Abstract
The aim of this investigation was to determine the biotransformation of bupropion by baboon hepatic and placental microsomes, identify the enzyme(s) catalyzing the reaction(s) and determine its kinetics. Bupropion was metabolized by baboon hepatic and placental microsomes to hydroxybupropion (OH-BUP), threo- (TB) and erythrohydrobupropion (EB). OH-bupropion was the major metabolite formed by hepatic microsomes (Km 36 ± 6 µM, Vmax 258 ± 32 pmol mg protein−1 min−1), however the formation of OH-BUP by placental microsomes was below the limit of quantification. The apparent Km values of bupropion for the formation of TB and EB by hepatic and placental microsomes were similar. The selective inhibitors of CYP2B6 (ticlopidine and phencyclidine) and monoclonal antibodies raised against human CYP2B6 isozyme caused 80% inhibition of OH-BUP formation by baboon hepatic microsomes. The chemical inhibitors of aldo-keto reductases (flufenamic acid), carbonyl reductases (menadione), and 11β-hydroxysteroid dehydrogenases (18β-glycyrrhetinic acid) significantly decreased the formation of TB and EB by hepatic and placental microsomes. Data indicate that CYP2B of baboon hepatic microsomes is responsible for biotransformation of bupropion to OH-BUP, while hepatic and placental short chain dehydrogenases/reductases and to a lesser extent aldo-keto reductases are responsible for the reduction of bupropion to TB and EB.
Introduction
Smoking is the largest modifiable risk factor for pregnancy-related morbidity and mortality in the U.S. [1]. Approximately 5–10% of prenatal deaths, 20–35% of low-birth-weight infants, and 8–15% of preterm deliveries have been attributed to smoking [2, 3]. Despite the substantial risks to the fetus, most pregnant smokers do not quit smoking during pregnancy because of the highly addictive nature of nicotine.
Bupropion is an antidepressant that has been successfully used as an alternative to nicotine replacement therapy to aid in smoking cession in non-pregnant patients. However, due to limited data on its safety and efficacy in pregnant women, its use in this patient population is restricted. Additionally, the onset of pregnancy is accompanied by changes in maternal physiology that affect the absorption, distribution, metabolism, and elimination of administered medications [4]. In humans, bupropion is extensively metabolized in vivo and less than 10% of the drug is excreted unchanged in urine and feces [5, 6]. Furthermore, recent preclinical data obtained from in vitro studies revealed that bupropion is also metabolized by human placenta [7]. Consequently, if pregnancy-induced changes alter the activity of enzymes metabolizing bupropion, the pharmacokinetics of bupropion reported for non-pregnant patients cannot be extrapolated to those who are pregnant.
There are several challenges associated with drug development for the pregnant patient: First, ethical and safety concerns for the mother and fetus. Second, the anatomical and functional differences between the human placenta and the placenta of other mammals limit availability of a recognized pregnant animal model that approximates drugs disposition in the pregnant human. In order to elucidate the effect of pregnancy on the metabolism of bupropion in vivo and due to the above mentioned concerns, the use of an animal model that best simulates drug metabolism and placentation in humans is required. Previously, interspecies differences in the biotransformation of bupropion between laboratory animals have been reported [8, 9] and it was concluded that the metabolic fate of bupropion in humans more closely resembles that of guinea pig than either rats or mice [9]. Although the use of a non-primate animal model to study drug disposition in vivo has its advantages (e.g. short gestation and lower expense), the distinct differences in placental development, structure, and functions limit its validity in extrapolating data to humans.
During the last 5 years, data obtained from our laboratory revealed similarities between baboon (Papio cynocephalus) and human hepatic and placental microsomes in their biotransformation of the 17α-hydroxyprogesterone caproate [10] and hypoglycemic drug glyburide [11]. The baboon was chosen as a non-human primate animal model because of its similarities with humans in placental structure, functions [12] as well as in fetal development [13].
Therefore, the aim of this investigation is to determine the biotransformation of bupropion by baboon hepatic and placental microsomes, identify the enzyme(s) catalyzing the reaction(s) and determine its kinetics.
2. Material and Methods
2.1. Chemicals and Biological Reagents
Hydroxybupropion (OH-BUP), erythro- (EB) and threohydrobupropion (TB) were purchased from Toronto Research Chemicals Inc. (North York, Canada). HPLC-grade methanol, acetic acid, 4-methylpyrazole, dicumarol, and menadione were purchased from Fisher Scientific (Fair Lawn, NJ). Phencyclidine hydrochloride was a gift from the National Institute on Drug Abuse drug supply unit. All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Polyclonal antibodies raised against CYPs 2C19, 2D6 and 3A4 were obtained from AbD Serotec (Oxford, UK). Monoclonal and polyclonal antibodies against CYPs 2B6, 2C9, 2E1, 2A6, 2C8, 1A2 and 1A1 were purchased from XenoTech, LLC (Lenexa, KS). Rabbit antiserum to human placental aromatase (CYP19) was purchased from Hauptman-Woodward Institute (Buffalo, NY).
2.2. Preparation of microsomes from baboon hepatic and placental tissues
Adult baboon livers (n=4) were obtained from female animals that were sacrificed for herd reduction. Placentas of baboons (n=3) were obtained by cesarean section. All tissues were obtained according to a protocol approved by the Institution Animal Care and Use Committee of the Southwest Foundation for Biomedical Research, San Antonio, TX. The hepatic and placental microsomal fractions were prepared by differential centrifugation as previously described [7, 11]. The microsomes were re-suspended in 0.1 M potassium phosphate buffer (pH 7.4) and stored at −80°C until used. Protein content of microsomes was determined by a commercially available kit (BioRad Laboratories, Hercules, CA) using bovine serum albumin as a standard.
