Abstract
Background
Human milk contains a wide variety of nutrients that contribute to the fulfillment of its functions, which include the regulation of newborn development. However, few studies have investigated the concentrations of S100B protein, brain-derived neurotrophic factor (BDNF), and glial cell line-derived neurotrophic factor (GDNF) in human milk. The associations of the concentrations of S100B protein, BDNF, and GDNF with maternal factors are not well explored.
Methodology/Principal Findings
To investigate the concentrations of S100B protein, BDNF, and GDNF in human milk and characterize the maternal factors associated with their levels in human milk, human milk samples were collected at days 3, 10, 30, and 90 after parturition. Levels of S100B protein, BDNF, and GDNF, and their mRNAs in the samples were detected. Then, these concentrations were compared with lactation and other maternal factors. S100B protein levels in human milk samples collected at 3, 10, 30, and 90 d after parturition were 1249.79±398.10, 1345.05±539.16, 1481.83±573.30, and 1414.39±621.31 ng/L, respectively. On the other hand, the BDNF concentrations in human milk samples were 10.99±4.55, 13.01±5.88, 13.35±6.43, and 2.83±5.47 µg/L, while those of GDNF were 10.90±1.65, 11.38±1., 11.29±3.10, and 11.40±2.21 g/L for the same time periods. Maternal post-pregnancy body mass index was positively associated with S100B levels in human milk (r = 0.335, P = 0.030<0.05). In addition, there was a significant correlation between the levels of S100B protein and BDNF (z = 2.09, P = 0.037<0.05). Delivery modes were negatively associated with the concentration of GDNF in human milk.
Conclusions
S100B protein, BDNF, and GDNF are present in all samples of human milk, and they may be responsible for the long term effects of breast feeding.
Introduction
The S100B protein is a member of the calcium-binding S100 family, which is characterized by a low molecular weight and a special EF-hand structure [1]. Like most members of this family, S100B has a homodimeric structure wherein each beta monomer is approximately 10.5 kDa. Each monomer has two EF hand sites for Ca2+ binding and independent sites for Zn2+ binding. It has two disulfide bridges, but the dimeric structure is maintained independently of this aspect.
Brain-derived neurotrophic factor (BDNF) is a small dimeric protein belonging to the neurotrophin family, which is widely expressed in the mammalian adult brain [2].
Glial cell line-derived neurotrophic factor (GDNF) is a distant member of the transforming growth factor β superfamily that was originally isolated from the rat B49 glial cell line [3]. This protein is a glycosylated, disulfide-bonded homodimerwith a molecular weight of 33–45 kDa. Its monomer has a molecular weight of 16 kDa after deglycosylation [4].
S100B, BDNF, and GDNF play a critical role in the development and maintenance of the nervous system, and in neuronal survival and proliferation [3], [5]–[19]. These proteins have been implicated in the modulation of learning and memory [20]–[24]. Human milk protects the infants from infection, modulates their immune function, and affects their overall development [25]. The present study investigates the concentration of S100B, BDNF, and GDNF in the milk of Chinese women after parturition to clarify the function of these cytokines.
Methods
Ethics Statement
Approval from the Ethical Committee of the Harbin Medical University was obtained prior to this study. Written informed consent was obtained before collection of milk samples from donors.
Participants
Samples for ELISA analysis were collected from 42 mothers: 31 of whom had abdominal delivery at term, while 11 delivered vaginally at term. Milk samples were collected at 3, 10, 30, and 90 d after parturition. Participants who had gestational hypertension, diabetes, infection, fever, metabolic diseases, breast diseases, central nervous system diseases, malnutrition, maternal allergy, fetal anomaly, and smoking habits were excluded.
Human milk was collected by hand into sterile 5-ml Eppendorf tubes. Upon collection, samples were refrigerated at 4°C in a polystyrene box containing ice. All samples were immediately transferred to the laboratory, where they were stored at −80°C.
ELISA analysis
After thawing at room temperature, milk samples were centrifuged at 1000 g for 10 min at 4°C. The supernatant was removed and re-centrifuged at 10,000 g for 30 min at 4°C, and the floating lipid layer and cellular sediments were removed. BDNF and GDNF concentration were measured in all samples by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Inc., United States of America) according to the manufacturer's instructions. ELISA (Wuhan EIAab Science Co., Ltd., China) was also used to determine the concentration of S100B protein. All samples were tested in duplicate and the averages were reported. Intra-assay and the inter-assay variation coefficients were <5% and <10%, respectively. The assay ranges of the S100B protein, BDNF and GDNF ELISA kits were 15.6–1000 ng/L, 1.5–110 g/L, and 2–60 g/L, respectively.
