43% (21/49) |
| Outflow tract septation defect at the distal region | 57% (28/49) |
| Interrupted aortic arch artery type B | 47% (23/49) |
| Retroesophageal right subclavian artery | 8% (4/49) |
| Ventricular septal defect | 100% (49/49) |
Defective Pharyngeal Arch Remodeling and Abnormal Aortic Arch Morphogenesis in the Mutant Embryos
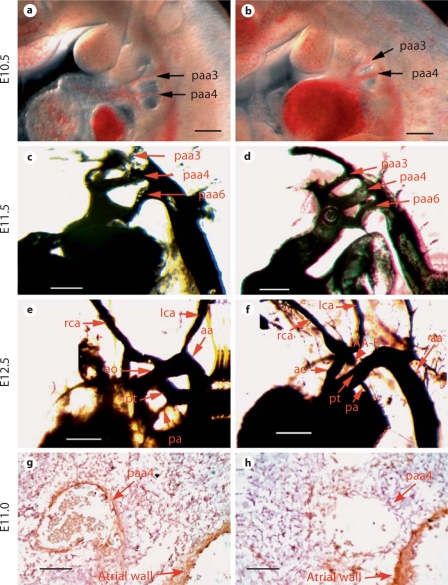
To determine whether these pharyngeal arch artery abnormities were due to early patterning defects, we examined embryos at E10.5, and found that pharyngeal arch arteries were well formed and patent in all mutant embryos examined (fig. 3a, b). We next performed cardiac ink injection on E11.5, E12.5 and E13.5 embryos to examine the remodeling status of the pharyngeal arch arteries. No pharyngeal arch artery defect was apparent in E11.5 embryos (fig. 3c, d). Starting from E12.5, we often detected complete interruption or regression of the aortic arch in the mutant embryos (4/7) (fig. 3e, f). These data suggest that inactivation of Bmp4 from Tbx1 domains does not affect the initial formation and patterning of pharyngeal arch arteries, but rather cause their abnormal remodeling. To better understand the cellular mechanism underlying the interruption of the 4th pharyngeal arch artery derivative, we performed immunohistochemistry analysis on sections of mutant and control embryos at E11.0 using an antibody against SMA (fig. 3g, h). We showed that the endothelial tube of the right 4th pharyngeal arch artery derivative in the control embryo is surrounded by a group of SMA-positive cells, while few SMA-positive cells are observed surrounding the 4th pharyngeal arch artery derivative of mutant embryos. Our results suggest that Bmp4 expressed in the Tbx1 expression domain is required for normal recruitment and/or differentiation of smooth muscle cells surrounding the endothelial tube of the 4th pharyngeal arch artery derivative.
Fig. 3.
Defective pharyngeal arch artery remodeling in Tbx1-cre;Bmp4tm1/loxP-lacZ embryos. a, b The 3rd and 4th pharyngeal arch artery derivatives were patent in both wild-type (a) and mutant (b) E10.5 embryos. c–f Cardiac ink injection was performed on E11.5 (c, d) and E12.5 (e, f) embryos. The remodeling of pharyngeal arch arteries was comparable between wild-type (c) and mutant (d) embryos at E11.5. While at E12.5, the remodeling defect became apparent in mutant embryos (f). The arrowhead in f indicates the IAA-B defect. g, h Sagittal sections of a wild-type (g) and a mutant (h) embryo at E11.0 were immunostained with an antibody against SMA. A group of SMA-positive cells surrounding the endothelial tube of the right 4th pharyngeal arch artery derivative were observed in the wild-type embryo but not in the mutant one. Expression of SMA was detected in the atrial wall of both control and mutant embryos. Scale bars = 200 μm (a–f); 30 μm (g, h).