2.3. Biotransformation of bupropion by baboon hepatic and placental microsomes
The activity of baboon hepatic and placental microsomes in the biotransformation of bupropion was determined in the reaction solution (250 µL) of 0.1 M potassium phosphate buffer (pH 7.4). Each reaction solution contained 0.25 mg protein of the hepatic or placental microsomes and bupropion at a final concentration of 250 µM was pre-incubated for 5 min at 37°C. The reaction was initiated by the addition of NADPH-regenerating system, made of 0.4 mM NADP+, 4 mM glucose 6-phosthate, 1 U/mL glucose-6-phosphate dehyrogenase, and 2 mM MgCl2 followed by incubation at 37°C for 30 min or 40 min in presence of either hepatic or placental microsomes, respectively. The reaction was terminated by the addition of 25 µL of 40% trichloroacetic acid (TCA). Phenacetin (10 µl of 2.4 µg/mL) was then added as an internal standard. The effect of bupropion, (10–400µM) on the reaction velocity was used to construct the saturation curve and to calculate the Vmax and apparent Km values.
2.4. Identification of the enzyme(s) catalyzing the hydroxylation of bupropion by baboon hepatic microsomes
2.4.1. Effect of chemical inhibitors on the formation of hydroxybupropion
The effect of chemical inhibitors selective for CYP isoforms [14] on the biotransformation of bupropion to OH-BUP by baboon’s hepatic microsomes was determined. The final concentration used for each inhibitor was approximately 10-fold its reported Ki value to maintain selectivity for its specific CYP isozyme and to obtain at least ≥ 80% inhibition of the reaction. These inhibitors were dissolved in several solvents: 1) in 0.1M potassium phosphate buffer: quinidine, CYP2D6 (4µM) [15], chlomethiazole hydrochloride, CYP 2E1 (120 µM) [16], (+)-nootkatone, CYP2C19 (5µM) [17], phencyclidine hydrochloride, CYP2B6 (100µM) and ticlopidine hydrochloride, CYP2B6 (2µM) [18, 19]; 2) in 0.5% (v/v) ethanol: aminoglutethimide, CYP19 (7µM) [20], sulfaphenazole, CYP2C9 (3µM) [15], α-naphthoflavone, CYP1A1 (0.1µM) [15], ketonconazole, CYP 3A4 (1.8µM) [15] and furafylline, CYP1A2 (8µM) [15]; 3) in 0.3% (v/v) DMSO: trimethoprim, CYP2C8 (320µM) [21] and trans-2-phenylcyclopropyl-amine hydrochloride, CYP2A6 (0.4µM) [22]. Furafylline (the mechanism-based inhibitor) was pre-incubated with hepatic microsomes (0.25 mg) and the NADPH-regeneration system at 37°C for 10 min, and then 50 µM (≈Km value) of bupropion was added to initiate the reaction. Other inhibitors were pre-incubated with hepatic microsomes (0.25 mg) and 50 µM of bupropion for 5 min at 37°C. The reaction was initiated by the addition of NADPH-regenerating system, incubated for 30 min at 37°C, and then terminated by the addition of TCA. The control reactions included all the above-mentioned components in presence of either 0.5% ethanol (v/v) or 0.3% (v/v) DMSO instead of inhibitors.
2.4.2. The effect of antibodies against CYP isozymes on the formation of hydroxybupropion
Monoclonal and polyclonal antibodies raised against human hepatic CYP isoforms 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, and rabbit antiserum to human CYP19 were used to identify the CYP isozymes responsible for the biotransformation of bupropion to OH-BUP. The reaction volume was 250 µL of 0.1M potassium phosphate buffer (pH 7.4). Each monoclonal or polyclonal antibody was added to the reaction components at the concentration causing 80% inhibition of the human CYP isoform it was raised against. Baboon hepatic microsomal proteins (13 µg) were pre-incubated with the antibody at 25°C for 10 min followed by the addition of bupropion at a final concentration of 300 µM. The reaction was initiated by the addition of the NADPH-regenerating system, incubated for 45 min at 37°C, and terminated by the addition of TCA. Mouse IgG replaced the antibodies in the control reaction.
2.5. Identification of the enzyme(s) catalyzing the reduction of the carbonyl group in bupropion by baboon hepatic and placental microsomes
Identification of hepatic and placental microsomal enzyme(s) catalyzing the reduction of the carbonyl group in bupropion was performed as previously reported [7]. The concentration used for each chemical inhibitor was based on their IC50 or Ki values reported for a specific carbonyl reductase. The following are the carbonyl reducing enzymes, their selective inhibitors and the concentrations used: alcoholdehydrogenase (ADH), 4-methylpyrazole (500µM) [23], aldehyde/aldose reductase, barbital (500µM) [24], aldo-ketoreductase (AKR), flufenamic acid (5µM) [25], carbonyl reductase (CR) menadione (100µM) [25], quinone oxidoreductase, dicumarol (500µM) [26], 11β-hydroxysteroid dehydrogenase (11β-HSD), 18β-glycyrrhetinic acid (18β-GA) (0.1µM) [27]. Stock solutions of inhibitors were prepared in 0.5% ethanol (v/v) and an aliquot of each was used to attain the final concentrations specified above. Each inhibitor was pre-incubated in presence of hepatic or placental microsomes (1 mg/ml) for 10 min at 37°C. The final concentration of bupropion in the reaction solution was 30 µM (≈Km value). The reaction was initiated by the addition of NADPH-regenerating system, incubated for 40 min at 37°C, and terminated by the addition of TCA. The control reaction included all the above-mentioned components in presence of 0.5% ethanol (v/v) instead of inhibitor.
The IC50 for menadione, flufenamic acid, and 18β-GA for their inhibition of bupropion reduction by hepatic and placental microsomes was determined. The concentration of bupropion in the reaction solution was 30 µM (≈Km), and each inhibitor was added in the following range of concentrations: menadione (5–250 µM), flufenamic acid (1–100 µM), and 18β-GA (1–300 nM). Each IC50 value was calculated from plots of the remaining activity as percent of control (absence of inhibitor) versus the log of its concentration.