Western blot analysis
Protein concentrations were determined using the Lowry method of protein assay [26] with bovine serum albumin as standard. About10 µL of human milk (1000 g supernatant) were separated on 15% SDS–PAGE and transferred to a nitrocellulose membrane. Immunoblotting was performed using rabbit BDNF and GDNF antibodies (Wuhan Boster Bioligical Technology.,LTD, China). The membrane was then incubated with the secondary alkaline phosphatase-conjugated IgG and detected with the Western Blue Stabilized Substrate for alkaline phosphatase (Promega).
RT-PCR analysis
Milk samples for reverse transcription-PCR (RT-PCR) analysis were collected from a mother at 3, 10, 30, and 90 d after parturition. The milks (15 ml) were centrifuged at 1000 g for 10 min at 4°C, and the RNA was extracted from the cell-pellet using TRIzol reagent (Invitrogen, Carlsbad, CA). RT-PCR (RNA PCR kit, TaKaRa Shuzo Co., Ltd., Japan) was conducted according to the manufacturer's manual. The quality of RNA extract was determined using the A260/A280 ratio, and was found to be 1.7–2.0 for all RNA preparations. A 1 μg portion of the total RNA was used for cDNA synthesis by reverse transcription (RT) with a final reaction mixture volume of 20 μl. RT was performed using a thermal program of 25°C for 10 min, 42°C for 30 min, and 95°C for 5 min. The cDNA was stored at −80°C for further use.
A 1.6 μl aliquot of the cDNA solution was used for the PCR assay (20 μl final volume). Samples were subjected to 36 cycles of PCR amplification: each cycle consisting of denaturation at 94°C for 30 s, annealing at a specified temperatures for 30 s, and extension at 72°C for 30 s. A final extension was performed at 72°C for 10 min.
Annealing temperatures (AT) and primer sequences are as follows: β2-actin (forward: 5′-CTCGCTGTCCACCTTCCA-3′; reverse: 5′-GCTGTCACCTTCACCGTTC-3′; size: 256 bp; AT: 56°C), S100B [27] (forward: 5′- CATTTCTTAGAGGAAATC-3′; reverse: 5′-ATGTTCAAAGAACTCGTG-3′; size: 147 bp; AT: 46°C), BDNF (forward: 5′-CAAACATCCGAGGACAAG-3′; reverse: 5′- GCCGTTACCCACTCACT-3′; size: 379 bp; AT: 56°C), and GDNF (forward: 5′- ACTTGGGTCTGGGCTATGAA-3′; reverse: 5′-TGTCACTCACCAGCCTTCTATT-3′; size: 132 bp; AT: 53°C). Amplification products were examined by electrophoresis on 1.5% agarose gel stained with ethidium bromide. All assays were performed with at least one replicate. The amplicons were matched with DL500 DNA Marker 100T (TaKaRa Shuzo Co., Ltd., Japan).
Statistical analysis
All data were expressed as the mean ±SD and were analyzed using Stata version 10.0 (StataCorp, United States of America). Statistical analysis was performed using the generalized estimating equation. A linear correlation was conducted to assess the relationship of S100B milk concentrations and the body mass index (BMI) of mothers. Statistical significance was indicated by P values less than 0.05.
Results
Mothers who participated in the study ranged from 19 to 38 years (mean age 25.26 years), with BMIs ranging from 21.7 to 34.8 (mean 27.57 kg/m2) and Gestational Ages between 37 and 42 weeks (mean 38.98 weeks). All mothers had their first accouchements at the time of the study and all showed normal clinical conditions.
Cytokine concentrations in the milk of the mothers, who gave samples at 30 (n = 40) and 90 (n = 24) d after parturition, are presented in Tables 1 and 2 .
Table 1. Cytokines in the human milk from Chinese women during day 3, 10, and 30 after parturition (n = 42).
| Cytokines | day 3 | day 10 | day 30* | P |
| S100B (ng/L) | 1249.79±398.10 | 1345.05±539.16 | 1481.83±573.30 | 0.034 |
| BDNF (µg/L) | 10.99±4.55 | 13.01±5.88 | 13.35±6.43 | 0.205 |
| GDNF (µg/L) | 10.90±1.65 | 11.38±1.78 | 11.29±3.10 | 0.831 |
*number of donors equal to 40.