Bmp4 Is Required for Normal Cellularization of Outflow Tract Cushions
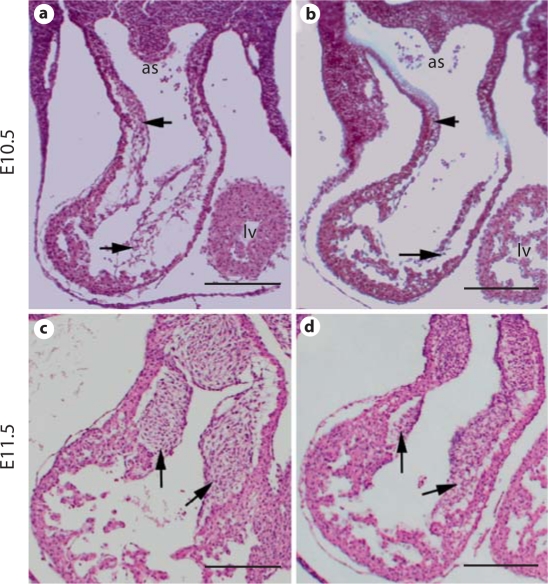
Outflow tract cushions are precursors of outflow tract septum and semilunar valves. We examined the outflow tract cushions at E10.5 and E11.5. Transverse sections of the embryos revealed that mutant outflow tract cushions were visibly hypoplastic and poorly cellularized compared with those of littermate controls (fig. 4). The outflow tract cushion mesenchymal cells are derived from both endocardial cells and incoming NCCs. We therefore further tested whether distribution of NCCs in the pharyngeal region was also impaired in mutant embryos. We examined expression of NCC markers including Crabp1 and AP-2 at E9.5, when cardiac NCCs have already colonized at the pharyngeal regions. Expression of Crabp1 and Ap-2α in the mutant embryos was comparable to the controls in the pharyngeal region between the 3rd an 6th pharyngeal arch artery derivatives (fig. 5), implying that cardiac NCC migration to the pharyngeal arches and their distribution in the pharyngeal region was largely unaffected by disruption of Bmp4.
Fig. 4.
Hypoplastic outflow tract cushion defect in Tbx1-cre;Bmp4tm1/loxP-lacZ embryos. Mutant (b, d) and littermate control (a, c) embryos were isolated at E10.5 (a, b) and 11.5 (c, d), and cross-sectioned followed by HE staining. The arrows indicate outflow tract cushions. Scale bars = 200 μm.
Fig. 5.
Normal expression of NCC markers in the pharyngeal region. Mutant (b, d) and control (a, c) embryos were isolated at E9.5 and subjected to wholemount in situ hybridization analysis using AP-2 (a, b) and Crabp1 (c, d) probes. The arrows indicate NCCs between the 4th and 6th pharyngeal arch artery derivatives, where cardiac NCCs are localized. Scale bars = 200 μm.
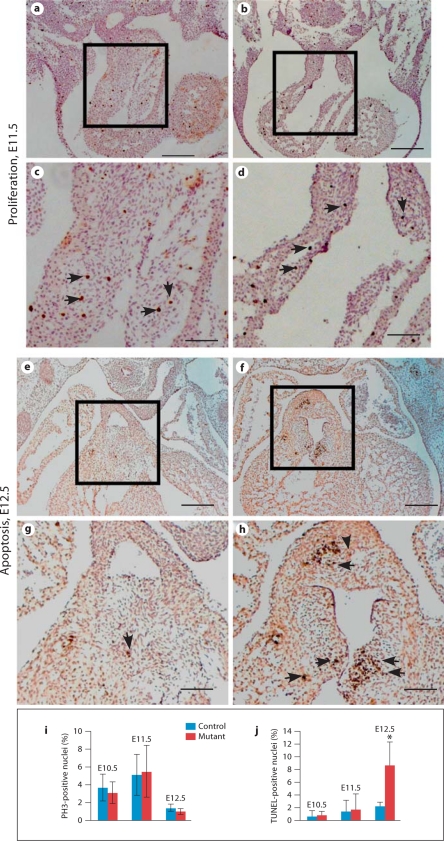
We next examined cell apoptosis/proliferation during outflow tract cushion morphogenesis. No obvious reduction in cell proliferation rate was observed in mutant outflow tract cushions at E11.5 (fig. 6a–d) or later stages (data not shown). We can clearly detect apoptotic cells in wild-type outflow tract cushions at E12.5, suggesting that active apoptosis is a normal process during outflow tract cushion remodeling. The number of apoptotic cells was visibly increased starting from E12.5 in mutant outflow tract cushions. Our observation is further confirmed with quantitative analysis (fig. 6i, j). Since the hypocellular outflow tract cushion defect was observed in E10.5 and E11.5 mutant embryos, the increased cell death at E12.5 cannot account for the initial cushion defect (also see Discussion).