2.6. Identification of bupropion metabolites formed by baboon hepatic and placental microsomes
The analysis of bupropion metabolites was achieved by using an Agilent HPLC 1200 series system coupled with an API 4000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). The HPLC system consisted of a degasser, binary pump delivery system and Hip-ALS auto-sampler controlled by Analyst™1.5 Software (MDS INC. and Applera Corporation, USA). The separation of metabolites was achieved by using a Waters Symmetry C18 column (150 × 4.6 mm, 5 µm) connected to a Phenomenex C18 guard column (4×3.0 mm). The aqueous mobile phase consisted of 10 mM ammonium acetate buffer (pH=6.0, adjusted with 0.1 M acetic acid). Samples were eluted at flow rate of 1.0 mL/min using a linear gradient from 5 to 90% methanol for 60 min. A volume of 250 µL of effluent was directed to the mass spectrometer.
The API 4000 triple quadrupole mass spectrometer equipped with a Turbo (V) ion source (ESI) was operated in positive mode. Optimal MS parameters were as follows: IonSpray Voltage, 2000 V; Curtain Gas, 30 L/h; Ion Source Gas1, 25 L/h; Ion Source Gas2, 50 L/h; Temperature, 300°C; Collision Gas 5 L/h; Declustering Potential, 20 V; Entrance Potential, 5 V; Collision Energy, 25 V; Collision Cell Exit Potential, 14 V. The mass scan range was setup from m/z 80 to m/z 450 for Q1 full scan, and the quasi molecular ion of each metabolite was transferred into Q3 to generate the product ion spectrum.
The formation of chromatographic peaks in the presence of NADPH-regenerating system that were not observed in its absence were considered as metabolites of bupropion. Further characterization of these peaks as corresponding to metabolites of bupropion was confirmed by MS/MS analysis.
2.7. Quantitative determination of bupropion metabolites in baboon hepatic and placental microsomes
Bupropion and its metabolites were extracted from reaction solution as previously reported by our laboratory [7]. The HPLC and MS instruments used consisted of Waters 600E multisolvent delivery system, 717 autosampler controlled by Empower™ 2 chromatography Data Software (Waters, Milford, MA). The mobile phase consisted of A: 40% methanol and B: 60% 10 mM ammonium acetate buffer (pH=6.0, adjusted with 0.1 M acetic acid). The separation of bupropion and metabolites was performed using Waters Symmetry C18 column (150 × 4.6 mm, 5 µm) connected to a Phenomenex C18 guard column (4×3.0 mm). Isocratic elution of metabolites was achieved at a rate of 1.0 mL/min and mobile phase was transferred into mass spectrometer with flow rate of 100 µL/min.
The mass spectrometer (Waters EMD 1000 single-quadrupole; Milford, MA) equipped with an electrospray ion source (ESI) was operated in positive mode. MS parameters were as follows: capillary voltage, 2.2 kV; cone voltage, 40 V; source temperature, 95°C; desolvation temperature, 350°C; desolvation gas flow rate, 450 L/h; cone gas flow rate, 100 L/h. The metabolites and internal standard were monitored by selective ion monitoring (SIM) at m/z 180 for phenacetin (IS), m/z 238 for OH-BUP and m/z 168 for TB and EB.
2.8. Data Analysis
For each inhibition experiment the control was set at 100% and the amount of metabolite formed in presence of an inhibitor (antibody or chemical) was expressed as percent of control. All data are presented as mean ± S.D. The variance (One way-ANOVA) was used for all multiple comparative analyses.
The Vmax and apparent Km values were determined using nonlinear regression analysis of the saturation equation (GraphPad Prism 5, Vision 5.01, Graph Pad Software, Inc.).
3. Results
3.1. Biotransformation of bupropion by baboon hepatic and placental microsomes
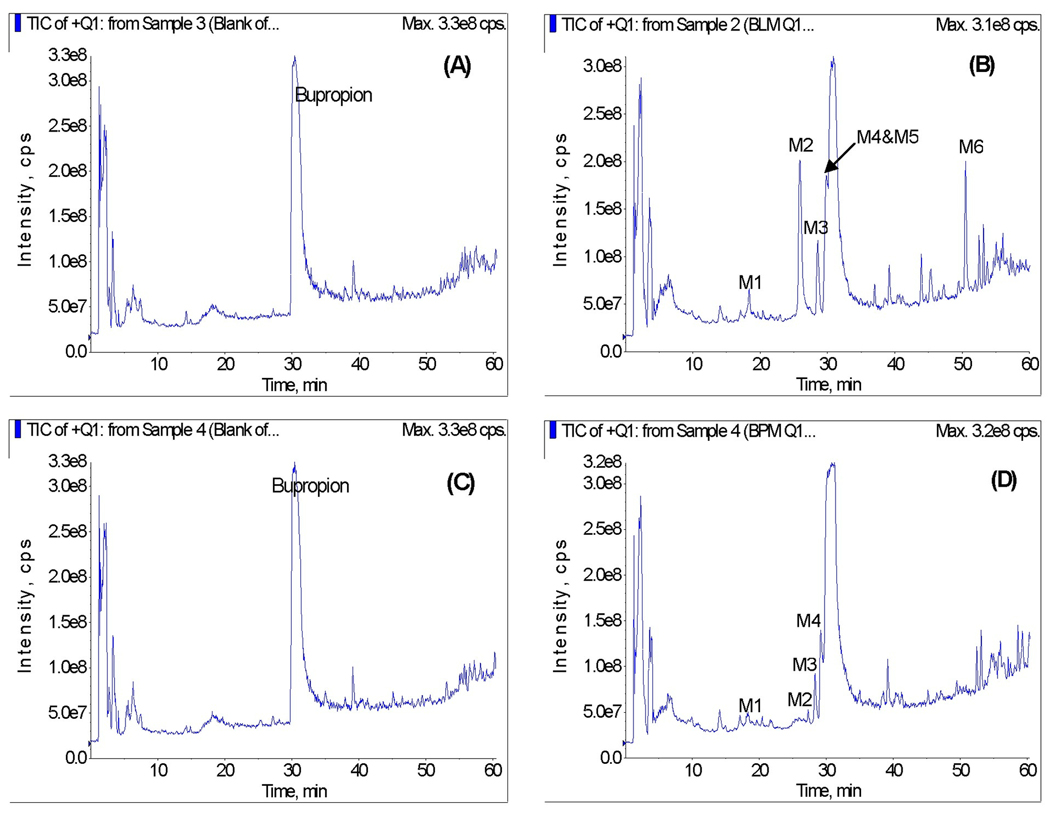
The incubation of bupropion in the presence of baboon hepatic microsomes and NADPH-regenerating system resulted in the formation of 6 metabolites (Figure 1A&B). The chromatographic peaks of the formed metabolites were eluted at retention times of 18.5 (M1), 25.8 (M2), 28.6 (M3), 29.5 (M4), 29.9 (M5), and 50.4 (M6) minutes, respectively.
