Abstract
Cell adhesion and migration are important events that occur during embryonic development, immune surveillance, wound healing and in tumor metastasis. It is a multi-step process that involves both mechanical and biochemical signaling that results in cell protrusion, adhesion, contraction and retraction. Each of these events generates mechanical forces into the environment measured as traction forces. We have previously found that the calpain small subunit, Calpain 4, is required for normal traction forces, and that this mechanism is independent of the catalytic activities of the holoenzymes that are formed between Calpain 4 and each of the proteolytic heavy chains of Calpain 1 and 2. To define a potential mechanism for the Calpain 4 regulation of traction force, we have evaluated the levels of tyrosine phosphorylation, a hallmark of force dependent signaling within focal adhesions. Using 2D gel electrophoresis we compared tyrosine phosphorylation profiles of Calpain 4 deficient mouse embryonic fibroblasts (MEFs) to the levels in wildtype MEFs and MEF’s deficient in the large catalytic subunits, Capn1 and Capn2. Of particular interest, was the identification of Galectin-3, a galactose binding protein known to interact with integrins. Galectin-3 has previously been shown to regulate cell adhesion and migration in both normal and tumor cells; however its full mechanism remains elusive. We have found that Calpain 4 is essential for the tyrosine phosphorylation of galectin-3, and its ultimate secretion from the cell, and speculate that its secretion interferes with the production of traction forces.
Keywords: migration, galectin-3, calpain 4, adhesion, traction force, secretion
1. Introduction
Galectins are a family of lectin proteins that bind to β-galactoside carbohydrate structures through their carbohydrate recognition domains (CRDs). Galectin-3 is a unique member of this family because it is chimeric, containing one CRD and a long N-terminal sequence rich in serine phosphorylation sites and glycine repeats, in addition to a tyrosine and proline rich collagen-like sequence (reviewed in [1]). Galectin-3 is ubiquitously expressed in most normal adult tissues and also found in a variety of tumor cell types. In tumor cells, galectin-3 expression varies with cell type and stage of cancer progression (reviewed in [2,3,4]. Within the cell, galectin-3 is localized to the nucleus, cytoplasm and cell surface and is also known to be secreted (reviewed in [5]). Cytoplasmic and nuclear localized galectin-3 is involved in apoptosis, cell proliferation, splicing, and Wnt/β-catenin signaling pathway, while cell surface and extracellular galectin-3 modulate cell adhesion (reviewed in [1,3,5]). Extracellular galectin-3 binds to extracellular matrix (ECM) proteins such as fibronectin, laminin, collagen IV, elastin, hensin, tenascin-C and –R (reviewed in [4]). Interestingly, galectin-3 also binds to a variety of integrin receptors located on the cell surface (reviewed in [4]).
Despite knowing that galectin-3 has multiple interactions in the extracellular environment, its function in this niche is not clear, and the exact mechanism by which galectin-3 is secreted is not understood. It is known that galectin-3 is secreted by a non-classical secretion pathway which bypasses the ER/Golgi, it also lacks a conventional secretion signal sequence ([6,7]). However, there are indications that the N-terminal 11-amino acids introduce structural changes in the protein that may mediate secretion [8,9,10]. There is some evidence of vesicle mediated secretion of galectin-3 and it has been found associated with exosomes [10,11]. Nonetheless, how extracellular galectin-3 effects cell adhesion is ambiguous. Previous studies have described either an enhancement or abrogation of cellular adhesion and spreading, dependent on cell type and galectin-3 concentration (reviewed in [4]). For example, extracellular galectin-3 may form a lattice along with Mgat5 to induce clustering and activation of β1 integrin. On the other hand, it has also been shown that extracellular galectin-3 is needed for internalization of the β1 integrin receptor [12,13,14]. A better understanding of how galectin-3 is secreted and what purpose it serves in the extracellular environment is needed. In this paper we have found a previously unknown link between secretion of galectin-3 and the calpain small subunit, calpain 4.