Table 2. Cytokines in human milk from Chinese women during day 3, 10, 30, and 90 after parturition (n = 24).
| Cytokines | day 3 | day 10 | day 30 | day 90 | P |
| S100B (ng/L) | 1221.63±338.27 | 1246.08±542.27 | 1381.28±525.48 | 1414.39±621.31 | 0.434 |
| BDNF (µg/L) | 10.64±5.402811 | 11.69±5.51 | 12.42±6.27 | 12.83±5.47 | 0.293 |
| GDNF (µg/L) | 10.95±1.87 | 11.37±1.93 | 11.47±2.65 | 11.40±2.21 | 0.735 |
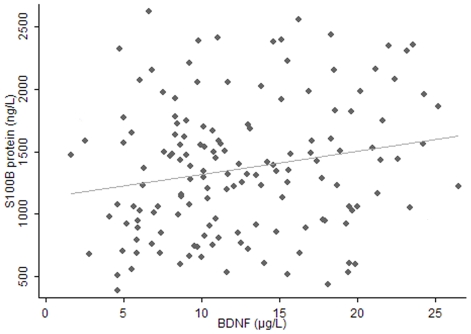
S100B protein, BDNF, and GDNF were present in all samples of human milk. The levels of S100B protein peaked at 30 d after parturition , while BDNF and GDNF levels did not show variations with time. A significant correlation was found between S100B protein and BDNF levels at the third month after birth (z = 2.09, P = 0.037<0.05, Figure 1 ).
Figure 1. Positive association between levels of S100B and BDNF in human milk.
The solid line represents the predicted regression line determined from repeated-measures analysis of S100B and BDNF concentrations 3 month after parturition. n = 24, P = 0.037<0.05.
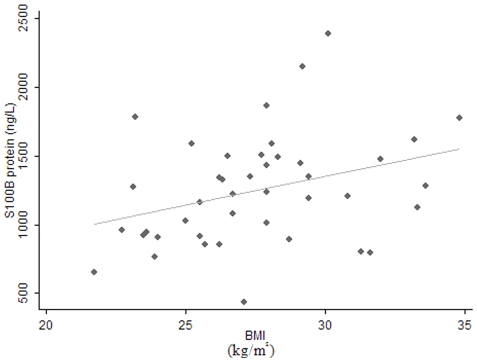
S100B protein levels in milk at 3 d after parturition were positively correlated with the maternal post pregnancy BMI (r = 0.335, P = 0.030<0.05) ( Figure 2 ). At one month after parturition, the GDNF levels from mothers who delivered vaginally at term were significantly lower than those who delivered abdominally at term (z = −2.19, P = 0.029<0.05). This correlation persisted until three months after birth (z = −2.17, P = 0.030<0.05). No correlations were found between the levels of other cytokines and age, height, weight, BMI, gestational age, or delivery modes.
Figure 2. S100B levels in human milk were closely correlated with BMI.
The solid line represents the predicted regression line determined from measures analysis of human milk collected 3 days after birth. Pearson correlation coefficient (r) was 0.335. P<0.05. n = 42.