Figure 1.
Representative total ion chromatograms of bupropion metabolites formed in vitro by baboon hepatic (A&B) and placental (C&D) microsomes in the absence (A&C) and presence (B&D) of NADPH-regenerating system.
The total ion chromatograms of the reaction solutions revealed 4 peaks corresponding to metabolites formed by baboon placental microsomes. The formation of bupropion metabolites by placental microsomes also required the presence of NADPH-regenerating system (Figure 1C&D). The retention times of the metabolites M1, M2, M3, and M4 formed by placental microsomes were identical to the retention times of the metabolites M1–M4 formed by hepatic microsomes.
Subsequent identification of the metabolites formed by baboon hepatic and placental microsomes was performed by MS/MS analysis.
3.2. Identification of bupropion metabolites formed by baboon hepatic and placental microsomes
The structural identification of metabolites was performed by comparing the fragment ion of metabolites with that of bupropion, and the proposed fragmentation pattern of metabolites is shown in Figure 2.
Figure 2.
Proposed fragmentation pathway of bupropion and its metabolites (M1–M6) formed by baboon microsomes.
Metabolites M1, M2, M5 and M6 shows the protonated molecular ion at m/z 256, which is 16Da higher than quasi molecular ion of bupropion (m/z 240), suggesting that these metabolites were hydroxylated derivatives of the parent bupropion.
The MS2 spectrum of M1 (Figure 3A) shows the fragment at m/z 200, which indicates the loss of the intact butyl group (m/z 56). The m/z 182 is the fragment ion derived from the ion at m/z 200 by losing water. The fragment ions at m/z 147 and 155 of M1 are 16Da greater than the fragment ions at m/z 131 and 139 of bupropion (Figure 3F), indicating the hydroxylation of aromatic ring of M1 [28].
Figure 3.
MS2 spectra of bupropion and its metabolites formed by baboon hepatic (M1–M6) and placental (M1–M4) microsomes. (A) M1 (aromatic hydroxylated metabolite); (B) M2 (hydroxybupropion); (C) M3 (erythrohydrobupropion); (D) M4 (threohydrobupropion; (E) M5 (methyl hydroxylated metabolite); (F) Bupropion; (G) M6 (methine hydroxylated metabolite).
Metabolite M2 has the fragment ion at m/z 131 indicating that the hydroxylation located at the methyl group in the side chain [28]. The MS2 analysis of M2 confirmed the identification of OH-BUP by comparing the MS2 fragmentation (Figure 3B) with the authentic bupropion and OH-BUP [29].
The MS2 spectrum of M5 (Figure 3E) shows the fragment ion at m/z 238, indicating the hydroxylation of the chain of the molecule. The fragment at m/z 200 indicates the loss of intact butyl group.
The MS2 spectrum of M6 (Figure 3G) shows the same fragment ions at m/z 200, 182 and 147 with those of M1 and M2. The ion at m/z 139 is derived from fragment ion at m/z 182 by losing C2H5N, indicating that hydroxylation is most likely located at the methine group in the side chain. The fragment ions at m/z 164 and 154 is not readily identifiable and may arise via a rearrangement process.
Metabolites M3 and M4 shows the protonated molecular ion at m/z 242, 2Da higher than quasi molecular ion of bupropion (m/z 240), suggesting that these metabolites were hydrogenated derivatives of the parent bupropion. The MS2 spectrum of M3 and M4 (Figure 3C and 3D) have the similar fragmentation patterns indicating that M3 and M4 are the stereo isomers. The identification of M3 as EB and M4 as TB was confirmed by comparing their MS2 spectrum with the authentic compounds.
3.3. Quantification of bupropion metabolites formed by baboon hepatic and placental microsomes
The individual baboon hepatic microsomes (n=4) and placental microsomes (n=3) were utilized to investigate the metabolism of bupropion. Metabolites M1, M5, and M6 formed by baboon microsomes were not quantified in the present study due to the lack of commercially available standard compounds of these metabolites. The metabolites OH-BUP (M2), EB (M3), and TB (M4) were quantitatively determined by using LC/MS-ESI method. The quantitative determination of these metabolites was validated for specificity, linearity, sensitivity, precision and accuracy as previously described from our laboratory [7].
The major metabolite formed by baboon hepatic microsomes was OH-BUP (6992 ± 982 pmol/mg protein) which accounted for 62% of the sum of the three metabolites formed. The amounts of TB (3241 ± 577 pmol/mg protein) and EB (1222 ± 233 pmol/mg protein) comprised 28% and 10%, respectively.
The amounts of bupropion metabolites formed by placental microsomes were significantly lower than those by hepatic microsomes. The major metabolite formed by placental microsomes was TB (1042 ± 880 pmol/mg protein) representing 82% of the sum of the three metabolites formed, while the formation of EB (243 ± 238 pmol/mg protein) accounted for 18%. The formation of OH-BUP was below the lower limit of quantification (LLOQ) of the analytical method used.