Fig. 6.
Cell proliferation and apoptosis in the outflow tract cushion of control (a, c, e, g) and mutant (b, d, f, h) embryos. a–d E11.5 embryos were cross-sectioned and immunostained with an antibody against phospho-histone H3 (PH3), which stains cells at the M phase. c and d correspond to a and b, respectively. The arrows indicate examples of positively stained nuclei. No visible reduction in cell proliferation rate was detected. Similar results were observed at other stages (data not shown). e–h E12.5 embryos were cross-sectioned and subjected to the TUNEL assay. g and h correspond to e and f, respectively. Arrows indicate examples of apoptotic cells in outflow tract cushions. More apoptotic cells were observed in mutant outflow tract cushions. i, j Quantitative analysis of cell proliferation (i) and apoptosis (j) in outflow tract cushions of control and mutant embryos from E10.5 to E12.5. Data were averaged from at least 3 independent embryos with error bars indicating standard derivation. * p < 0.01 (Student's t test). Scale bars = 200 μm (a, b, e, f); 80 μm (c, d, g, h).
Discussion
In this study, we provide convincing evidence demonstrating that inactivation of Bmp4 from the Tbx1 expression domain leads to a spectrum of cardiovascular defects resembling DS. No defect is observed in the myocardium of mutant embryos, even though expression of Bmp4 from the outflow tract myocardium is visibly reduced in Tbx1-Cre;Bmp4tm1/loxP-lacZ mutant embryos (fig. 1). The lack of myocardial defect is consistent with previous studies using different Cre lines (cTnt-Cre, Nkx2.5-Cre and Mef2c-AHF-Cre) to conditionally inactivate Bmp4 [Jiao et al., 2003; Liu et al., 2004; McCulley et al., 2008]. These studies collectively support the notion that Bmp4 expressed in the outflow tract myocardium and pharyngeal region is not essential for cardiomyocyte development, but is required for remodeling of outflow tract cushions and pharyngeal arch arteries. A previously published study demonstrated that inactivation of Bmp4 specifically from the anterior heart field by Mef2c-AHF-Cre led to abnormal outflow tract development, while no defect in pharyngeal arch arteries was observed in mutant embryos [McCulley et al., 2008]. We noticed that Tbx1-Cre and Mef2c-AHF-Cre both inactivate Bmp4 expression in similar pharyngeal regions including pharyngeal arch mesenchymal cells and the outflow tract myocardial cells, and yet defects in Tbx1-Cre;Bmp4tm1/loxP-lacZ embryos were not restricted to the outflow tract region. In addition to abnormal outflow tract morphogenesis, Tbx1-Cre;Bmp4tm1/loxP-lacZ embryos displayed severe pharyngeal arch artery defects including IAA-B and retroesophageal right subclavian artery, which are both characteristic vascular abnormalities associated with DS. We further showed that Bmp4 expressed from the Tbx1 expression domain is essential for forming the smooth muscle cell wall surrounding the 4th pharyngeal arch artery derivative. The different results between these two studies are likely due to differences in Cre activity and/or the extent of target cell population between the two lines.