Calpains are a family of intracellular calcium-dependent proteases involved in a plethora of physiological processes including cell migration, apoptosis, and cell proliferation to name a few (reviewed in [15]. The calpain system includes the Calpain 1 and 2 holoenzymes, and their endogenous inhibitor calpastatin. Calpain 1 and 2 holoenzymes are comprised of the large catalytic subunits calpain 1 and 2 and a common small regulatory subunit, calpain 4, often referred to as Css-1 (reviewed in [16]). This specific system impacts cell adhesion and migration, likely by controlling the turnover of focal adhesions (reviewed in [16,17,18,19]. We have previously found that the small subunit not only acts as a regulatory protein, but also functions independent of the catalytic activity of the large subunits to produce mechanical forces on the ECM, known as traction forces [20]. Calpain 4 deficient mouse embryonic fibroblasts (MEF) produce less traction force in comparison to wildtype MEF, MEF deficient in the large catalytic subunits or MEF overexpressing the endogenous inhibitor calpastatin.
Exactly how traction forces are produced is currently an area of intense research in which mechanisms involving tyrosine phosphorylation have become a focus. Early studies established that enhanced tyrosine phosphorylation occurs at focal adhesions upon the application of mechanical stress [21,22]. Subsequent studies have substantiated these original observations (as reviewed in [23]). To further elucidate the force generation pathway, we have compared the tyrosine phosphorylation patterns of intracellular proteins from calpain 4 deficient MEFs to wildtype MEFs. We have identified galectin-3 as differentially tyrosine phosphorylated in these cells. Most significantly, we have made the unique observation that calpain 4 alone, and not the catalytic subunits of the Calpain 1 and 2 holoenzymes, as essential for galectin-3 secretion.
2. Materials and Methods
2.1 Cell Culture and Plasmids
Mouse embryonic fibroblasts (MEF) cells, Capn4 −/− MEFs, Capn1 knockdown MEFs, Capn2 knockdown MEFs were used in this study [24,25,26]. All cell lines were cultured and maintained in Dulbecco’s Modified Eagle’s Medium - high glucose (Sigma), supplemented with 10% fetal bovine serum (Hyclone) and 1% Penicillin/Streptomycin /Glutamine (Gibco) and incubated at 37°C under 5% humidified CO2. 0.1 % Trypsin-EDTA was used for cell passages, never exceeding eight passages for a given cell line. Calpain 4 deletion was rescued by nucleofection of Capn4 −/− MEFs with the plasmid pSBC-r28kDa encoding the full-length rat calpain small subunit as described by Dourdin et al., 2001 [25]. Calpastatin was over-expression in wildtype MEF cells from the plasmid hrEGFP-calpastatin [27].
2.2 Protein Extraction and Collection of Conditioned Media
Proteins were extracted from each cell line with triple detergent lysis buffer (100mM Tris-Cl, 300mM NaCl, 0.5% sodium deoxycholate, 0.2% SDS, 2% NP 40) containing Protease Inhibitor Cocktail (Sigma) and also Halt™ Phosphatase Inhibitor Cocktail. Protein from cells grown to 80% confluency on three 100mm cell culture dishes were extracted, concentrated and further prepared for two-dimensional polyacrylamide gel electrophoresis as described below.
To test for the secretion of galectin-3, conditioned media was collected from two 80% confluent 60mm culture dishes containing 2.5ml of culture media. Equal volume of conditioned media from each cell type was loaded onto standard 4–20% Tris-HEPES-SDS polyacrylamide gels and used for western blot or for Coomassie Brilliant Blue staining to ensure equal loading.