3.4. Kinetic parameters of bupropion metabolism by baboon hepatic microsomes
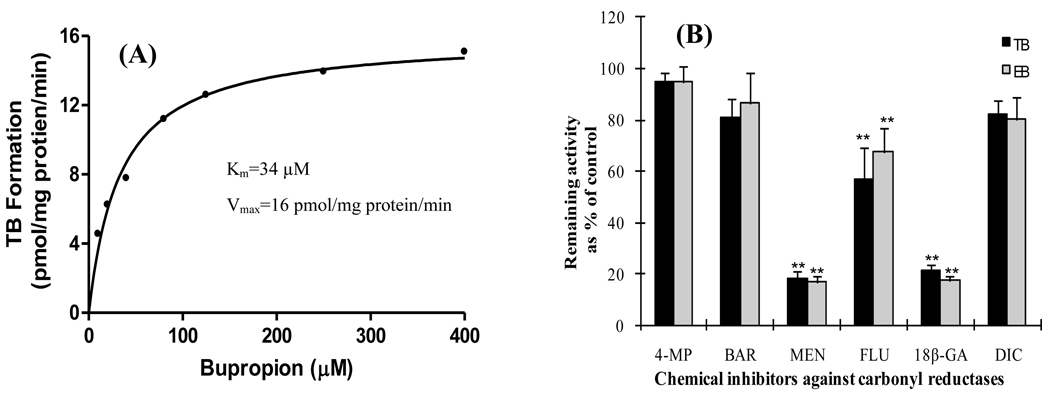
The rate of bupropion biotransformation to its three metabolites OH-BUP, TB and EB was dependent on its concentration and exhibited saturation kinetics. The hydroxylation of bupropion by hepatic microsomes revealed an apparent Km value of 36 ± 6 µM and Vmax value of 258 ± 32 pmol mg protein−1 min−1 (Figure 4). The reduction of carbonyl group of bupropion to TB and EB revealed an apparent Km values of 28 ± 2 µM and 32 ± 3 µM, respectively. The rates of TB and EB formation were lower than that for OH-BUP with mean Vmax values of 116 ± 19 and 46 ± 8 pmol mgprotein−1 min−1, respectively.
Figure 4.
A representative saturation curve of OH-BUP formation by baboon hepatic microsomes. The rate of bupropion hydroxylation to OH-BUP was dependent on bupropion concentration and exhibited typical saturation kinetics.
3.5. Identification of the hepatic enzymes responsible for hydroxylation and reduction of bupropion
3.5.1. Identification of baboon hepatic enzyme(s) catalyzing the hydroxylation of bupropion
Chemical inhibitors selective for the various CYP 450 isoforms were utilized to identify the enzyme(s) catalyzing the hydroxylation of bupropion by baboon hepatic microsomes. Phencyclidine (64%, p < 0.01) and ticlopidine hydrochloride (77%, p < 0.01) selective for CYP2B6 caused the maximum inhibition of OH-BUP formation (Figure 5A). Chemicals selective for CYP2E1 (chlomethiazole hydrochloride) and CYP2C19 ((+)-nootkatone inhibited the formation of OH-BUP by 10–15%, whereas other compounds did not affect its formation.
Figure 5.
Effect of chemical inhibitors selective for CYP isoforms on the formation of OH-BUP (A) and effect of chemical inhibitors selective for carbonyl reductases on the formation of threo- and erythrohydrobupropion (B) by baboon hepatic microsomes.
The inhibitors of CYP450 isoforms (A) are: sulfaphenazole (SUL, 3µM), chlomethiazole (CHL, 120µM), quinidine (QUI, 4µM), trimethoprim (TRI, 320µM), α-naphthoflavone (NAP, 0.4µM), (+)-nootkatone (NOK, 5µM), phencyclidine (PHE, 8µM), ticlopidine hydrochloride (TIC, 2µM), trans-2-henmylcyclopropyl-anmie (TRN, 0.4µM), furafylline (FUR, 8µM), ketonconazole (KET, 1.8µM) and aminoglutethimide (AMG, 7µM). The inhibitors on carbonyl reductases (B) are: 4-methylpyrazole (4-MP, 500µM), barbital (BAR, 500µM), flufenamic acid (FLU, 5µM), dicumarol (DIC, 500µM), menadione (MEN, 100µM), and 18β-glycyrrhetinic acid (18β-GA, 0.1µM). The rates of hydroxybupropion (OH-BUP), threo-(TB) and erythrohydrobupropion (EB) are expressed as percent of control (absence of inhibitor) and represent the mean ± S.D. of triplicate experiments. ** Statistical significance of p < 0.01 as determined by one-way ANOVA with Tukey’s comparison.
Monoclonal and polyclonal antibodies raised against human hepatic CYP isoforms 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, and rabbit antiserum to human CYP19 were used to confirm the identity of the microsomal enzyme(s) catalyzing the biotransformation of bupropion to OH-BUP. Antibodies against human CYP2B6 were the most potent and inhibited the formation of OH-BUP by 82% (p < 0.01). The remaining antibodies had null effect on the hydroxylation of bupropion.
3.5.2. Identification of baboon hepatic enzyme(s) catalyzing the reduction of bupropion
Chemical inhibitors known as selective for carbonyl-reducing isoforms were used to identify baboon hepatic enzyme(s) catalyzing the reduction of bupropion to TB and EB (Figure 5B). The formation of TB by baboon hepatic microsomes was inhibited by menadione (80%, p< 0.01), 18β-glycyrrhetinic acid (80%, p < 0.01), while flufenamic acid caused 40% of inhibition (p < 0.01). On the other hand, these three compounds inhibited the formation of EB by approximately 40%, p<0.01. Barbital, 4 methylpyrazole and dicumarol did not affect TB and EB formation.
The IC50 values calculated for the effect of 18β-glycyrrhetinic acid, flufenamic acid, and menadione on the TB formation were 15 nM, 11.4 µM, and 47 µM, respectively (Figure 6). The IC50 value of flufenamic acid for inhibition of EB formation was 7.7 µM, whereas IC50 values of 18β-glycyrrhetinic acid and menadione on the formation of EB cannot be determined in the concentration range tested.
Figure 6.
The effect of increasing concentrations of (A) 18β-glycyrrhetinic acid, (B) flufenamic acid and (C) menadione on erythro- and threohydrobupropion formation by baboon hepatic microsomes. The rates of erythro- and threohydrobupropion formation are expressed as a percent of their formation in the absence of inhibitors (control) which was set at 100%. Each data point represents the mean ± S.D. of triplicate experiments.
