Abstract
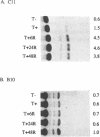
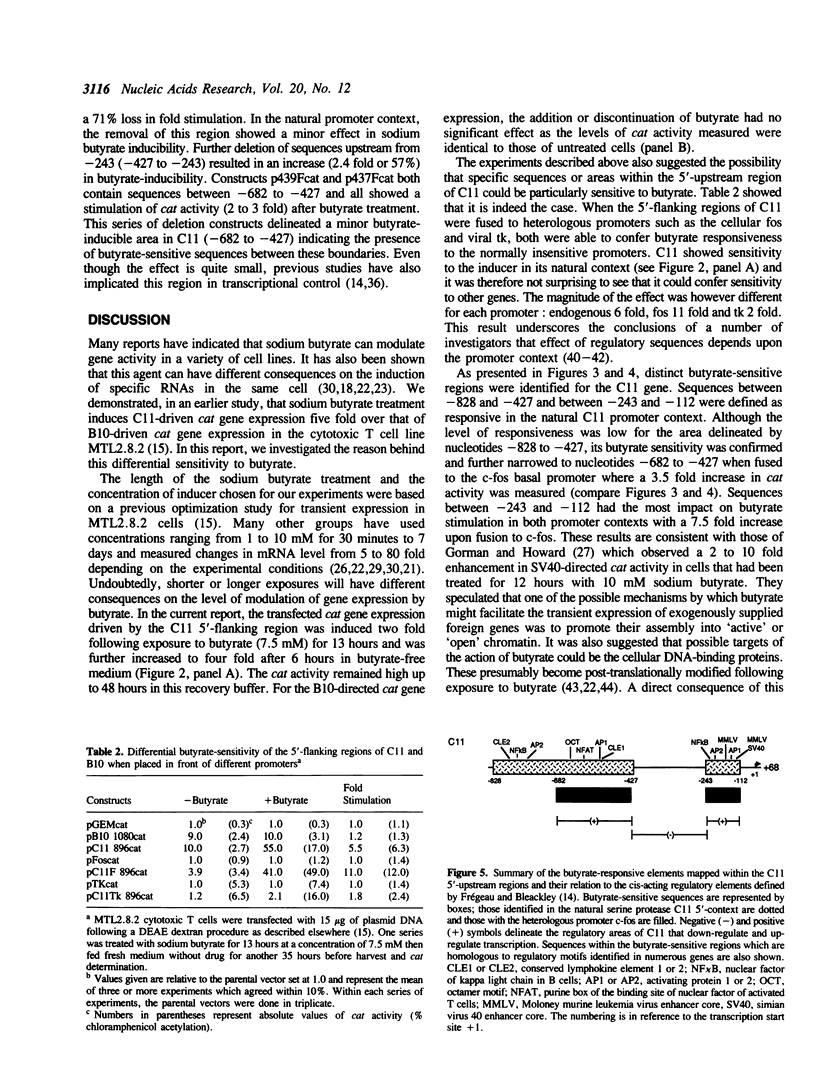
The 5'-flanking regions of two cytotoxic cell protease genes, CCP1 and 2, are sufficient to confer cytotoxic T lymphocyte-specific expression when fused to a reporter gene. The two regulatory regions are, however, differentially sensitive to treatment of the recipient cell, MTL 2.8.2, with sodium butyrate. With CCP1 a six-fold increase in cat expression was observed, whereas CCP2 was insensitive to the butyrate treatment. One major butyrate-sensitive regions was defined in the CCP1 5'-flanking sequence between -243 to -112 and another less effective one between-682 to -427. These fragments of DNA were also able to confer responsiveness to butyrate when ligated to a heterologous fos promoter. These sequences within the 5' flank of CCP1 share homology with other elements that have been defined as butyrate-responsive. We believe that our results argue against a pleiotropic affect of butyrate such as histone acetylation. More likely sodium butyrate is mediating a specific stimulation of transcription through modification of the activities of selected transcriptional regulatory proteins that in turn affect their interactions with proteins bound to the promoter.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Berke G. The cytolytic T lymphocyte and its mode of action. Immunol Lett. 1989 Feb;20(3):169–178. doi: 10.1016/0165-2478(89)90075-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren B. W., Herschman H. R. Regulation of the rat metallothionein-I gene by sodium butyrate. Nucleic Acids Res. 1986 Jan 24;14(2):853–867. doi: 10.1093/nar/14.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren B. W., Taplitz S. J., Herschman H. R. Butyrate-induced changes in nuclease sensitivity of chromatin cannot be correlated with transcriptional activation. Mol Cell Biol. 1987 Nov;7(11):3863–3870. doi: 10.1128/mcb.7.11.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleackley R. C., Havele C., Paetkau V. Cellular and molecular properties of an antigen-specific cytotoxic T lymphocyte line. J Immunol. 1982 Feb;128(2):758–767. [PubMed] [Google Scholar]
- Bleackley R. C., Lobe C. G., Duggan B., Ehrman N., Fregeau C., Meier M., Letellier M., Havele C., Shaw J., Paetkau V. The isolation and characterization of a family of serine protease genes expressed in activated cytotoxic T lymphocytes. Immunol Rev. 1988 Mar;103:5–19. doi: 10.1111/j.1600-065x.1988.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Gruss R. J., Allfrey V. G. Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem. 1981 Sep 25;256(18):9612–9621. [PubMed] [Google Scholar]
- Bohan C. A., Robinson R. A., Luciw P. A., Srinivasan A. Mutational analysis of sodium butyrate inducible elements in the human immunodeficiency virus type I long terminal repeat. Virology. 1989 Oct;172(2):573–583. doi: 10.1016/0042-6822(89)90200-6. [DOI] [PubMed] [Google Scholar]
- Bohan C., York D., Srinivasan A. Sodium butyrate activates human immunodeficiency virus long terminal repeat--directed expression. Biochem Biophys Res Commun. 1987 Nov 13;148(3):899–905. doi: 10.1016/s0006-291x(87)80217-6. [DOI] [PubMed] [Google Scholar]
- Boral A. L., Okenquist S. A., Lenz J. Identification of the SL3-3 virus enhancer core as a T-lymphoma cell-specific element. J Virol. 1989 Jan;63(1):76–84. doi: 10.1128/jvi.63.1.76-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Sartwell A. D., Lei K. J., Plouzek C. A. Effects of sodium butyrate on the synthesis of human pregnancy-specific beta 1-glycoprotein. J Biol Chem. 1990 May 25;265(15):8788–8794. [PubMed] [Google Scholar]