Pharyngeal arch arteries are initially formed as bilateral symmetric vessels. During remodeling, some pharyngeal arch artery segments degenerated, while other segments remained part of the major vessels. Asymmetric remodeling of outflow tract and pharyngeal arch artery is a complex process governed by a signaling network that remains poorly understood. Our data provide additional evidence that Bmp4 is an important participant within this network. Interestingly, tissue-specific disruption of Bmp4 from Tbx1-expressing cells does not affect early morphogenesis of the outflow tract and pharyngeal arch arteries, but leads to abnormal pharyngeal arch artery and outflow tract remodeling. This is in contrast to Tbx1 mutant embryos. Most Tbx1+/– embryos display hypoplastic fourth pharyngeal arch arteries at E10.5, and the failure of recovery from this early defect at least partially contributes to the pharyngeal arch artery defects at later developmental stages [Vitelli et al., 2002a]. Therefore, Bmp4 may promote remodeling of outflow tract cushions and pharyngeal arch arteries through a pathway not directly associated with Tbx1. This idea is further supported by our observation that expression of Tbx1 was not visibly altered in Tbx1-Cre;Bmp4tm1/loxP-lacZ embryos (data not shown). However, these results do not exclude the potential crosstalk between Tbx1- and Bmp4-mediated pathways. A recent study showed that Tbx1 can modulate Bmp-signaling activities by binding BMP receptor-activated Smads, which are nuclear mediators of BMP signaling, to prevent them from forming a complex with the Smad4 transcriptional coactivator [Fulcoli et al., 2009]. In addition, several previous studies have demonstrated that Tbx1 is required for normal expression during development [Raft et al., 2004; Moraes et al., 2005; Arnold et al., 2006a; Aggarwal et al., 2010]. In Tbx1–/– embryos, the expression level and pattern of Bmp4 was disturbed in otocysts and mandibular arch [Raft et al., 2004; Moraes et al., 2005; Aggarwal et al., 2010]. The requirement of Tbx1 for normal Bmp4 expression in otic vesicle was also demonstrated through tissue-specific gene ablation studies [Arnold et al., 2006a]. These results suggest that Tbx1 acts upstream of Bmp4 to regulate its expression; however, it is currently unclear whether Bmp4 is a direct downstream target gene of Tbx1. Our immunohistochemical assay showed that Bmp4 is required for proper recruitment and/or differentiation of smooth muscle cells surrounding the endothelial tube of the 4th pharyngeal arch artery derivative on the right side (fig. 3). The absence of an intact smooth muscle cell layer may cause fragility of pharyngeal arch artery 4 and result in interruption during remodeling. Our result is consistent with the previous report in which a smooth muscle reporter mouse line was used to show that inactivation of Bmp4 by Nkx2.5-Cre leads to a deficiency in recruiting smooth muscle cells to the pharyngeal arch artery region [Liu et al., 2004].
We observed the severe hypocellular abnormality in both proximal and distal regions of mutant outflow tract cushions as early as E10.5 and E11.5, and yet no obvious defect in cushion mesenchyme proliferation/death was detected at these stages (fig. 6). Therefore, the initial cushion defect in mutant embryos is not caused by aberrant cell proliferation and apoptosis. The mesenchymal cells within the proximal truncus arteriosus region of the outflow tract are mainly derived from endocardial cells through epithelial-mesenchymal transformation (EMT) [Jiang et al., 2000; Person et al., 2005]. Thus, the hypocellular defect of the proximal outflow tract region is likely caused by impaired EMT in mutant embryos. The role of BMP signaling in promoting EMT has been well documented in atrioventricular cushions [Ma et al., 2005; Wang et al., 2005; Park et al., 2006; Rivera-Feliciano and Tabin, 2006; Song et al., 2007d from the Tbx1 expression domain is required for normal EMT in the outflow tract; however, confirmation of this hypothesis will require further vigorous experimental testing. In addition to endocardial cell-derived mesenchyme, a large portion of mesenchymal cells within the distal conus arteriosus region of the outflow tract are derived from cardiac NCCs, which have migrated into outflow tract cushions from the dorsal neural tube through PA3, 4 and 6. Our in situ hybridization analysis using probes against Crabp1 and Ap-2α showed normal distribution of NCCs in the pharyngeal region (fig. 5), suggesting that NCCs can properly migrate into pharyngeal arches from the neural crest. We therefore infer that migration of cardiac NCCs from pharyngeal arches into outflow tract cushions is impaired by depletion of Bmp4 and contributes to the hypocellular outflow tract cushion defect in the distal region of mutant embryos. A previous study has demonstrated that Fgf signaling promotes Bmp4 expression in the mesodermal cells of second heart field in an autocrine manner, and disruption of Fgf signaling in the second heart field significantly reduced Bmp4 expression in the outflow tract and led to outflow tract cushion defects [Park et al., 2008]. Our study further suggests that Bmp4 in pharyngeal mesodermal cells acts on endocardial cells, outflow tract cushion mesenchymal cells and NCCs to promote normal outflow tract development.
