Abstract
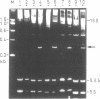
Four different type 1 ribosome-inactivating proteins (RIPs) with RNA N-glycosidase activity were tested for their ability to attack the large rRNA of plant ribosomes derived from tobacco plants, as well as from the plant species from which the particular RIP had been isolated. Incubation of tobacco ribosomes with RIPs isolated from either Phytolacca americana L. (pokeweed), Dianthus barbatus L. (carnation), Spinacia oleracea L. (spinach) or Chenopodium amaranthicolor Coste and Reyn. (chenopodium) rendered the 25S rRNA sensitive to aniline-catalyzed hydrolysis, generating a single rRNA-fragment of about 350 nucleotides. The same fragment was generated when rRNAs from pokeweed, carnation, spinach or chenopodium ribosomes were aniline-treated without any deliberate treatment of the ribosomes with the respective RIP. This indicated that ribosomes from all RIP-producing plants were already inactivated by their own RIPs during preparation. These results demonstrate that plant ribosomes are generally susceptible to RIP attack, including modification by their own RIPs. Direct sequencing of the newly generated fragments revealed that a single N-glycosidic bond at an adenosine residue within the highly conserved sequence 5'-AGUACGAGAGGA-3' was cleaved by all of the RIPs investigated, a situation also found in animal, yeast and Escherichia coli ribosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigotti M., Rambelli F., Zamboni M., Montanaro L., Sperti S. Effect of alpha-sarcin and ribosome-inactivating proteins on the interaction of elongation factors with ribosomes. Biochem J. 1989 Feb 1;257(3):723–727. doi: 10.1042/bj2570723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987 Apr 25;262(12):5908–5912. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K., Lambert J. M. The site of action of six different ribosome-inactivating proteins from plants on eukaryotic ribosomes: the RNA N-glycosidase activity of the proteins. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1032–1036. doi: 10.1016/0006-291x(88)90733-4. [DOI] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987 Jun 15;262(17):8128–8130. [PubMed] [Google Scholar]
- Endo Y., Wool I. G. The site of action of alpha-sarcin on eukaryotic ribosomes. The sequence at the alpha-sarcin cleavage site in 28 S ribosomal ribonucleic acid. J Biol Chem. 1982 Aug 10;257(15):9054–9060. [PubMed] [Google Scholar]
- Hartley M. R., Legname G., Osborn R., Chen Z., Lord J. M. Single-chain ribosome inactivating proteins from plants depurinate Escherichia coli 23S ribosomal RNA. FEBS Lett. 1991 Sep 23;290(1-2):65–68. doi: 10.1016/0014-5793(91)81227-y. [DOI] [PubMed] [Google Scholar]
- Irvin J. D. Purification and partial characterization of the antiviral protein from Phytolacca americana which inhibits eukaryotic protein synthesis. Arch Biochem Biophys. 1975 Aug;169(2):522–528. doi: 10.1016/0003-9861(75)90195-2. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Asano K., Svensson B., Poulsen F. M., Nygård O. Reduced turnover of the elongation factor EF-1 X ribosome complex after treatment with the protein synthesis inhibitor II from barley seeds. Biochim Biophys Acta. 1986 Oct 16;868(1):62–70. doi: 10.1016/0167-4781(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Treatment of abrin and ricin with -mercaptoethanol opposite effects on their toxicity in mice and their ability to inhibit protein synthesis in a cell-free system. FEBS Lett. 1972 Nov 15;28(1):48–50. doi: 10.1016/0014-5793(72)80674-4. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Bruening G., Shepherd R. J. A possible mechanism for the inhibition of plant viruses by a peptide from Phytolacca americana. Virology. 1973 Nov;56(1):390–393. doi: 10.1016/0042-6822(73)90319-x. [DOI] [PubMed] [Google Scholar]
- Prestle J., Hornung E., Schönfelder M., Mundry K. W. Mechanism and site of action of a ribosome-inactivating protein type 1 from Dianthus barbatus which inactivates Escherichia coli ribosomes. FEBS Lett. 1992 Feb 10;297(3):250–252. doi: 10.1016/0014-5793(92)80549-v. [DOI] [PubMed] [Google Scholar]
- Ready M. P., Adams R. P., Robertus J. D. Dodecandrin, a new ribosome-inhibiting protein from Phytolacca dodecandra. Biochim Biophys Acta. 1984 Dec 21;791(3):314–319. doi: 10.1016/0167-4838(84)90342-x. [DOI] [PubMed] [Google Scholar]
- Ready M. P., Brown D. T., Robertus J. D. Extracellular localization of pokeweed antiviral protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5053–5056. doi: 10.1073/pnas.83.14.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready M., Bird S., Rothe G., Robertus J. D. Requirements for antiribosomal activity of pokeweed antiviral protein. Biochim Biophys Acta. 1983 May 20;740(1):19–28. doi: 10.1016/0167-4781(83)90116-1. [DOI] [PubMed] [Google Scholar]
- Sperti S., Montanaro L., Rambelli F., Zamboni M. Interaction of alpha-sarcin and gelonin with cibacron blue. Biosci Rep. 1986 Oct;6(10):901–908. doi: 10.1007/BF01116244. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Bailey S., Miller S. P., Bodley J. W. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988 Feb 25;16(4):1349–1357. doi: 10.1093/nar/16.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Barbieri L. Ribosome-inactivating proteins up to date. FEBS Lett. 1986 Jan 20;195(1-2):1–8. doi: 10.1016/0014-5793(86)80118-1. [DOI] [PubMed] [Google Scholar]
- Stirpe F. On the action of ribosome-inactivating proteins: are plant ribosomes species-specific? Biochem J. 1982 Jan 15;202(1):279–280. doi: 10.1042/bj2020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. E., Irvin J. D. Depurination of plant ribosomes by pokeweed antiviral protein. FEBS Lett. 1990 Oct 29;273(1-2):144–146. doi: 10.1016/0014-5793(90)81070-5. [DOI] [PubMed] [Google Scholar]