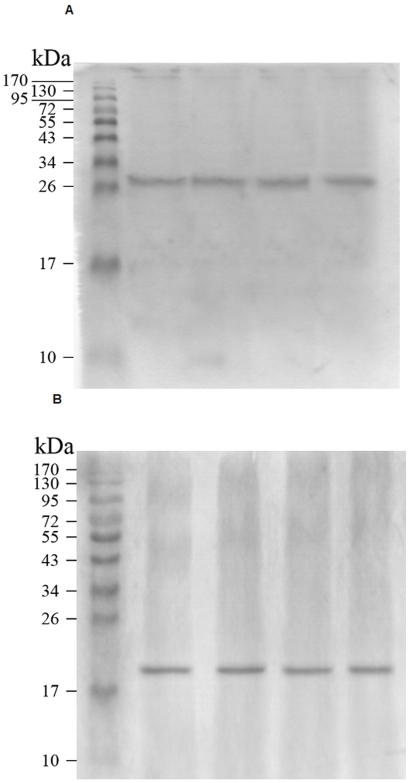
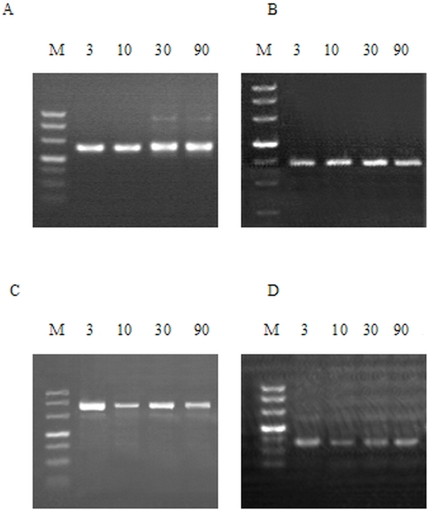
Antiserum against BDNF revealed a single band at approximately 27 kDa at all sampling days examined ( Figure 3 ), while the antibody against GDNF labeled a band at approximately 20 kDa ( Figure 3 ). RT-PCR products from milk RNA were subjected to subsequent gel electrophoresis, which showed the expected bands of 147, 379, and 132 bp ( Figure 4 ). Bands of cytokines from milk collected at 3, 10, 30, and 90 d after parturition were not found to vary with time ( Figure 4 ).
Figure 3. Western blot analysis of BDNF and GDNF in human milk.
A: BDNF band at approximately 27 kDa. B: GDNF band at approximately 20 kDa.
Figure 4. RT-PCR amplified fragments of S100B, BDNF and GDNF mRNA from human milk.
M = DL500 DNA Marker. A: bands of β-actin at 256 bp. B: bands of S100B at 147 bp. C: bands of BDNF at 379 bp. D: bands of GDNF at 132 bp. The bands of cytokines from milks collected at day 3, 10, 30, 90 after parturition were not found to vary with time.
Discussion
This is the first study to report on BDNF and GDNF concentrations in the milk of lactating women. This study investigated changes in these concentrations during lactation, in addition to the measurement of S100B protein in the milk of Chinese women. RT-PCR analysis detected S100B, BDNF, and GDNF mRNA in human milk collected at 3, 10, 30, and 90 d after birth ( Figure 4 ). Western blot analysis was used to confirm the immunoreactivity observed in ELISA assay.
Findings of this study indicate that BDNF and GDNF can be added to the list of bioactive factors (e.g. IL-1b, IL-2, IL-4, IL-5, Lactoferrin, transferrin) [28] present in human milk. S100B protein has been previously documented in human milk at 30 to 929 µg/L, indicating that the lactating human breast secretes S100B protein [29]–[31]. In view of the broad range of S100B protein concentrations reported and the lack of data on human milk from Chinese women, the S100B protein levels in milk from Chinese subjects were quantified in this study. We found that the S100B protein concentrations in milk collected within three months after giving birth is within 390.7–2623.9 ng/L. At day 3 after birth, the S100B protein concentration in milk was much lower than those in milk from Burkinabe and Sicilian women (204.31±63.25 and 199.42±45.28 µg/L, respectively) [31]. However, this does not mean that Chinese infants consume less S100B protein; the overall amount of milk production is independent of ethnicity [31], [32]. S100B, BDNF and GDNF concentrations in milk were much higher than in the serum [33]–[35]. Although the biological significance of the factors in human milk for breastfeeding infants remains unknown, studies suggest that they may serve potential neurotrophic function that may modulate the function and integrity of the GI tract [36], [37] and may exert a stimulating effect on neurodevelopment during breast-feeding or long afterwards [38], [39]. Previous studies have shown that these factors are critical molecules that support the process of neuronal growth, development, protection, and repair [3], [5]–[19], and the modulation of learning and memory [20]–[24]. BDNF plays an important role in the development of the enteric nervous system, defense against intestinal infection, and the modulation of gastrointestinal motility [40], [41]. GDNF has been shown to support the development of human enteric nervous system and intestinal epithelial barrier integrity [42], [43].
Human milk is known to contain leukocytes expressing BDNF and GDNF [44]–[46], which may be reasonably supposed to be the sources of BDNF and GDNF mRNA detected by RT-PCR and of the factors detected by ELISA and Western blot assays. There has been no evidence demonstrating that BDNF and GDNF in human milk are derived entirely from the serum or mammary gland cells. Studies have verified that a significant part of S100B protein present in milk is secreted by mammary epithelial cells and that S100B can be expressed by human milk cells [29].
