Abstract
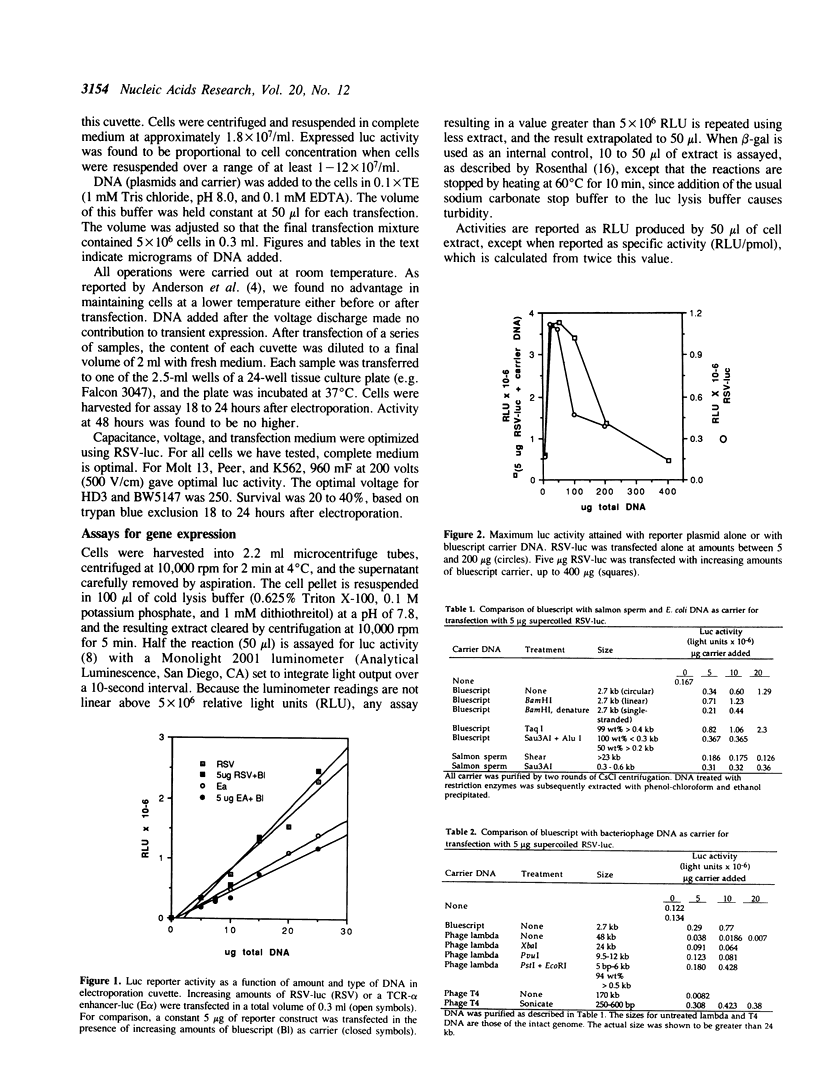
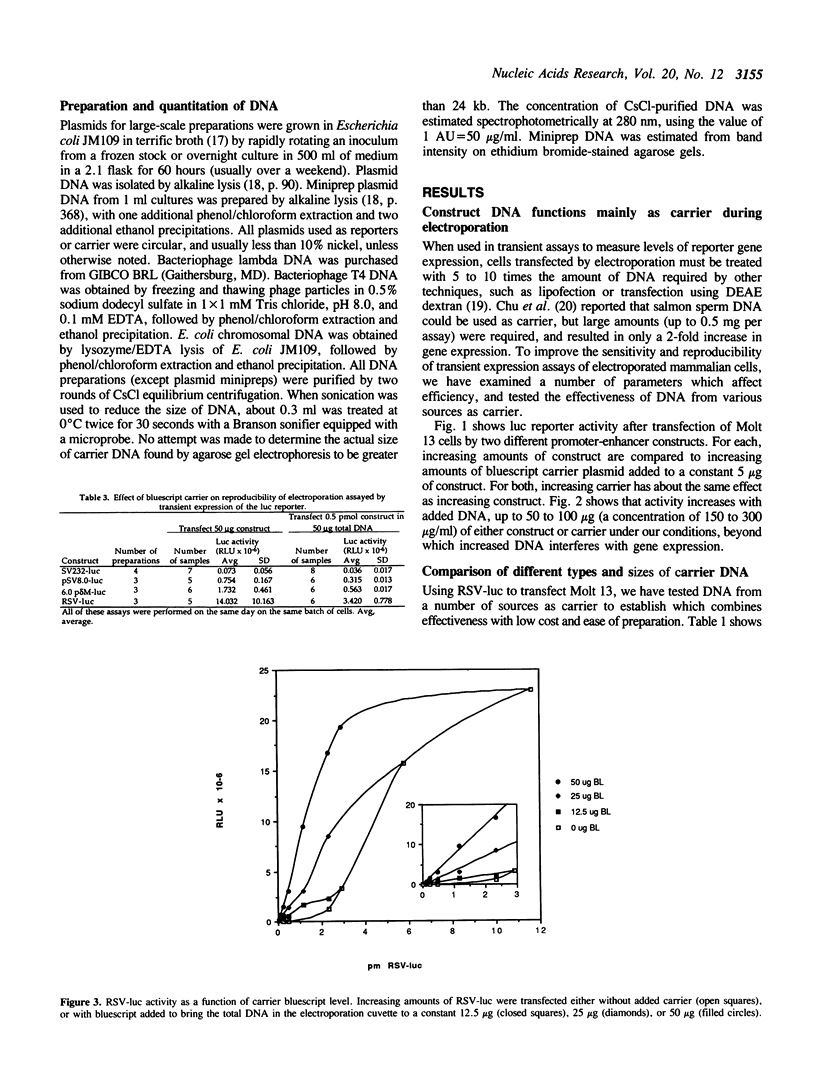
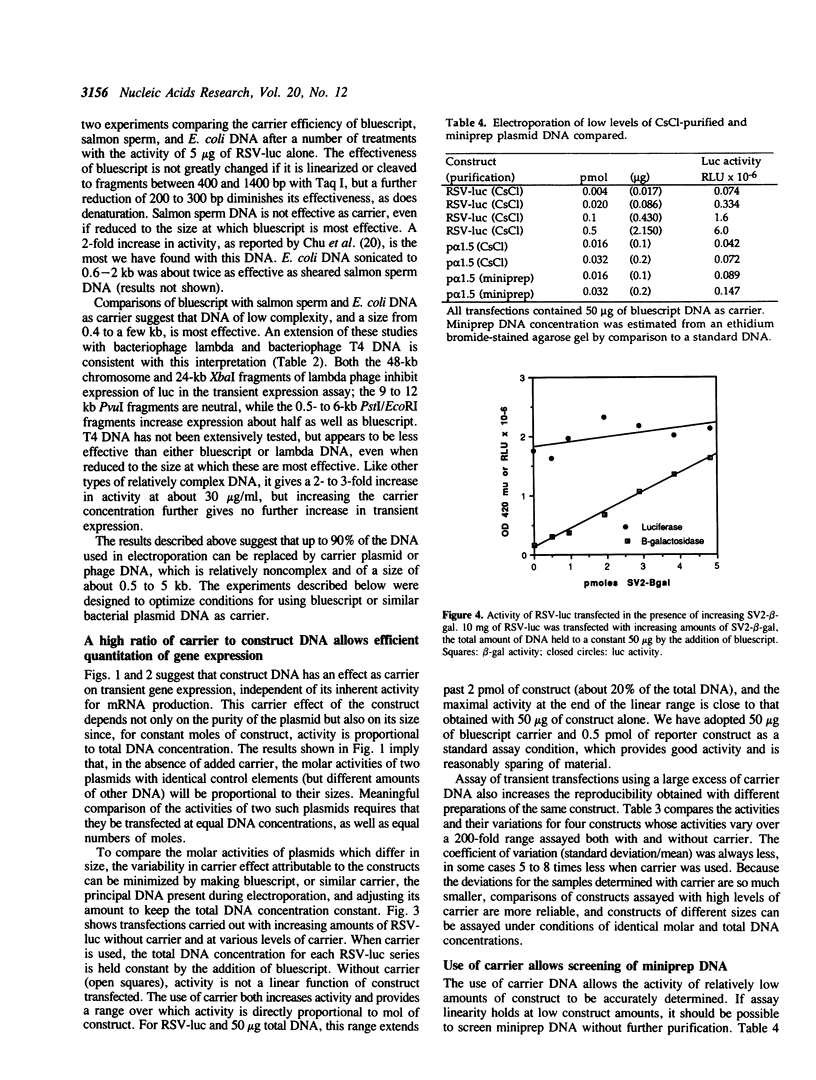
The combination of a photometric reporter-gene assay, with transfection by electroporation, is potentially a rapid and sensitive tool for the study of genetic regulatory elements in many types of cells. We have found that the sensitivity, accuracy, and reproducibility of the technique is greatly improved by the inclusion of appropriately chosen carrier DNA as the primary DNA species present during electroporation. By using high levels of carrier, the activities of constructs of differing sizes can be quantitatively compared, active constructs can be assayed with sub-microgram amounts of plasmid, and the activities of the constructs are linear over a wide concentration of DNA. In addition, the activity of miniprep DNA can be screened without purification on CsCl gradients giving activities equal to CsCl-purified DNA. This is extremely useful when doing preliminary screening of large numbers of constructs for promoter or enhancer activities. We report the results of testing various types of DNA as carrier, and the parameters for optimizing its use.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Hidaka K., Siminovitch L. Expression of bacterial beta-galactosidase in animal cells. Mol Cell Biol. 1982 Dec;2(12):1628–1632. doi: 10.1128/mcb.2.