Abstract
Nitrogen mustard alkylating agents react with isolated DNA in a sequence selective manner, and the substituent attached to the drug reactive group can impose a distinct sequence preference. It is not clear however to what extent the observed DNA sequence preferences are preserved in intact cells. The highly reiterated sequence of human alpha DNA has been used to determine the sites of guanine-N7 alkylation following treatment of cells with three nitrogen mustards, mechlorethamine, uracil mustard and quinacrine mustard, known to react in isolated DNA with distinctly different sequence preferences. Alpha DNA from drug treated cells was extracted, purified, end-labeled, and a 296 base pair, singly end-labelled, fragment isolated. Following the quantitative conversion of alkylation sites to strand breaks the fragments were separated on DNA sequencing gels. Clear differences were observed between the alkylation patterns of the three compounds, and the selectivities were qualitatively similar to those predicted and observed in the same sequence alkylated in vitro. In particular the unique preferences of uracil and quinacrine mustards for 5'-PyGC-3' and 5'-GT/GPu-3' sequences, respectively, were preserved in intact cells suggesting that the pattern of sequence dependent reactivity is not grossly affected by the nuclear milieu.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Chun E. H., Gonzales L., Lewis F. S., Jones J., Rutman R. J. Differences in the in vivo alkylation and cross-linking of nitrogen mustard-sensitive and -resistant lines of Lettré-Ehrlich asites tumors. Cancer Res. 1969 Jun;29(6):1184–1194. [PubMed] [Google Scholar]
- Ewig R. A., Kohn K. W. DNA damage and repair in mouse leukemia L1210 cells treated with nitrogen mustard, 1,3-bis(2-chloroethyl)-1-nitrosourea, and other nitrosoureas. Cancer Res. 1977 Jul;37(7 Pt 1):2114–2122. [PubMed] [Google Scholar]
- Gilman A., Philips F. S. The Biological Actions and Therapeutic Applications of the B-Chloroethyl Amines and Sulfides. Science. 1946 Apr 5;103(2675):409–436. doi: 10.1126/science.103.2675.409. [DOI] [PubMed] [Google Scholar]
- Grunberg S. M., Haseltine W. A. Use of an indicator sequence of human DNA to study DNA damage by methylbis(2-chloroethyl)amine. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6546–6550. doi: 10.1073/pnas.77.11.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
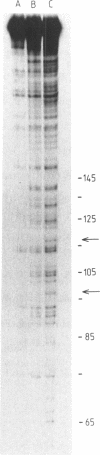
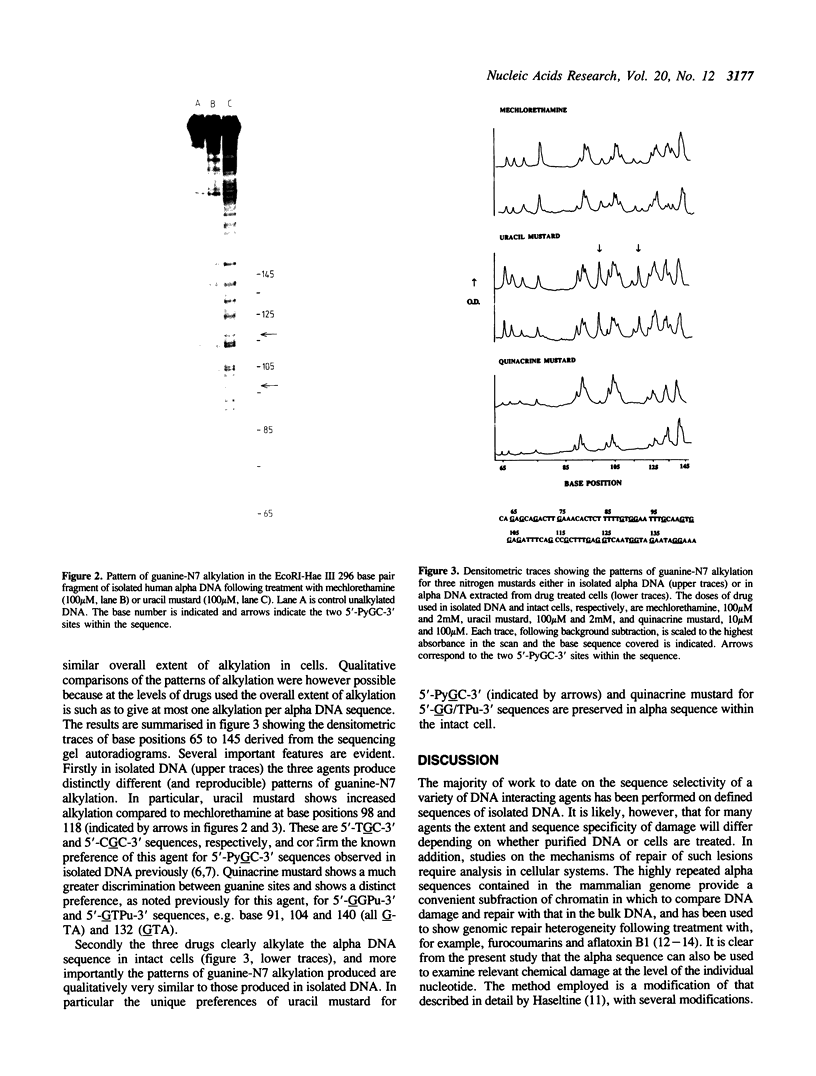
- Hartley J. A., Gibson N. W., Kohn K. W., Mattes W. B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986 Apr;46(4 Pt 2):1943–1947. [PubMed] [Google Scholar]
- Kohn K. W., Hartley J. A., Mattes W. B. Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res. 1987 Dec 23;15(24):10531–10549. doi: 10.1093/nar/15.24.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A., Zolan M. E., Hanawalt P. C. Restricted repair of aflatoxin B1 induced damage in alpha DNA of monkey cells. Nucleic Acids Res. 1983 Aug 25;11(16):5675–5689. doi: 10.1093/nar/11.16.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards. Nucleic Acids Res. 1986 Apr 11;14(7):2971–2987. doi: 10.1093/nar/14.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. Mechanism of DNA strand breakage by piperidine at sites of N7-alkylguanines. Biochim Biophys Acta. 1986 Oct 16;868(1):71–76. doi: 10.1016/0167-4781(86)90088-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ponti M., Forrow S. M., Souhami R. L., D'Incalci M., Hartley J. A. Measurement of the sequence specificity of covalent DNA modification by antineoplastic agents using Taq DNA polymerase. Nucleic Acids Res. 1991 Jun 11;19(11):2929–2933. doi: 10.1093/nar/19.11.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. C., Manuelidis L. Sequence definition and organization of a human repeated DNA. J Mol Biol. 1980 Sep 25;142(3):363–386. doi: 10.1016/0022-2836(80)90277-6. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Smith C. A., Hanawalt P. C. Formation and repair of furocoumarin adducts in alpha deoxyribonucleic acid and bulk deoxyribonucleic acid of monkey cells. Biochemistry. 1984 Jan 3;23(1):63–69. doi: 10.1021/bi00296a010. [DOI] [PubMed] [Google Scholar]