Detailed information on the fate of these cytokines in the gastrointestinal tract is needed, although we can assume that they participate in the nutritional effects of milk because previous studies have shown that human milk proteins are utilized exceptionally well [47]. Several factors may contribute to these nutritional effects. For instance, human milk contains proteins that bind essential nutrients, thus keeping nutrients in solution and facilitating their uptake by the intestinal mucosa. In addition, protease inhibitors limit the activity of proteolytic enzymes, thereby preserving the physiologic function of some relatively stable binding proteins and some enzymes that can affect the digestion and utilization of macronutrients.
In this study, we found a positive correlation between S100B protein levels in human milk and BMI. This result corroborates previous reports of a direct relationship between S100B serum levels and BMI [48]. More direct evidence of the potential role of S100B in fat metabolism comes from animal studies that have demonstrated the presence of S100B in adipose tissue of rats [49] and that serum S100B levels are significantly influenced by adipose tissue [50].
GDNF levels in milk from mothers who delivered vaginally at term were significantly lower than those who delivered abdominally. This could be attributed to the protective role that breast feeding plays in neonates delivered abdominally. Therefore, cesarean deliveries with no labor complications remain at a much higher risk of neonatal mortality than planned vaginal deliveries [51], because emergency and elective cesarean deliveries are similarly associated with a decreased rate of exclusive breastfeeding compared with vaginal delivery [52].However, no reports have conclusively proven this assumption and it remains to be an important research topic.
This study also found a significant correlation between the levels of S100B protein and BDNF in human milk. At present, no other study had reported this finding, and this association was found in 22 women only. Thus further studies are needed to confirm this relationship.
The present study was constrained by the limited amount of milk samples. Maternal sera were not simultaneously collected due to the peripartum folk customs in China. Furthermore, the absence of previous reports on the basal concentrations of BDNF and GDNF in lactating women prevented comparison of the results. Despite these limitations, the present study was the first to determine basal BDNF and GDNF concentrations in milk from lactating women.
In conclusion, our findings indicate that S100B protein, BDNF, and GDNF are present in human milk. Although their exact functions in human milk are not yet certain, present findings suggest that the physiological function of these cytokines possibly includes a trophic role. The relationship between S100B concentrations in human milk and the BMI of the lactating women has not been described before. This suggests that the S100B protein is a new adipokine. In addition, delivery modes were negatively associated with GDNF concentration in human milk. A positive correlation exists between the levels of S100B protein and BDNF in human milk. Additional investigations are required to clarify the physiologic roles of S100B protein, BDNF, and GDNF in human milk.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Heizmann CW, Fritz G, Schäfer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–68. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 2.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–64. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 4.Lin LF, Zhang TJ, Collins F, Armes LG. Purification and initial characterization of rat B49 glial cell line-derived neurotrophic factor. J Neurochem. 1994;63:758–68. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- 5.Kligman D, Marshak DR. Purification and characterization of a neurite extension factor from bovine brain. Proc Natl Acad Sci U S A. 1985;82:7136–9. doi: 10.1073/pnas.82.20.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winningham-Major F, Staecker JL, Barger SW, Coats S, Van Eldik LJ. Neurite extension and neuronal survival activities of recombinant S100 beta proteins that differ in the content and position of cysteine residues. J Cell Biol. 1989;109:3063–71. doi: 10.1083/jcb.109.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haglid KG, Yang Q, Hamberger A, Bergman S, Widerberg A, et al. S-100beta stimulates neurite outgrowth in the rat sciatic nerve grafted with acellular muscle transplants. Brain Res. 1997;753:196–201. doi: 10.1016/s0006-8993(96)01463-1. [DOI] [PubMed] [Google Scholar]
