Abstract
We have developed a miniature integrated optical coherence tomography (OCT) ultrasound (US) probing system for intravascular imaging applications. In the OCT probe, the light coming out of a single mode fiber is focused by a gradient-index lens and then reflected by a right-angle prism from the side of the probe into the sample. It was combined with a 35 MHz PMN-PT side-viewing ultrasound transducer to obtain the ultrasound image as well. The OCT and ultrasound probes were integrated as a single probe to obtain OCT and ultrasound images simultaneously. The integrated probe has an outer diameter of 0.69 mm which, to our knowledge, is the smallest integrated OCT-US probe reported. Fast data acquisition and processing was implemented for real-time imaging. In vitro OCT and US images of human coronary artery with pathology, as well as in vivo images of normal rabbit abdominal aorta, were obtained using the integrated OCT-US probe to demonstrate its capability.
Keywords: optical coherence tomography, intravascular ultrasound, integrated dual-modality imaging system
Introduction
Atherosclerosis is the leading cause of morbidity and mortality in the United States and is becoming the pre-eminent health problem worldwide. Detection and diagnosis of atherosclerosis relies on a medical imaging technique.1 Intravascular ultrasound (IVUS) imaging has become a standard imaging modality for atherosclerosis diagnosis since it provides direct visualization of vessel walls. Recently, optical coherence tomography (OCT) has been applied to intravascular imaging because it offers new insights into the microstructure of atherosclerosis and tissue responses to stent implantation.4, 5, 6 Research has been conducted to compare diagnostic accuracy of OCT and IVUS during which separate OCT and IVUS systems were used to image the same sites of interest.7, 8 It has been pointed out that OCT and IVUS are complementary in the application of intravascular imaging, and the combination of the two can offer advantages which cannot be achieved by using either modality alone.9
An integrated OCT ultrasound (US) system will be capable of offering high resolution, which is essential for visualizing the microstructure of a plaque, and also large penetration depth which is necessary for visualizing structures deep within the vessel wall. Thus, enhanced diagnostic accuracy can be obtained. Moreover, improved safety for the patient can be achieved since only a minimum amount of flushing agent will be needed for OCT under the guidance of US. An integrated OCT-US probe can provide both OCT and ultrasound imaging simultaneously so that both cost and the physician's time will be reduced significantly compared to using separate probes.
Previously, our group has developed different types of integrated intravascular imaging probes combining OCT with US.10, 11, 12 In Yin et al. and Yang et al.,11, 12 the OCT probes and the US transducers were put side by side and had outer diameters of 2.4 and 2.8 mm, respectively. In Li et al.,10 the OCT probe was inserted into a centric hole of the US transducer to achieve co-registered OCT-US imaging. This probe had an outer diameter of 2.5 mm. The imaging capabilities of previous OCT-US probes were demonstrated by successful imaging of normal rabbit aorta. In this paper, we report on a novel probe design and improvement of the whole system. By arranging the OCT probe and US transducer sequentially, the overall size of the integrated OCT-US probes has been decreased significantly. Furthermore, data acquisition and image display are in real-time for both OCT and US due to modified hardware and software design. The capability of our integrated OCT-US imaging system is demonstrated by in vitro imaging of a human coronary artery specimen and in vivo imaging of a rabbit abdominal aorta.
Materials and Methods
The major challenges behind the integration of the OCT-IVUS system come from three aspects. First, an integrated probe that combines OCT components with US components needs to be designed and fabricated. Second, an integrated rotary joint is required to allow transmission of both optical and electrical signals between the stationary and rotary parts of the system. Last but not least, a truly integrated system requires simultaneous OCT and US data acquisition, processing, and image display.