Several lines of evidence suggest that outflow tract morphogenesis is differently regulated along the proximal-distal segments [Kim et al., 2001; Liu et al., 2004]. Although Bmp4 is highly expressed throughout the entire outflow tract myocardial tube, Bmp4 deficiency from the outflow tract most severely affected the proximal portion. All Tbx1-Cre;Bmp4tm1/loxP-lacZ embryos examined displayed septation defects in the proximal portion whereas 57% of them were well separated in the distal portion. Previous studies showed that the distal portion of the outflow tract can also be regulated by other Bmp ligands, such as Bmp6 and Bmp7 [Kim et al., 2001; Liu et al., 2004]. Double knockout of Bmp6 and Bmp7 leads to separation defects in the distal outflow tract [Kim et al., 2001]. Therefore, functional redundancy among Bmp ligands may explain the incomplete penetrance of separation defects on the distal portion of the mutant outflow tract.
In summary, our data highlight the importance of the Bmp4 ligand produced from the Tbx1 expression domain in regulating outflow tract and pharyngeal arch artery remodeling. This study provides a unique mouse model for further studying the Bmp4 downstream regulatory targets in the pharyngeal arch mesenchymal cells and the outflow tract cardiomyocytes that are critical for cardiovascular development.
Acknowledgements
We thank Dr. T. Williams (U. Colorado, Denver) for providing the AP-2α in situ probe, Dr. V. Kaartinen (U. Michigan) for valuable suggestions on India ink injection and the members of the Jiao laboratory for assistance in the project. This project is supported by the Scientist Development Grant from AHA (National Center), a R01 grant (HL095783-01A1) from NHLBI, and a HSF-GEF Scholar Award to K.J.
Abbreviations used in this paper
- BMP
bone morphogenic protein
- DS
DiGeorge syndrome
- EMT
epithelial-mesenchymal transformation
- IAA-B
interrupted aortic arch artery type B
- NCC
neural crest cell
- SMA
smooth muscle actin
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Abbreviations used in figures 1, 2, 3, 4, 5, 6
- aa
aortic arch artery
- ao
aorta (or ascending aorta)
- as
aortic sac
- da
descending aorta
- la
left atrium
- lca
left carotid artery
- lsa
lef subclavian artery
- lv
left ventricle
- oft
outflow tract
- pa
pulmonary artery
- paa1–4, paa6
1st–4th and 6th pharyngeal arch artery derivatives
- paa
pharyngeal arch artery
- pt
pulmonary trunk
- PTA
persistent truncus arteriosus
- ra
right atrium
- rca
right carotid artery
- rsa
right subclavian artery
- rv
right ventricle
- vsd
ventricular septal defect
- vs
ventricular septum
References
- Abu-Issa R., Smyth G., Smoak I., Yamamura K., Meyers E.N. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Aggarwal V.S., Carpenter C., Freyer L., Liao J., Petti M., Morrow B.E. Mesodermal Tbx1 is required for patterning the proximal mandible in mice. Dev Biol. 2010;344:669–681. doi: 10.1016/j.ydbio.2010.05.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J.S., Braunstein E.M., Ohyama T., Groves A.K., Adams J.C., Brown M.C., Morrow B.E. Tissue-specific roles of Tbx1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum Mol Genet. 2006a;15:1629–1639. doi: 10.1093/hmg/ddl084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J.S., Werling U., Braunstein E.M., Liao J., Nowotschin S., Edelmann W., Hebert J.M., Morrow B.E. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006b;133:977–987. doi: 10.1242/dev.02264. [DOI] [PubMed] [Google Scholar]
- Baldini A. DiGeorge syndrome: an update. Curr Opin Cardiol. 2004;19:201–204. doi: 10.1097/00001573-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Black B.L. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.B., Wenning J.M., Lu M.M., Epstein D.J., Meyers E.N., Epstein J.A. Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev Biol. 2004;267:190–202. doi: 10.1016/j.ydbio.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Buckingham M., Meilhac S., Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Chapman D.L., Garvey N., Hancock S., Alexiou M., Agulnik S.I., Gibson-Brown J.J., Cebra-Thomas J., Bollag R.J., Silver L.M., Papaioannou V.E. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Datto M., Wang X.F. The Smads: transcriptional regulation and mouse models. Cytokine Growth Factor Rev. 2000;11:37–48. doi: 10.1016/s1359-6101(99)00027-1. [DOI] [PubMed] [Google Scholar]
