Abstract
We propose a useful method to boost the imaging speed for spectral domain optical coherence tomography (SDOCT) by multiplying a number of high-speed spectrometers used in the system with selective precise control of data-recording and data-reading phases for spectral cameras employed in each spectrometer. To demonstrate the proposed method, we use two spectrometers built in a 1310 nm-band SDOCT system, each equipped with a high-speed InGaAs line-scan camera capable of 92-kHz line-scan rate, to achieve an unprecedented imaging speed at 184,000 lines/s. We validate the multiplied imaging speed by measuring Doppler-induced phase shift in the spectrograms using a flow phantom.
Keywords: spectral domain optical coherence tomography, spectrometer, InGaAs line-scan camera
Fourier domain detection techniques in optical coherence tomography (OCT), with either spectrometer-based systems [spectral domain OCT (SDOCT)] or frequency swept laser-based systems [swept source OCT (SSOCT)], have enabled OCT imaging with unprecedented sensitivities and speeds.1, 2, 3, 4, 5 High-speed imaging is particularly important for in vivo imaging applications because it can minimize motion artifacts. In recent years, impressive progress in increasing the imaging speed has been made to SSOCT. For example, Huber et al. proposed a frequency domain mode locking (FDML) technology that used an optical delay to improve the imaging speed of the SSOCT system.6 Combining FDML with a multi-spot concept, a record imaging speed of ∼20 MHz line-scan rate (LSR) was achieved at 1310-nm wavelength band.7 In addition, SSOCT at 1060 nm wavelength band was recently reported to have ∼400 kHz LSR, which is of clinical significance in ophthalmology.8 In contrast, there is limited effort to date that has allowed improvement in the imaging speed in SDOCT.
The imaging speed of SDOCT largely depends on the availability of line-scan cameras used in the spectrometer. At 850 nm wavelength band, a recent use of the state-of-the-art CMOS cameras (e.g., Basler spL4096–140k) has made the SDOCT imaging at a LSR of ∼300 kHz.9 However, for the wavelength longer than 1 micron, the fastest imaging speed reported so far was 47 kHz LSR that used an InGaAs linear detector (SU-LDH-1.7, Goodrich Ltd., USA) [e.g., Refs. 10 and 11]. With the current available line-scan cameras, a significant limitation of the imaging speed comes from the time required to read the recorded data from the camera, i.e., the dead time, Δtd. For example, when employing a Basler spL4096–140k camera to achieve ∼300 kHz A-lines rate, Δtd = ∼1.2 μs, representing almost 40% of the total time, Δt, was required to capture one axial scan. If this dead time period could be efficiently utilized to capture the interferograms, the imaging speed of an SDOCT system would be improved.
In this letter, we propose a useful method to boost the imaging speed of an SDOCT system, by employing multiple spectrometers in an SDOCT system while using precise timing sequences to control the recording and reading phases of the cameras used in each spectrometer. Theoretically, more than two spectrometers can be used to multiply the imaging speed; here we report the use of dual-spectrometers to demonstrate the concept. We will focus on the use of state-of-the-art InGaAs line-scan camera to realize an unprecedented imaging speed for an SDOCT system at 1310 nm. The camera used is capable of a maximum ∼92 kHz line-scan rate that was only recently made available by Goodrich Ltd., USA. When running at 92 kHz, a fixed timing period of Δt = 10.88 μs is required to capture one axial scan. With this in mind, if there are two spectrometers (cameras) in an SDOCT system, we can use two 92-kHz square waveforms (50% duty cycle) to externally trigger each camera, meaning that the first 50% timing period (5.44 μs) is used for light integration (data recording) for the first camera while for data transmission for the second camera, and vice versa for the second 50% time period. As a result, Δt is 100% fully utilized for the SDOCT system to capture the useful signals for imaging, leading to the final line-scan rate of 184 kHz.