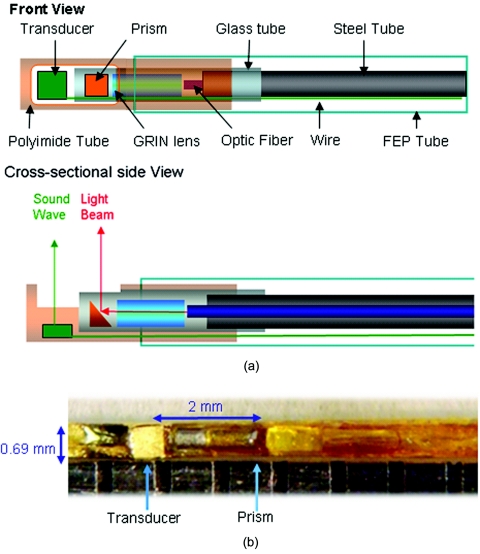
The schematic of the miniature OCT-US probe is shown in Fig. 1. Unlike our previously published designs which had a parallel or coaxial arrangement, this new version of the probe design features a sequential arrangement of the US transducer and OCT probe so that overall probe size is significantly decreased by roughly 4-fold. Within the OCT probe, a 0.35-mm diameter gradient index lens (NSG America, Inc., Somerset, New Jersey) was used for light focusing, followed by a 0.3-mm diameter micro-prism (Tower Optical Corp., Boynton Beach, Florida) for reflecting the focused light beam into tissue. All the optical components were fixed in a polyimide tube with an outer diameter (OD) of 0.41 mm and wall thickness of 0.02 mm (Small Parts, Inc., Miramar, Florida). The working distance of the OCT probe was about 3 mm. The ultrasonic transducer with an aperture size of 0.5 mm×0.5 mm was built using a PMN-PT single crystal (H.C. Materials, Bolingbrook, Illinois) which has superior piezoelectric properties for building high sensitivity US transducers in a small size. The center frequency of the ultrasound transducer was 35 MHz with a fractional bandwidth of 51%. The two way insertion loss was measured to be 15 dB at the center frequency. The transducer was fixed in the proximal end of a thin-wall polyimide tube (OD 0.69 mm, wall thickness 0.025 mm, Small Parts, Inc., Miramar, Florida) within which the OCT probe was also fixed. A window was made on the tube to let both the light beam and soundwave exit. Finally, the transducer wire and optical fiber were sealed in a thin-wall fluorinate dethylenepropylene tube (OD 0.61 mm, wall thickness 0.05 mm, Small Parts, Inc., Miramar, Florida). The maximum outer diameter of the fully integrated probe was 0.69 mm. It was known beforehand that the transducer and prism were 2 mm apart, thus co-registered OCT and US images could easily be matched from a 3D data set by offsetting OCT and US images by this distance. The measured sensitivity of the OCT system with rotary joint and probe was 103 dB. The measured axial and lateral resolutions (based on FWHM) of the OCT system with the probe were 8 and 30 μm, respectively. Resolutions of the US part were 60 and 420 μm, respectively, measured from a 6 μm wire phantom.
Figure 1.
(a) Schematic and (b) photo of miniature OCT-US probe.
The OCT-US probe was connected to the integrated OCT-US system via a semi-homemade rotary joint which consisted of a fiber optic rotary joint (Princetel, Inc., Pennington, New Jersey) and an electric slip ring (Prosperous. Co., Hangzhou, China). The optical portion of the OCT sub-system has been previously reported.10, 11, 12 As for the US sub-system, a Panametrics pulser/receiver (Olympus NDT, Inc., Kennewick, Washington) was used to drive the ultrasound transducer and also to receive the echo signals. The light source generated a 20 KHz trigger signal which drove a function generator (Agilent Technologies, Inc., Santa Clara, California) serving as a frequency divider to provide 4 KHz triggers to synchronize the data acquisition board and the pulser/receiver. Both OCT and US signals were digitized by a two-channel, 12 bit data acquisition board (Alazar Technologies Inc., Pointe-Claire, QC, Canada) working at a sampling rate of 250 MHz. OCT-US software was developed to handle both OCT and US data acquisition, processing, image display, and data saving. A real-time OCT-US image display was achieved thanks to multithreaded computing techniques. Our home-developed OCT-US program was capable of handling simultaneous OCT-US data processing and image display at a speed of 20 frames/s (1000 A-lines/frame for both OCT and US). Due to the rotational speed limitation of the slip ring, actual imaging speed was turned down to 4 frames/s. Three-dimensional scanning was obtained by using a rotational motor and a translational stage.
A post-mortem human coronary artery sample was fixed in formalin and then preserved in phosphate buffer. During the in vitro experiment, the specimen was pinned to a piece of cork and immersed in water. For the in vivo experiment, a male New Zealand white rabbit weighing 3.9 kg was anesthetized and intubated during the surgical and imaging procedures. Details of the surgical procedure can be found in Hoang et al.13 Perfluorocarbon (PFC) was used as a flushing agent for OCT. During both in vitro and in vivo experiments, the probe was spinning within a 3.6 Fr catheter sheath (maximum outer diameter 1.18 mm, Boston Scientific Corp., Natick, Massachusetts) to avoid contamination and causing trauma to the vessel wall.
Results and Discussion
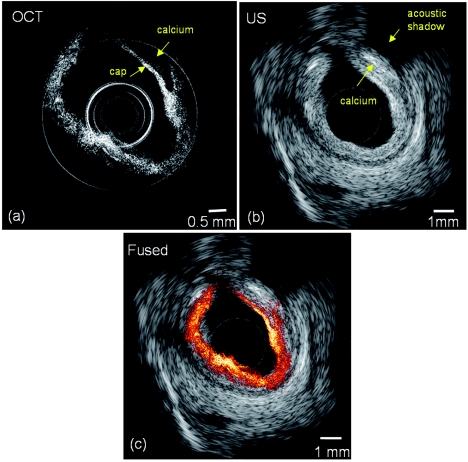
OCT and US images of a human coronary artery specimen with calcified plaque is shown in Fig. 2. The plaque can be identified in both images. From the OCT image, the plaque cap, as highlighted by the arrow, can be clearly identified due to the high axial resolution and high contrast of OCT. However, the OCT system could not visualize the entire depth of the vessel wall, and its maximum penetration depth was about 1 mm. On the other hand, in the US image, the acoustic shadow as pointed by the arrow indicates the location of the plaque. However, one can hardly distinguish the border between calcium and the surrounding tissue due to the inferior resolution of the US, but its penetration depth was much deeper than that of OCT (the radius of the image is 4.5 mm). The contour of the vessel in the OCT image matches very well with that in the US image, as shown in Fig. 2c which indicates that the two images were taken at approximately the same site.