- de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Dolle P., Ruberte E., Leroy P., Morriss-Kay G., Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990;110:1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- Dyer L.A., Kirby M.L. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg L.M., Markwald R.R. Cellular recruitment and the development of the myocardium. Dev Biol. 2004;274:225–232. doi: 10.1016/j.ydbio.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Epstein J.A. Developing models of DiGeorge syndrome. Trends Genet. 2001;17:S13–S17. doi: 10.1016/s0168-9525(01)02450-7. [DOI] [PubMed] [Google Scholar]
- Feng W., Williams T. Cloning and characterization of the mouse AP-2 epsilon gene: a novel family member expressed in the developing olfactory bulb. Mol Cell Neurosci. 2003;24:460–475. doi: 10.1016/s1044-7431(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Feng X.H., Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Frank D.U., Fotheringham L.K., Brewer J.A., Muglia L.J., Tristani-Firouzi M., Capecchi M.R., Moon A.M. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Dehart D.B., Sulik K.K., Hogan B.L. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development. 2002;129:4685–4696. doi: 10.1242/dev.129.20.4685. [DOI] [PubMed] [Google Scholar]
- Fulcoli F.G., Huynh T., Scambler P.J., Baldini A. Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner. PLoS One. 2009;4:e6049. doi: 10.1371/journal.pone.0006049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Graham A. The development and evolution of the pharyngeal arches. J Anat. 2001;199:133–141. doi: 10.1046/j.1469-7580.2001.19910133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. The neural crest. Curr Biol. 2003;13:R381–R384. doi: 10.1016/s0960-9822(03)00315-4. [DOI] [PubMed] [Google Scholar]
- Grossfeld P.D. The genetics of congenital heart disease. J Nucl Cardiol. 2003;10:71–76. doi: 10.1067/mnc.2003.129294. [DOI] [PubMed] [Google Scholar]
- Hiruma T., Nakajima Y., Nakamura H. Development of pharyngeal arch arteries in early mouse embryo. J Anat. 2002;201:15–29. doi: 10.1046/j.1469-7580.2002.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J.I. Incidence of congenital heart disease. II. Prenatal incidence. Pediatr Cardiol. 1995;16:155–165. doi: 10.1007/BF00794186. [DOI] [PubMed] [Google Scholar]
- Hoffman J.I., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Hu T., Yamagishi H., Maeda J., McAnally J., Yamagishi C., Srivastava D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development. 2004;131:5491–5502. doi: 10.1242/dev.01399. [DOI] [PubMed] [Google Scholar]
- Jerome L.A., Papaioannou V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Jiang X., Rowitch D.H., Soriano P., McMahon A.P., Sucov H.M. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiao K., Kulessa H., Tompkins K., Zhou Y., Batts L., Baldwin H.S., Hogan B.L. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R.G. Molecular inroads into the anterior heart field. Trends Cardiovasc Med. 2005;15:51–56. doi: 10.1016/j.tcm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kim R.Y., Robertson E.J., Solloway M.J. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Kochilas L., Merscher-Gomez S., Lu M.M., Potluri V., Liao J., Kucherlapati R., Morrow B., Epstein J.A. The role of neural crest during cardiac development in a mouse model of DiGeorge syndrome. Dev Biol. 2002;251:157–166. doi: 10.1006/dbio.2002.0819. [DOI] [PubMed] [Google Scholar]
- Kulessa H., Hogan B.L. Generation of a loxP flanked bmp4loxP-lacZ allele marked by conditional lacZ expression. Genesis. 2002;32:66–68. doi: 10.1002/gene.10032.abs. [DOI] [PubMed] [Google Scholar]
- Lindsay E.A., Baldini A. Congenital heart defects and 22q11 deletions: which genes count? Mol Med Today. 1998;4:350–357. doi: 10.1016/s1357-4310(98)01302-1. [DOI] [PubMed] [Google Scholar]