3.6. Kinetic parameters of bupropion metabolism by baboon placental microsomes
The rates of TB and EB formation were dependent on bupropion concentrations and exhibited saturation kinetics (Figure 7A). The reduction of bupropion to TB and EB by placental microsomes revealed a mean apparent Km values of 20 ± 14 µM and 32± 14 µM, respectively. The Vmax value for formation of TB was of 28 ± 24 pmol mg protein−1 min−1, while the rate for formation of EB (7±7 pmol mg protein−1 min−1) was approximately 25% that of TB formation.
Figure 7.
The representative saturation curve of threohydrobupropion formation by baboon placental microsomes (A) and the effect of chemical inhibitors selective to carbonyl reductases on the formation of threo- and erythrohydrobupropion by baboon placental microsomes (B).
The inhibitors of carbonyl reductase are: 4-methylpyrazole (4-MP, 500µM), barbital (BAR, 500µM), flufenamic acid (FLU, 5µM), dicumarol (DIC, 500µM), menadione (MEN, 100µM), and 18β-glycyrrhetinic acid (18β-GA, 0.1µM). The rates of threo-(TB) and erythrohydrobupropion (EB) are expressed as percent of control (absence of inhibitor) and represent the mean ± S.D. of triplicate experiments. ** Statistical significance of p < 0.01 as determined by one-way ANOVA with Tukey’s comparison.
3.7. Identification of placental enzymes responsible for reduction of bupropion
The chemical inhibitors known as selective for carbonyl-reducing isoforms were used to identify baboon enzyme(s) catalyzing the reduction of bupropion to TB and EB by placental microsomes (Figure 7B). Menadione and 18β-GA inhibited (80%, p < 0.01) the formation of TB and EB, while flufenamic acid caused 40% (p < 0.01) inhibition. On the other hand, 4-methylpyrazole, barbital, and dicumarol did not affect the formation of TB and EB. The IC50 for the inhibition of the TB and EB by 18β-GA was 25 and 22 nM, by menadione 27.9 and 30.32 µM, and by flufenamic acid 9.9 and 5.6 µM, respectively.
4. Discussion
The aim of this investigation was to determine the biotransformation of bupropion by baboon hepatic and placental microsomes, identify the enzyme(s) catalyzing the reaction(s) and determine its kinetics.
Data obtained in this investigation revealed that the biotransformation of bupropion by baboon hepatic and placental microsomes resulted in the formation of six and four metabolites, respectively. These metabolites, depending on the order of their elution on LC/MS chromatograms, were named M1 to M6 (Figure 1). Based on the similar retention times and MS2 spectrum as compared to the standards compounds, the metabolites M2, M3, and M4 formed by baboon hepatic and placental microsomes were identified as hydroxybupropion, erythro- and threohydrobupropion. Metabolite M1 formed by both hepatic and placental microsomes was identified by its fragmentation pattern as an aromatic hydroxylated metabolite of bupropion. The two metabolites of bupropion (M5 and M6) were formed only by hepatic microsomes and based on their fragmentation patterns were identified as methyl and methine hydroxylated metabolites, respectively. The retention times and the fragmentation pattern of the six metabolites formed by baboon hepatic microsomes (M1–M6) and the four metabolites formed by placental microsomes (M1–M4) were identical to those formed by human hepatic and placental microsomes under the same experimental (in the presence of NADPH) and chromatographic conditions (Figures 8 & 9, see supplementary notes). Therefore, the data obtained in this investigation revealed similarities between baboon and human hepatic and placental microsomes for their in vitro biotransformation of bupropion. However, it is plausible that the hydroxylated metabolites of bupropion identified in vitro can undergo further metabolism by phase II enzymes in vivo.
The formation of three hydroxylated metabolites of bupropion identified in the current investigation (M1, M5, and M6) in addition to previously described OH-BUP (M2) [30] is in agreement with the recently published study by Chen et al [28]. Using QTRAP-mass spectrometry, the authors reported a total of nine metabolites of bupropion formed by cDNA-expressed P450s and human hepatic microsomes [28]. Furthermore, metabolites M1, M2, M3, and M4 described in this investigation were also identified among 20 reported metabolites of bupropion in human urine samples by using time-of-flight mass spectrometry [29].
The second part of this investigation focused on the characterization of the metabolites of bupropion (OH-BUP, TB and EB) formed by baboon hepatic and placental microsomes as well as the identification of the enzymes involved in their formation.
The data obtained in this investigation revealed that hydroxybupropion (62%) is the major hepatic metabolite while threohydrobupropion (82%) is the major placental metabolite of bupropion formed in vitro by baboons. Accordingly, in baboons, the primary metabolic pathway for the biotransformation of bupropion in the liver is by hydroxylation and in the placenta by reduction. Similar tissue specific differences in the in vitro biotransformation of bupropion were reported earlier for human liver and placenta [7, 31–33].
The major human hepatic enzyme responsible for the hydroxylation of bupropion to hydroxybupropion was identified as CYP2B6 [33]. In this investigation, two experimental approaches were utilized to identify the enzyme(s) responsible for hydroxylation of bupropion by baboon hepatic microsomes, namely, inhibition of the reaction by compounds that are known as selective inhibitors of specific CYP isoforms as well as by antibodies raised against each isozym. It was assumed that an antibody raised against a human CYP isoform will cross-react with the same CYP subfamily of baboon isozymes.
Phencyclidine and ticlopidine hydrochloride (Figure 5A), at their concentrations selective for CYP2B6, were the most potent inhibitors of OH-BUP formation by baboons. In addition, the formation of OH-BUP was inhibited (82%) by monoclonal antibodies raised against human CYP2B6. Taken together these data indicate that the main enzyme responsible for the hydroxylation of bupropion to OH-BUP by baboon hepatic microsomes is very similar or identical to human CYP2B6 and most likely belongs to the CYP2B subfamily.