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. L., Spandidos D. A., Coggins J. R. Electroporation of lymphoid cells: factors affecting the efficiency of transfection. J Biochem Biophys Methods. 1991 Apr;22(3):207–222. doi: 10.1016/0165-022x(91)90069-9. [DOI] [PubMed] [Google Scholar]
- Andreason G. L., Evans G. A. Introduction and expression of DNA molecules in eukaryotic cells by electroporation. Biotechniques. 1988 Jul-Aug;6(7):650–660. [PubMed] [Google Scholar]
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Borrás T., Peterson C. A., Piatigorsky J. Evidence for positive and negative regulation in the promoter of the chicken delta 1-crystallin gene. Dev Biol. 1988 May;127(1):209–219. doi: 10.1016/0012-1606(88)90202-3. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioe L., McNab A., Hubbell H. R., Meo P., Curtis P., Rovera G. Differential expression of the globin genes in human leukemia K562(S) cells induced to differentiate by hemin or butyric acid. Cancer Res. 1981 Jan;41(1):237–243. [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Harvey R. C., Martinerie C., Sun L. H., Williams D., Showe L. C., Marteneire C. Translocations and rearrangements in T-cell acute leukemias with the t(11;14) (p13;q11) chromosomal translocations. Oncogene. 1989 Mar;4(3):341–349. [PubMed] [Google Scholar]
- Ho I. C., Yang L. H., Morle G., Leiden J. M. A T-cell-specific transcriptional enhancer element 3' of C alpha in the human T-cell receptor alpha locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6714–6718. doi: 10.1073/pnas.86.17.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E. Y., Lanier L. L., Turck C. W., Littman D. R., Davis M. M., Chien Y. H., Weiss A. Identification and sequence of a fourth human T cell antigen receptor chain. Nature. 1987 Dec 10;330(6148):569–572. doi: 10.1038/330569a0. [DOI] [PubMed] [Google Scholar]
- McNally M. A., Lebkowski J. S., Okarma T. B., Lerch L. B. Optimizing electroporation parameters for a variety of human hematopoietic cell lines. Biotechniques. 1988 Oct;6(9):882–886. [PubMed] [Google Scholar]
- Redondo J. M., Hata S., Brocklehurst C., Krangel M. S. A T cell-specific transcriptional enhancer within the human T cell receptor delta locus. Science. 1990 Mar 9;247(4947):1225–1229. doi: 10.1126/science.2156339. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Weiss A., Newton M., Crommie D. Expression of T3 in association with a molecule distinct from the T-cell antigen receptor heterodimer. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6998–7002. doi: 10.1073/pnas.83.18.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]