- Dorner A. J., Wasley L. C., Kaufman R. J. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989 Dec 5;264(34):20602–20607. [PubMed] [Google Scholar]
- Frégeau C. J., Bleackley R. C. Factors influencing transient expression in cytotoxic T cells following DEAE dextran-mediated gene transfer. Somat Cell Mol Genet. 1991 May;17(3):239–257. doi: 10.1007/BF01232820. [DOI] [PubMed] [Google Scholar]
- Frégeau C. J., Bleackley R. C. Transcription of two cytotoxic cell protease genes is under the control of different regulatory elements. Nucleic Acids Res. 1991 Oct 25;19(20):5583–5590. doi: 10.1093/nar/19.20.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauber J. G., Wandersee N. J., Little J. A., Ginder G. D. 5'-flanking sequences mediate butyrate stimulation of embryonic globin gene expression in adult erythroid cells. Mol Cell Biol. 1991 Sep;11(9):4690–4697. doi: 10.1128/mcb.11.9.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Grusby M. J., Nabavi N., Wong H., Dick R. F., Bluestone J. A., Schotz M. C., Glimcher L. H. Cloning of an interleukin-4 inducible gene from cytotoxic T lymphocytes and its identification as a lipase. Cell. 1990 Feb 9;60(3):451–459. doi: 10.1016/0092-8674(90)90596-7. [DOI] [PubMed] [Google Scholar]
- Haddad P., Jenne D., Tschopp J., Clément M. V., Mathieu-Mahul D., Sasportes M. Structure and evolutionary origin of the human granzyme H gene. Int Immunol. 1991 Jan;3(1):57–66. doi: 10.1093/intimm/3.1.57. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Jenne D., Rey C., Haefliger J. A., Qiao B. Y., Groscurth P., Tschopp J. Identification and sequencing of cDNA clones encoding the granule-associated serine proteases granzymes D, E, and F of cytolytic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4814–4818. doi: 10.1073/pnas.85.13.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Lazar M. A. Sodium butyrate selectively alters thyroid hormone receptor gene expression in GH3 cells. J Biol Chem. 1990 Oct 15;265(29):17474–17477. [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B. Enhanced phosphorylation of high-mobility-group proteins in nuclease-sensitive mononucleosomes from butyrate-treated HeLa cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2189–2193. doi: 10.1073/pnas.78.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe C. G., Shaw J., Fregeau C., Duggan B., Meier M., Brewer A., Upton C., McFadden G., Patient R. K., Paetkau V. Transcriptional regulation of two cytotoxic T lymphocyte-specific serine protease genes. Nucleic Acids Res. 1989 Jul 25;17(14):5765–5779. doi: 10.1093/nar/17.14.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey D. M., Aebischer T., Olsen K., Lichtenheld M., Rupp F., Hengartner H., Podack E. R. Cloning, analysis, and expression of murine perforin 1 cDNA, a component of cytolytic T-cell granules with homology to complement component C9. Proc Natl Acad Sci U S A. 1989 Jan;86(1):247–251. doi: 10.1073/pnas.86.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mickley L. A., Bates S. E., Richert N. D., Currier S., Tanaka S., Foss F., Rosen N., Fojo A. T. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem. 1989 Oct 25;264(30):18031–18040. [PubMed] [Google Scholar]
- Naranjo J. R., Mellström B., Auwerx J., Mollinedo F., Sassone-Corsi P. Unusual c-fos induction upon chromaffin PC12 differentiation by sodium butyrate: loss of fos autoregulatory function. Nucleic Acids Res. 1990 Jun 25;18(12):3605–3610. doi: 10.1093/nar/18.12.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva R., Bazett-Jones D. P., Locklear L., Dixon G. H. Histone hyperacetylation can induce unfolding of the nucleosome core particle. Nucleic Acids Res. 1990 May 11;18(9):2739–2747. doi: 10.1093/nar/18.9.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Clark W. R. Evidence for multiple lytic pathways used by cytotoxic T lymphocytes. J Immunol. 1989 Oct 1;143(7):2120–2126. [PubMed] [Google Scholar]