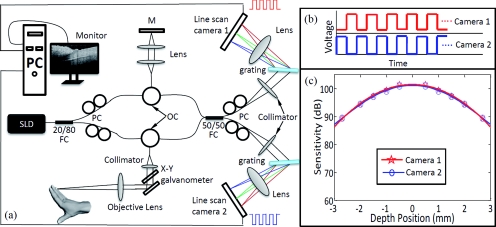
The system schematic is illustrated in Fig. 1a, where a 1310-nm superluminescent diode (SLD) was used to illuminate a fiber-optic interferometer system based on Mach–Zehnder configuration. The SLD (10 mW output power) had a spectral bandwidth of 60 nm, giving an axial resolution of ∼12 μm in air. A 20/80 fiber coupler was used to split the lights from the SLD with 20% going to the reference mirror and 80% to the sample. The lights received from the reference and sample arms are routed, via the optical circulators, to a 50/50 fiber coupler, which split the lights into two equal portions before being sent to two purpose-built high-speed spectrometers for detection of interferograms. The spectrometers were carefully adjusted to have almost equal imaging performance, each equipped with a state-of-the-art InGaAs line-scan camera (SU1024LDH2, Goodrich Ltd., USA) capable of a 92-kHz line-scan rate. Each spectrometer had a designed spectral resolution of 0.14 nm, giving ∼3 mm imaging depth in air. The system had a lateral resolution of 15 μm determined by the objective lens used to deliver the light to the sample.
Figure 1.
(a) System setup where the cameras are externally triggered by the trigger-sequences shown in (b). (c) Measured system-sensitivity for each spectrometer. SLD: super luminescent diode, OC: optical circulator, PC: polarization controller, and M: mirror.
In order to sequentially control the two cameras to achieve the system line-scan rate of 184 kHz, two trigger sequences [Fig. 1b] were generated to control the cameras. At first, a 92-kHz trigger-sequence [i.e., square waveform with a time interval 10.88 μs, top in Fig. 1b] was generated by a digital to analog output card (NI PCI 6713) to trigger the first camera with a 50% duty cycle, i.e., data recording when the signal is “on” in the first half and data transmission otherwise. Second, an identical, but reversed, trigger sequence to the first camera was used to control the second camera [the bottom trigger sequence in Fig. 1b]. Consequently, with the combined use of these two trigger sequences, the two cameras can capture two consecutive A-lines during one cycle of the trigger sequence. In this way, when the system continuously acquires the A-lines to form one B-scan frame, the odd-numbered A-lines would be captured by the first camera while the even-numbered A-lines by the second camera. This indicates that the SDOCT system captures the spectral interferograms at 100% duty cycle with the help of two cameras, achieving ∼180 kHz A-lines sampling rate. Under this condition, the system sensitivity curve was measured and plotted in Fig. 1c, where the star and circle signs represent the measured sensitivity values obtained from the spectrometers that employed the first and second cameras, respectively. As shown, these two spectrometers demonstrated almost identical sensitivity performance, both of which have ∼102-dB sensitivity around zero-delay line and ∼95 dB at 1.5-mm imaging-depth position.