- 8.Van Eldik LJ, Christie-Pope B, Bolin LM, Shooter EM, Whetsell WO Neurotrophic activity of S-100 beta in cultures of dorsal root ganglia from embryonic chick and fetal rat. Brain Res. 1991;542:280–5. doi: 10.1016/0006-8993(91)91579-p. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya A, Oppenheim RW, Prevette D, Moore BW, Brackenbury R, et al. S100 is present in developing chicken neurons and Schwann cells and promotes motor neuron survival in vivo. J Neurobiol. 1992;23:451–66. doi: 10.1002/neu.480230410. [DOI] [PubMed] [Google Scholar]
- 10.Nishi M, Whitaker-Azmitia PM, Azmitia EC. Enhanced synaptophysin immunoreactivity in rat hippocampal culture by 5-HT 1A agonist, S100b, and corticosteroid receptor agonists. Synapse. 1996;23:1–9. doi: 10.1002/(SICI)1098-2396(199605)23:1<1::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Ueda S, Kokotos Leonardi ET, Bell J, Azmitia EC. Serotonergic sprouting into transplanted C-6 gliomas is blocked by S-100 beta antisense gene. Brain Res Mol Brain Res. 1995;29:365–8. doi: 10.1016/0169-328x(94)00269-k. [DOI] [PubMed] [Google Scholar]
- 12.Alexanian AR, Bamburg JR. Neuronal survival activity of s100betabeta is enhanced by calcineurin inhibitors and requires activation of NF-kappaB. FASEB J. 1999;13:1611–20. doi: 10.1096/fasebj.13.12.1611. [DOI] [PubMed] [Google Scholar]
- 13.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 14.Barnabé-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–60. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. 2003;258:319–33. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–9. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 18.Ledda F, Paratcha G, Sandoval-Guzmán T, Ibáñez CF. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- 19.Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, et al. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–61. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 20.Gromov LA, Syrovatskaya LP, Ovinova GV. Functional role of the neurospecific S-100 protein in the processes of memory. Neurosci Behav Physiol. 1992;22:25–9. doi: 10.1007/BF01186664. [DOI] [PubMed] [Google Scholar]
- 21.Lewis D, Teyler TJ. Anti-S-100 serum blocks long-term potentiation in the hippocampal slice. Brain Res. 1986;383:159–64. doi: 10.1016/0006-8993(86)90016-8. [DOI] [PubMed] [Google Scholar]
- 22.O'Dowd BS, Zhao WQ, Ng KT, Robinson SR. Chicks injected with antisera to either S-100 alpha or S-100 beta protein develop amnesia for a passive avoidance task. Neurobiol Learn Mem. 1997;67:197–206. doi: 10.1006/nlme.1997.3766. [DOI] [PubMed] [Google Scholar]
- 23.Ernfors P, Bramham CR. The coupling of a trkB tyrosine residue to LTP. Trends Neurosci. 2003;26:171–3. doi: 10.1016/S0166-2236(03)00064-X. [DOI] [PubMed] [Google Scholar]
- 24.Gerlai R, McNamara A, Choi-Lundberg DL, Armanini M, Ross J, et al. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur J Neurosci. 2001;14:1153–63. doi: 10.1046/j.0953-816x.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamosh M. Bioactive factors in human milk. Pediatr Clin North Am. 2001;48:69–86. doi: 10.1016/s0031-3955(05)70286-8. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.27. [PubMed] [Google Scholar]
- 27.Riol H, Tardy M, Rolland B, Lévesque G, Murthy MR. Detection of the peripheral nervous system (PNS)-type glial fibrillary acidic protein (GFAP) and its mRNA in human lymphocytes. J Neurosci Res. 1997;48:53–62. [PubMed] [Google Scholar]
- 28.Chirico G, Marzollo R, Cortinovis S, Fonte C, Gasparoni A. Antiinfective properties of human milk. J Nutr. 2008;138:1801S–1806S. doi: 10.1093/jn/138.9.1801S. [DOI] [PubMed] [Google Scholar]
- 29.Gazzolo D, Monego G, Corvino V, Bruschettini M, Bruschettini P, et al. Human milk contains S100B protein. Biochim Biophys Acta. 2003;1619:209–12. doi: 10.1016/s0304-4165(02)00499-3. [DOI] [PubMed] [Google Scholar]
- 30.Gazzolo D, Bruschettini M, Lituania M, Serra G, Santini P, et al. Levels of S100B protein are higher in mature human milk than in colostrum and milk-formulae milks. Clin Nutr. 2004;23:23–6. doi: 10.1016/s0261-5614(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 31.Musumeci M, Betta P, Magro E, Isaia T, Simpore J, et al. S100B concentration in colostrums of Burkinabe and Sicilian women. Nutr Metab (Lond) 22; 2008;5:15. doi: 10.1186/1743-7075-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Amici D, Gasparoni A, Guala A, Klersy C. Does ethnicity predict lactation? A study of four ethnic communities. Eur J Epidemiol. 2001;17:357–62. doi: 10.1023/a:1012731713393. [DOI] [PubMed] [Google Scholar]