- Lindsay E.A., Vitelli F., Su H., Morishima M., Huynh T., Pramparo T., Jurecic V., Ogunrinu G., Sutherland H.F., Scambler P.J., Bradley A., Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Liu W., Selever J., Murali D., Sun X., Brugger S.M., Ma L., Schwartz R.J., Maxson R., Furuta Y., Martin J.F. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Liu W., Selever J., Wang D., Lu M.F., Moses K.A., Schwartz R.J., Martin J.F. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Lu M.F., Schwartz R.J., Martin J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Massagué J., Gomis R.R. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Massagué J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- McCulley D.J., Kang J.O., Martin J.F., Black B.L. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merscher S., Funke B., Epstein J.A., Heyer J., Puech A., Lu M.M., Xavier R.J., Demay M.B., Russell R.G., Factor S., Tokooya K., Jore B.S., Lopez M., Pandita R.K., Lia M., Carrion D., Xu H., Schorle H., Kobler J.B., Scambler P., Wynshaw-Boris A., Skoultchi A.I., Morrow B.E., Kucherlapati R. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Moraes F., Novoa A., Jerome-Majewska L.A., Papaioannou V.E., Mallo M. Tbx1 is required for proper neural crest migration and to stabilize spatial patterns during middle and inner ear development. Mech Dev. 2005;122:199–212. doi: 10.1016/j.mod.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Heldin C.H. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Nakajima Y. Second lineage of heart forming region provides new understanding of conotruncal heart defects. Congenital Anomalies. 2010;50:8–14. doi: 10.1111/j.1741-4520.2009.00267.x. [DOI] [PubMed] [Google Scholar]
- Nie X., Deng C.X., Wang Q., Jiao K. Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Dev Biol. 2008;316:417–430. doi: 10.1016/j.ydbio.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Lavine K., Mishina Y., Deng C.X., Ornitz D.M., Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Park E.J., Watanabe Y., Smyth G., Miyagawa-Tomita S., Meyers E., Klingensmith J., Camenisch T., Buckingham M., Moon A.M. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development. 2008;135:3599–3610. doi: 10.1242/dev.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person A.D., Klewer S.E., Runyan R.B. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Plageman T.F., Jr., Yutzey K.E. T-box genes and heart development: putting the ‘T’ in heart. Dev Dyn. 2005;232:11–20. doi: 10.1002/dvdy.20201. [DOI] [PubMed] [Google Scholar]
- Raft S., Nowotschin S., Liao J., Morrow B.E. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–1812. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J., Tabin C.J. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K., Chin A.J. T-box genes and cardiac development. Birth Defects Res C Embryo Today. 2003;69:25–37. doi: 10.1002/bdrc.10001. [DOI] [PubMed] [Google Scholar]
- Scambler P.J. 22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development. Pediatr Cardiol. 2010;31:378–390. doi: 10.1007/s00246-009-9613-0. [DOI] [PubMed] [Google Scholar]
- Shi Y., Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Song L., Fassler R., Mishina Y., Jiao K., Baldwin H.S. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301:276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Hill C.S. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Vitelli F., Morishima M., Taddei I., Lindsay E.A., Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet. 2002a;11:915–922. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- Vitelli F., Taddei I., Morishima M., Meyers E.N., Lindsay E.A., Baldini A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development. 2002b;129:4605–4611. doi: 10.1242/dev.129.19.4605. [DOI] [PubMed] [Google Scholar]
- Wang J., Sridurongrit S., Dudas M., Thomas P., Nagy A., Schneider M.D., Epstein J.A., Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Morishima M., Wylie J.N., Schwartz R.J., Bruneau B.G., Lindsay E.A., Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Yagi H., Furutani Y., Hamada H., Sasaki T., Asakawa S., Minoshima S., Ichida F., Joo K., Kimura M., Imamura S., Kamatani N., Momma K., Takao A., Nakazawa M., Shimizu N., Matsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Srivastava D. Unraveling the genetic and developmental mysteries of 22q11 deletion syndrome. Trends Mol Med. 2003;9:383–389. doi: 10.1016/s1471-4914(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Cerrato F., Xu H., Vitelli F., Morishima M., Vincentz J., Furuta Y., Ma L., Martin J.F., Baldini A., Lindsay E. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Huynh T., Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development. 2006;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]