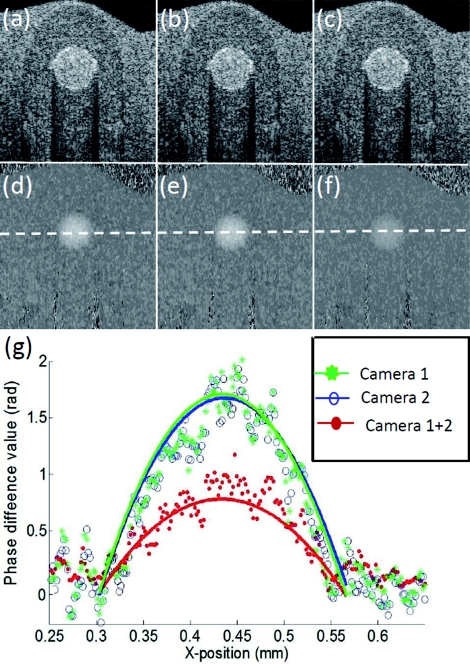
To demonstrate the proposed system capable of a 184-kHz line-scan rate by employing dual 92-kHz spectrometers, we elected to image a purpose-made flow phantom to assess the system on two aspects: microstructure imaging and flow imaging using phase-resolved measurement. Because we know that if under the condition of 184-kHz line-scan rate, the phase differences assessed between two adjacent A-lines must be half that when the system runs at 92 kHz by the use of the triggering protocol described above. To make the flow phantom, an inner diameter of a ∼250-μm plastic tube was buried within a tissue phantom made by mixing the gelatin with 2% milk. A ∼1% intralipid water solution was driven to flow in the plastic tube by a precision syringe pump. In the experiment, we captured 1000 A-lines to form one B-scan covering ∼1 mm. With the triggering protocol described above, 500 odd-numbered A-lines were captured by the first camera and the other 500 A-lines by the second camera. The results are shown in Fig. 2, where (a) and (b) are the cross-sectional micro-structure images obtained from the first and second spectrometers, respectively, while (c) is from the combined system but twice the imaging speed. Due to the same performance of the spectrometers used, the image qualities from the individual and combined spectrometers are almost identical to each other as expected, both in system sensitivity and imaging depth.
Figure 2.
Cross-sectional structure and phase-difference images obtained from the flow-phantom experiments. (a) and (b) SDOCT structural images resulting from the first and second spectrometers, respectively. (c) Structural image from the dual-spectrometer system. (d), (e) and (f) Corresponding PRODT phase difference maps evaluated from the system employing the first, second, and dual-spectrometers, respectively. (g) Phase-difference plot along the dashed line (see middle images), passing through the center of the flow-tube. The solid curves are the parabolic fitting to the measured data.
Next we applied the phase-resolved optical Doppler tomography (PRODT) algorithm to obtain phase difference maps of the flow to verify the system sampling rate. Figure 2d shows the phase difference map obtained from the data captured by the first camera, while Fig. 2e is from the second camera. Because of the identical performance of two spectrometers, the phase-difference values within the flow regions are without visible difference between the two, both giving an average flow velocity of 5.68 mm/s after converting the phases into the velocities, agreeing well with the pre-set value (5.8 mm/s, Doppler angle corrected) at the pump. Figure 2f gives the phase-difference map obtained from the merged dataset from the first and second spectrometers. Compared to Figs. 2d, 2e, the phase values in Fig. 2f are halved due to being twice the A-line rate, however, the evaluated average flow velocity (5.66 mm/s) is about the same as that of the single spectrometer. The phase values can be better illustrated in Fig. 2g by plotting them along the dashed line passing through the tube center. It is clear that the phase-difference values at the flow region are statistically the same for the first and second spectrometers. However, these values are twice as high as those from the dual-spectrometers. These results demonstrate that the dual-spectrometer system doubled the sampling rate of the single-spectrometer system.
We have demonstrated the 184-kHz line-scan rate for a SDOCT system working at 1310-nm band by the use of a dual-spectrometer system. The concept can be extended to include more spectrometers in a SDOCT system, including that at other wavelength bands, to further increase the imaging speed. With a speed of 184 kHz as demonstrated here, a 3D imaging cube of 512×512×512 pixels can be acquired within ∼0.7 s, important for in vivo imaging applications where the subject movement is inevitable. With such high speed, multiple advantages can be envisioned for in vivo imaging. For example, high speed makes rapid survey of large tissue volumes possible as well as increasing the imaging throughput. In addition, high speed also allows for multi-frame averaging or acquisition of high-pixel-density images, which can be used to improve image quality or reduce speckle. However, the price that has to be paid for the proposed approach is the increased cost of the likely system, which may become a main factor limiting its clinical applications.