- Partington G. A., Yarwood N. J., Rutherford T. R. Human globin gene transcription in injected Xenopus oocytes: enhancement by sodium butyrate. EMBO J. 1984 Dec 1;3(12):2787–2792. doi: 10.1002/j.1460-2075.1984.tb02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. A., Annunziato A. T. Influence of histone acetylation on the solubility, H1 content and DNase I sensitivity of newly assembled chromatin. Nucleic Acids Res. 1989 Jun 12;17(11):4275–4291. doi: 10.1093/nar/17.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius C., Cabañas C., Aller P. The induction of vimentin gene expression by sodium butyrate in human promonocytic leukemia U937 cells. Exp Cell Res. 1990 May;188(1):129–134. doi: 10.1016/0014-4827(90)90287-k. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., MacDermott R. P., Bartley G., Bertovich M., Amato D. A., Austen K. F., Schlossman S. F., Stevens R. L., Ritz J. Specific release of proteoglycans from human natural killer cells during target lysis. Nature. 1985 Nov 21;318(6043):289–291. doi: 10.1038/318289a0. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Kamada M. M., Serafin W. E. Structure and function of the family of proteoglycans that reside in the secretory granules of natural killer cells and other effector cells of the immune response. Curr Top Microbiol Immunol. 1989;140:93–108. doi: 10.1007/978-3-642-73911-8_8. [DOI] [PubMed] [Google Scholar]
- Stoddart J. H., Lane M. A., Niles R. M. Sodium butyrate suppresses the transforming activity of an activated N-ras oncogene in human colon carcinoma cells. Exp Cell Res. 1989 Sep;184(1):16–27. doi: 10.1016/0014-4827(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Tang D. C., Taylor M. W. Transcriptional activation of the adenine phosphoribosyltransferase promoter by an upstream butyrate-induced Moloney murine sarcoma virus enhancer-promoter element. J Virol. 1990 Jun;64(6):2907–2911. doi: 10.1128/jvi.64.6.2907-2911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S. J., Ko L. W., Lee Y. H., Wang F. F. Induction of fos and sis proto-oncogenes and genes of the extracellular matrix proteins during butyrate induced glioma differentiation. Biochim Biophys Acta. 1990 Jan 30;1048(1):59–65. doi: 10.1016/0167-4781(90)90022-t. [DOI] [PubMed] [Google Scholar]
- Taylor I. C., Kingston R. E. Factor substitution in a human HSP70 gene promoter: TATA-dependent and TATA-independent interactions. Mol Cell Biol. 1990 Jan;10(1):165–175. doi: 10.1128/mcb.10.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiesen H. J., Bösze Z., Henry L., Charnay P. A DNA element responsible for the different tissue specificities of Friend and Moloney retroviral enhancers. J Virol. 1988 Feb;62(2):614–618. doi: 10.1128/jvi.62.2.614-618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Wang W. D., Gralla J. D. Differential ability of proximal and remote element pairs to cooperate in activating RNA polymerase II transcription. Mol Cell Biol. 1991 Sep;11(9):4561–4571. doi: 10.1128/mcb.11.9.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987 Sep 10;329(6135):174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]
- Young J. D. Killing of target cells by lymphocytes: a mechanistic view. Physiol Rev. 1989 Jan;69(1):250–314. doi: 10.1152/physrev.1989.69.1.250. [DOI] [PubMed] [Google Scholar]