The enzyme(s) catalyzing the reduction of bupropion to threo- and erythrohydrobupropion by baboon hepatic and placental microsomes were identified using chemical inhibitors selective for human carbonyl-reducing enzymes. However, it is plausible that members of the same subfamily of enzymes from different species may exhibit different substrate selectivity. Inhibition of aldo-keto reductases (by flufenamic acid), carbonyl reductases (by menadione), and 11β-hydroxysterioid dehydrogenase (by 18β-GA) significantly decreased the formation of threo- and erythrohydrobupropion by baboon hepatic and placental microsomes (Figure 5B and 7B). Unfortunately, further identification/confirmation of carbonyl-reducing enzymes catalyzing the biotransformation of bupropion to threo- and erythrohydrobupropion by baboon hepatic and placental microsomes is not feasible due to the lack of commercially available isoforms of these enzymes. Taken together, it appears that baboon hepatic and placental 11β-HSD, carbonyl reductases and to a lesser extent aldo-ketoreductases are responsible for the reduction of the carbonyl group of bupropion to threo- and erythrohydrobupropion. This is in agreement with the report demonstrating that carbonyl-reducing enzymes are involved in the biotransformation of bupropion to threo- and erythrohydrobupropion by human placental microsomes [5]. Furthermore, the similarities between human and baboon placental 11β-HSD in catalyzing the metabolism of cortisol and cortisone has been also reported [34].
Therefore, data obtained in this investigation revealed that the major enzyme responsible for hydroxylation of bupropion to OH-bupropion in baboon liver is similar to human hepatic CYP2B6 and belongs to the CYP2B subfamily of enzymes. Moreover, the formation of the reduced metabolites of bupropion, namely, threo- and erythrohydrobupropion is catalyzed by hepatic and placental 11β-HSD, carbonyl reductases and to a lesser extent aldo-ketoreductases. The existence of two metabolic pathways (hydroxylation and reduction) in the biotransformation of bupropion by baboons revealed tissue-specific differences between their liver and placenta and are similar to those reported for human liver and placenta [7].
In summary, the revealed in vitro similarities in the biotransformation of bupropion by hepatic and placental microsomes of humans and baboons support the use of the pregnant baboon as an animal model for investigation of bupropion disposition during pregnancy.
Supplementary Material
Supplementary data Figure 8. Representative total ion chromatograms of bupropion metabolites formed in vitro by human hepatic (A&B) and placental (C&D) microsomes in the absence (A&C) and presence (B&D) of NADPH-regenerating system.
Supplementary data Figure 9. MS2 spectra of bupropion and its metabolites formed by human hepatic (M1–M6) and placental (M1–M4) microsomes. (A) M1 (aromatic. hydroxylated metabolite); (B) M2 (hydroxybupropion); (C) M3 (erythrohydrobupropion); (D) M4 (threohydrobupropion); (E) M5 (methyl hydroxylated metabolite); (F) (bupropion); (G) M6 (methine hydroxylated metabolite).
Acknowledgement
The authors sincerely appreciate the support of the physicians and nurses of the Labor & Delivery Ward of the John Sealy Hospital, the teaching hospital at UTMB, Galveston, Texas. We also appreciate the assistance of the Perinatal Research Division and Publication, Grant, & Media Support Office of the Department of Obstetrics & Gynecology.
A list of non-standard abbreviations
- Vmax
maximum reaction velocity
- Km
substrate concentration causing 50% of the reaction velocity
- P450
cytochrome P450
- IC50
concentration of compound causing 50% inhibition of the reaction
- OH-BUP
hydroxybupropion
- TB
threohydrobupropion
- EB
erythrohydrobupropion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benowitz NL, Dempsey DA. Pharmacotherapy for smoking cessation during pregnancy. Nicotine Tob Res. 2004;6 suppl 2:S189–S202. doi: 10.1080/14622200410001669169. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Smoking during pregnancy-United States, 1990–2002. Morb Mortal Wkly Rep. 2004;53:911–915. [PubMed] [Google Scholar]
- 3.Fang WL, Goldstein AO, Butzen AY, Hartsock A, Hartmann KE, et al. Smoking cessation in pregnancy: A review of postpartum relapse prevention strategies. JABFP. 2004;17:264–275. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- 4.Loebstein R, Koren G. “Clinical relevance of therapeutic drug monitoring during pregnancy.”. Ther Drug Monit. 2002;24:15–22. doi: 10.1097/00007691-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rotzinger S, Bourin M, Akimoto Y, Coutts RT, Baker GB. Metabolism of some "second"- and "fourth"-generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine. Cell Mol Neurobiol. 1999;19:427–442. doi: 10.1023/A:1006953923305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai AA, Schroeder DH. Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry. 1983;44:82–84. [PubMed] [Google Scholar]
- 7.Wang XM, Abdelrahman DR, Zharikova OL, Patrikeeva SL, Hankins GDV, Ahmed MS, et al. Bupropion metabolism by human placenta. Biochem Pharmacol. 2010;79:1684–1690. doi: 10.1016/j.bcp.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butz RF, Schroeder DH, Welch RM, Mehta NB, Phillips AP, Findlay JW. Radioimmunoassay and pharmacokinetic profile of bupropion in the dog. J Pharmacol Exp Ther. 1981;217:602–610. [PubMed] [Google Scholar]
- 9.Suckow RF, Smith TM, Perumal AS, Cooper AT. Pharmacokinetics of bupropion and metabolites in plasma and brain of rats, mice, and guinea pigs. Drug Metab Dispos. 1986;14:692–697. [PubMed] [Google Scholar]