In summary, we have proposed a useful method to boost the imaging speed of a SDOCT system, in which multiple spectrometers can be employed to multiply the system line-scan rate by precise timing control of the reading and recording phases in the cameras. We have demonstrated the concept by use of a dual-spectrometer that employed the state-of-the-art InGaAs line-scan cameras to achieve an unprecedented imaging speed of 184,000 lines per second at the 1310-nm wavelength band.
This work was supported in part by research Grants Nos. R01HL093140, R01EB009682, and R01DC010201 from the National Institutes of Health.
References
- Choma M. A., Sarunic M. V., Yang C. H., and Izatt J. A., “Sensitivity advantage of swept source and Fourier domain optical coherence tomography,” Opt. Express 11, 2183–2189 (2003). 10.1364/OE.11.002183 [DOI] [PubMed] [Google Scholar]
- de Boer J. F., Cense B., Park B. H., Pierce M. C., Tearney G. J., and Bouma B. E., “Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography,” Opt. Lett. 28, 2067–2069 (2003). 10.1364/OL.28.002067 [DOI] [PubMed] [Google Scholar]
- Leitgeb R., Hitzenberger C. K., and Fercher A. F., “Performance of Fourier domain vs. time domain optical coherence tomography,” Opt. Express 11, 889–894 (2003). 10.1364/OE.11.000889 [DOI] [PubMed] [Google Scholar]
- Wojtkowski M., Bajraszewski T., Gorczynska I., Targowski P., Kowalczyk A., Wasilewski W., and Radzewicz C., “Ophthalmic imaging by spectral optical coherence tomography,” Am. J. Ophthalmol. 138, 412–419 (2004). 10.1016/j.ajo.2004.04.049 [DOI] [PubMed] [Google Scholar]
- Yun S. H., Boudoux C., Tearney G. J., and Bouma B. E., “High-speed wavelength-swept semiconductor laser with a polygon-scanner-based wavelength filter,” Opt. Lett. 28, 1981–1983 (2003). 10.1364/OL.28.001981 [DOI] [PubMed] [Google Scholar]
- Huber R., Wojtkowski M., and Fujimoto J. G., “Fourier domain mode locking (FDML): a new laser operating regime and applications for optical coherence tomography,” Opt. Express 14, 3225–3237 (2006). 10.1364/OE.14.003225 [DOI] [PubMed] [Google Scholar]
- Wieser W., Biedermann B. R., Klein T., Eigenwillig C. M., and Huber R., “High-quality 3-D imaging with multimegahertz OCT,” Opt. Express 18, 14685–14704 (2010). 10.1364/OE.18.014685 [DOI] [PubMed] [Google Scholar]
- Potsaid B., Baumann B., Huang D., Barry S., Cable A. E., Schuman J. S., Duker J. S., and Fujimoto J. G., “Ultrahigh speed 1050 nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second,” Opt. Express 18, 20029–20048 (2010). 10.1364/OE.18.020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potsaid B., Gorczynska I., Srinivasan V. J., Chen Y. L., Jiang J., Cable A., and Fujimoto J. G., “Ultrahigh speed spectral/Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second,” Opt. Express 16, 15149–15169 (2008). 10.1364/OE.16.015149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanathasan P., Forbes P., Ren Z., Malchow D., Boyd S., and Bizheva K., “High-speed, high-resolution Fourier-domain optical coherence tomography system for retinal imaging in the 1060 nm wavelength region,” Opt. Lett. 33, 2479–2481 (2008). [DOI] [PubMed] [Google Scholar]
- An L., Subhash H. M., and Wang R. K., “Full range complex spectral domain optical coherence tomography for volumetric imaging at 47,000 A scans per second,” J. Opt. 12, 084003 (2010). 10.1088/2040-8978/12/8/084003 [DOI] [PMC free article] [PubMed] [Google Scholar]