- 33.Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. 2006;398:215–9. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Zhang Z, Xie C, Xi G, Zhou H, et al. Effect of treatment on serum glial cell line-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:886–90. doi: 10.1016/j.pnpbp.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Portela L VC, Tort A BL, Schaf DV, Ribeiro L, Nora DB, et al. The serum S100B concentration is age dependent. Clin Chem. 2002;48:950–952. [PubMed] [Google Scholar]
- 36.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103:1150–1157. doi: 10.1542/peds.103.6.1150. [DOI] [PubMed] [Google Scholar]
- 37.Bhandari N, Bahl R, Mazumdar S, Martines J, Black RE, et al. Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomized controlled trial. Infant Feeding Study Group. Lancet. 2003;361:1418–1423. doi: 10.1016/S0140-6736(03)13134-0. [DOI] [PubMed] [Google Scholar]
- 38.Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ. 1998;317:1481–1487. doi: 10.1136/bmj.317.7171.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7–8 years. Arch Dis Child Fetal Neonatal Ed. 2001;84:F23–F27. doi: 10.1136/fn.84.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boesmans W, Gomes P, Janssens J, Tack J, Vanden Berghe P. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut. 2008;57:314–322. doi: 10.1136/gut.2007.131839. [DOI] [PubMed] [Google Scholar]
- 41.Delafoy L, Gelot A, Ardid D, Eschalier A, Bertrand C, et al. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut. 2006;55:940–945. doi: 10.1136/gut.2005.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wartiovaara K, Salo M, Sainio K, Rintala R, Sariola H. Distribution of glial cell line-derived neurotrophic factor mRNA in human colon suggests roles for muscularis mucosae in innervation. J Pediatr Surg. 1998;33:1501–1506. doi: 10.1016/s0022-3468(98)90485-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhang DK, He FQ, Li TK, Pang XH, Cui DJ, et al. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol. 2010;222:213–222. doi: 10.1002/path.2749. [DOI] [PubMed] [Google Scholar]
- 44.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enstrom A, Onore C, Tarver A, Hertz-Picciotto I, Hansen R, et al. Peripheral blood leukocyte production of bdnf following mitogen stimulation in early onset and regressive autism. Am J Biochem & Biotech. 2008;4:121–129. doi: 10.3844/ajbbsp.2008.121.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto M, Ito T, Fukumitsu H, Nomoto H, Furukawa Y, et al. Stimulation of production of glial cell line-derived neurotrophic factor and nitric oxide by lipopolysaccharide with different dose-responsiveness in cultured rat macrophages. Biomed Res. 2005;26:223–229. doi: 10.2220/biomedres.26.223. [DOI] [PubMed] [Google Scholar]
- 47.Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–1543S. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- 48.Steiner J, Schiltz K, Walter M, Wunderlich MT, Keilhoff G, et al. S100B serum levels are closely correlated with body mass index: an important caveat in neuropsychiatric research. Psychoneuroendocrinology. 2010;35:321–4. doi: 10.1016/j.psyneuen.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Haimoto H, Kato K, Suzuki F, Nagura H. The ultrastructural changes of S-100 protein localization during lipolysis in adipocytes. An immunoelectron-microscopic study. Am J Pathol. 1985;121:185–91. [PMC free article] [PubMed] [Google Scholar]
- 50.Netto CB, Conte S, Leite MC, Pires C, Martins TL, et al. Serum S100B protein is increased in fasting rats. Arch Med Res. 2006;37:683–686. doi: 10.1016/j.arcmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 51.MacDorman MF, Declercq E, Menacker F, Malloy MH. Neonatal mortality for primary cesarean and vaginal births to low-risk women: application of an "intention-to-treat" model. Birth. 2008;35:3–8. doi: 10.1111/j.1523-536X.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 52.Zanardo V, Svegliado G, Cavallin F, Giustardi A, Cosmi E, et al. Elective cesarean delivery: does it have a negative effect on breastfeeding? Birth. 2010;37:275–279. doi: 10.1111/j.1523-536X.2010.00421.x. [DOI] [PubMed] [Google Scholar]