- 10.Yan R, Nanovskaya TN, Zharikova OL, Mattison DR, Hankins GD, Ahmed MS. Metabolism of 17 alpha-hydroxyprogesterone caproate by hepatic and placental microsomes of human and baboons. Biochem Pharmacol. 2008;75:1848–1857. doi: 10.1016/j.bcp.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zharikova OL, Ravindran S, Naovskaya TN, Hill RA, Hankins GDV, Ahmed MS. Kinetics of glyburide metabolism by hepatic and placental microsomes of human and baboon. Biochem Pharmacol. 2007;73:2012–2019. doi: 10.1016/j.bcp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Houston ML. The development of the baboon (Papio sp.) placenta during the fetal period of gestation. Am J Anat. 1969;126:17–29. doi: 10.1002/aja.1001260103. [DOI] [PubMed] [Google Scholar]
- 13.Enders AC, Lantz KC, Peterson PE, Hendrickx AG. From blastocyst to placenta: the morphology of implantation in the baboon. Hum Reprod Update. 1997;3:561–573. doi: 10.1093/humupd/3.6.561. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. Drug Development and Drug Interactions: Table of Su bstrates, Inhibitors and Inducers. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/
- 15.Bourré M, Meunier V, Berger F, Fabre G. Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human liver microsomes. Drug Metab. Dispos. 1996;277:321–332. [PubMed] [Google Scholar]
- 16.Gebhardt AC, Lucas D, Menez J, Seitz HK. Chlormethiazole inhibition of cytochrome P450 2E1 as assessed by chlorzoxazone hydroxylation in humans. Hepatology. 2003;26:957–961. doi: 10.1002/hep.510260423. [DOI] [PubMed] [Google Scholar]
- 17.Tassaneeyakul W, Guo LQ, Fukuda K, Ohta T, Yamazoe Y. Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys. 2000;378:356–363. doi: 10.1006/abbi.2000.1835. [DOI] [PubMed] [Google Scholar]
- 18.Jushchyshyn MI, Kent UM, Hollenberg PF. The mechanism-based inactivation of human cytochrome P450 2B6 by phencyclidine. Drug Metab Dispos. 2003;31:46–52. doi: 10.1124/dmd.31.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Turpeinen M, Nieminen R, Juntunen T, Taavitsainen P, Raunio H, Pelkonen O. Selective inhibition of CYP2B6-catalyzed bupropion hydroxylation in human liver microsomes in vitro. Drug Metab Dispos. 2004;32:626–631. doi: 10.1124/dmd.32.6.626. [DOI] [PubMed] [Google Scholar]
- 20.Stresser DM, Turner SD, McNamara J, Stocker P, Miller VP, Crespi CL, et al. A high-throughput screen to identify inhibitors of aromatase (CYP19) Anal Biochem. 2000;284:427–430. doi: 10.1006/abio.2000.4729. [DOI] [PubMed] [Google Scholar]
- 21.Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos. 2002;30:631–635. doi: 10.1124/dmd.30.6.631. [DOI] [PubMed] [Google Scholar]
- 22.Koenigs LL, Peter RM, Thompson SJ, Rettie AE, Trager WF. Mechanism-based inactivation of human liver cytochrome P450 2A6 by 8-methoxypsoralen. Drug Metab Dispos. 1997;25:1407–1415. [PubMed] [Google Scholar]
- 23.Salaspuro MP, Lindros KO, Pikkarainen P. Ethanol and galactose metabolism as influenced by 4-methylpyrazole in alcoholics with and without nutritional deficiencies. Preliminary report of a new approach to pathogenesis and treatment in alcoholic liver disease. Ann Clin Res. 1975;7:269–272. [PubMed] [Google Scholar]
- 24.Shimada H, Hirashima T, Imamura Y. Effects of quinones and flavonoids on the reduction of all-trans retinal to all-trans retinol in pig heart. Eur J Pharmacol. 2006;540:46–52. doi: 10.1016/j.ejphar.2006.04.050. [DOI] [PubMed] [Google Scholar]
- 25.Atalla A, Breyer-Pfaff U, Maser E. Purification and characterization of oxidoreductases-catalyzing carbonyl reduction of the tobacco-specific nitrosamine 4-methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK) in human liver cytosol. Xenobiotica. 2000;30:755–769. doi: 10.1080/00498250050119826. [DOI] [PubMed] [Google Scholar]
- 26.Cullen JJ, Hinkhouse MM, Grady M, Gaut AW, Liu J, Zhang YP, et al. Dicumarol inhibition of NADPH:quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res. 2003;63:5513–5520. [PubMed] [Google Scholar]
- 27.Diederich S, Grossman C, Hanke B, Quinkler M, Herrmann M, Bahr V, et al. In the search for specific inhibitors of human 11β-hydroxysteroid dehydrogenase (11β-HSDs): chenodeoxycholic acid selectively inhibits 11β-HSD-1. Eur J Endocrinol. 2000;142:200–207. doi: 10.1530/eje.0.1420200. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Liu HF, Liu L, Nguyen K, Jones EB, Fretland AJ. The in vitro metabolism of bupropion revisited: concentration dependent involvement of cytochrome P450 2C19. Xenobiotica. 2010;40:536–546. doi: 10.3109/00498254.2010.492880. [DOI] [PubMed] [Google Scholar]
- 29.Petsalo a, Turpeinen M, Tolonen A. Identification of bupropion urinary metabolites by liquid chromatography/mass spectrometry. Rapid Commun. Mass spectrum. 2007;21:2547–2554. doi: 10.1002/rcm.3117. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder DH. Metabolism and kinetics of bupropion. J Clin Psychiatry. 1983;44:79–81. [PubMed] [Google Scholar]
- 31.Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan BF, Laethem RL, et al. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–1230. [PubMed] [Google Scholar]
- 32.Faucette SR, Hawke RL, Shord SS, Lecluyse EL, Lindley CM. Evaluation of the contribution of cytochrome P450 3A4 to human liver microsomal bupropion hydroxylation. Drug Metab Dispos. 2001;29:1123–1129. [PubMed] [Google Scholar]
- 33.Hess LM, Venkatakrishnan K, Court MH, Von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. CYP 2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- 34.Pepe GJ, Albrecht ED. Comparison of cortisol-cortisone interconversion in vitro by the human and baboon placenta. Steroids. 1984;44:229–240. doi: 10.1016/0039-128x(84)90004-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data Figure 8. Representative total ion chromatograms of bupropion metabolites formed in vitro by human hepatic (A&B) and placental (C&D) microsomes in the absence (A&C) and presence (B&D) of NADPH-regenerating system.
Supplementary data Figure 9. MS2 spectra of bupropion and its metabolites formed by human hepatic (M1–M6) and placental (M1–M4) microsomes. (A) M1 (aromatic. hydroxylated metabolite); (B) M2 (hydroxybupropion); (C) M3 (erythrohydrobupropion); (D) M4 (threohydrobupropion); (E) M5 (methyl hydroxylated metabolite); (F) (bupropion); (G) M6 (methine hydroxylated metabolite).