Figure 2.
(a) OCT, (b) ultrasound, and (c) combined OCT-US images of a human coronary artery specimen.
Furthermore, in vivo imaging of the normal rabbit abdominal aorta was performed using this miniature probe and integrated system. Figures 3a, 3b show the OCT and US images without flushing. Since blood is highly scattering for OCT but serves as natural transmission media for US, no vessel structure could be seen in the OCT image [Fig. 3a] but was clearly visualized in the US image [Fig. 3b]. When using PFC as a flushing agent, a clear view was obtained for OCT as shown in Fig. 3c. Again, the fused image as shown in Fig. 3e shows that the OCT and US images match well. We can see the aorta surface with fine resolution in the OCT image and deep penetration depth into the aortic wall in the US image.
Figure 3.
(a) OCT and (b) ultrasound images of a rabbit abdominal aorta without flushing. (c) OCT, (d) ultrasound and (e) combined (e) OCT-US images with flushing.
Conclusion
We have successfully developed a miniature integrated OCT-US probe with an outer diameter of 0.69 mm which is suitable for in vivo imaging. A further integrated OCT-US imaging system has been realized by adopting a two-channel data acquisition board and creating real-time OCT-US software. Both in vitro and in vivo imaging have been performed to demonstrate the feasibility of our system in intravascular imaging.
Acknowledgments
We would like to thank Ms. Tanya Burney and Mr. David Yoon for their assistance during the surgical procedure. This work is based on research supported by the NIH (Grant Nos. R01EB10090, R01EB-00293, R01HL105215, RR-01192, and P41-EB2182) and the U.S. Air Force Office of Scientific Research, Medical Free-Electron Laser Program Grant No. FA9550-08-1-0384. Xiang Li is partially supported by USC Provost Fellowship. Institutional support from the Beckman Laser Institute Endowment is also gratefully acknowledged.
References
- Pasterkamp G., Falk E., Woutman H., and Borst C., “Techniques characterizing the coronary atherosclerotic plaque: Influence on clinical decision making,” J. Am. Coll. Cardiol. 36(1), 13–21 (2000). 10.1016/S0735-1097(00)00677-X [DOI] [PubMed] [Google Scholar]
- Farooq M. U., Khasnis A., Majid A., and Kassab M. Y., “The role of optical coherence tomography in vascular medicine,” Vas. Med. 14(1), 63–71 (2009). 10.1177/1358863X08095153 [DOI] [PubMed] [Google Scholar]
- Tearney G. J., Jang I. K., and Bouma B. E., “Optical coherence tomography for imaging the vulnerable plaque,” J. Biomed. Opt. 11(2), 021002 (2006). 10.1117/1.2192697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. , “Assessment by optical coherence tomography of stent struts across side branch—comparison of bare-metal stents and drug-eluting stents,” Circ. J. 75(1), 106–112 (2011). 10.1253/circj.CJ-10-0574 [DOI] [PubMed] [Google Scholar]
- Kawasaki M. et al. , “Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques,” J. Am. Coll. Cardiol. 48(1), 81–88 (2006). 10.1016/j.jacc.2006.02.062 [DOI] [PubMed] [Google Scholar]
- Rieber J. et al. , “Diagnostic accuracy of optical coherence tomography and intravascular ultrasound for the detection and characterization of atherosclerotic plaque composition in ex-vivo coronary specimens: a comparison with histology,” Coron. Artery Dis. 17(5), 425–430 (2006). 10.1097/00019501-200608000-00005 [DOI] [PubMed] [Google Scholar]
- Sawada T. et al. , “Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma,” Eur. Heart J. 29(9), 1136–1146 (2008). 10.1093/eurheartj/ehn132 [DOI] [PubMed] [Google Scholar]
- Li X., Yin J. C., Hu C. H., Zhou Q. F., Shung K. K., and Chen Z. P., “High-resolution coregistered intravascular imaging with integrated ultrasound and optical coherence tomography probe,” Appl. Phys. Lett. 97(13), 133702 (2010) . 10.1063/1.3493659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. C., Yang H. C., Li X., Zhang J., Zhou Q. F., Hu C. H., Shung K. K., and Chen Z. P., “Integrated intravascular optical coherence tomography ultrasound imaging system,” J. Biomed. Opt. 15(1), 010512 (2010). 10.1117/1.3308642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. C., Yin J. C., Hu C. H., Cannata J., Zhou Q. F., Zhang J., Chen Z. P., and Shung K. K., “A dual-modality probe utilizing intravascular ultrasound and optical coherence tomography for intravascular imaging applications,” IEEE Trans. Ultrasonics Ferroelectrics and Frequency Control 57(12), 2839–2843 (2010). 10.1109/TUFFC.2010.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang K. C. et al. , “Use of an oxygen-carrying blood substitute to improve intravascular optical coherence tomography imaging,” J.Biomed. Opt. 14(3), 034028 (2009). 10.1117/1.3153895 [DOI] [PMC free article] [PubMed] [Google Scholar]