2.3 Two-Dimensional Polyacrylamide Gel Electrophoresis (2-D PAGE)
The protocol used for 2-D PAGE has been described in detail with minor modification to the sample preparation [28]. Briefly, the protein extracts from each cell line was concentrated using Amicon Ultra-4 5K filter units of 5000 Da Nominal Molecular Weight Limit (NMWL). The concentrated proteins were then solubilized in sample buffer (8M Urea, 50mM DTT, 4% CHAPS, 0.2% Carrier ampholytes, 0.0002% Bromophenol Blue). 200 micrograms of protein for each sample was rehydrated into isoelectric focusing strips with a pH range of 3–10 (Bio-Rad). Isoelectric focusing was then performed at 35,000 V-h in a PROTEAN IEF Cell (Bio-Rad). Following this, second-dimension SDS PAGE was performed using 4–12% Bis-Tris precast polyacrylamide gels (Bio-Rad).
2.4 Western Blot
Protein samples resolved either by the 2-D PAGE method or by standard SDS-PAGE (4–20% gradient Tris-HEPES-SDS precast polyacrylamide gel system, Pierce) were transferred (semi-dry) onto PVDF membranes (Bio-Rad). Buffers used for the transfer have been previously described [29]. The blots were probed with one of the following antibodies; anti-phosphotyrosine antibody clone PY20 (Millipore), monoclonal rat anti-Galectin-3 antibody and polyclonal rabbit anti-Galectin-3 antibody (gifts from Dr. A. Raz, Karmanos Cancer Institute, MI). Commercially available HRP conjugated secondary antibodies were detected with ECL Plus Western Blotting Detection Reagents (Amersham).
2.5 Identification of Differentially Tyrosine Phosphorylated Proteins
Protein samples from MEF cells and Capn4 −/− MEF cells were resolved by 2-D PAGE. The proteins were partially electro transferred onto PVDF membranes. As mentioned above, the blots were probed using anti-phosphotyrosine antibody clone PY20 (Millipore). The gel containing residual proteins were stained using SYPRO-Ruby Protein Gel Stain (Bio-Rad). Images from the immunoblot and stained gels were superimposed to select protein spots that were differentially phosphorylated in the two cell types (Described previously by Kang et al., 2005 [28]). These spots were then excised and identified by mass spectrometric analysis by the Protein Core Facility, Columbia University.
2.6 Immunofluorescence and Microscopy
Cultured cells were fixed for immunofluorescence using 4% paraformaldehyde and 0.1% Triton X-100 for 10 min, blocked for one hour with 5% BSA in PBS, followed by Anti-Galectin-3 antibodies and the species appropriate secondary Alexa Fluor 546 antibody. All images were acquired using an Olympus IX81 ZDC inverted microscope. Images were captured using a Diagnostic Instruments Boost EM-CCD-BT2000 back-thinned camera driven by IPLab software.
3. Results
3.1 Identification of Galectin-3 as a Non-Tyrosine Phosphorylated Protein in Calpain 4 Deficient Cells
Protein lysates were prepared from four different cell lines; wildtype MEF cells, MEF cells deficient in either the large Calpain 1 or 2 subunits, and MEF’s deficient in the small subunit Calpain 4. These lysates were resolved by two-dimensional gel electrophoresis as described in the materials and methods section. Western blotting with an anti-tyrosine antibody revealed numerous protein spots that differed in their levels of tyrosine phosphorylation between the four cell lines (Fig 1). A total of six protein spots were selected based on their differential presence on the four gels and analyzed by mass spectrometry. An isolated protein spot within the 30kDa range which was present in wildtype cells and Capn1 and 2 deficient cells, but absent in Capn4−/− lysates was identified as galectin-3. Using an anti-Galectin-3 antibody, we confirmed that the protein was expressed in Capn4 −/− cells and at levels equivalent to its expression in wildtype MEF cells (Fig 2). Expression levels of galectin-3 were also unchanged when the two large catalytic subunits, Capn1 and Capn2, were silenced (data not shown). These results strongly indicate that calpain 4 is essential for the tyrosine phosphorylation of galectin-3.
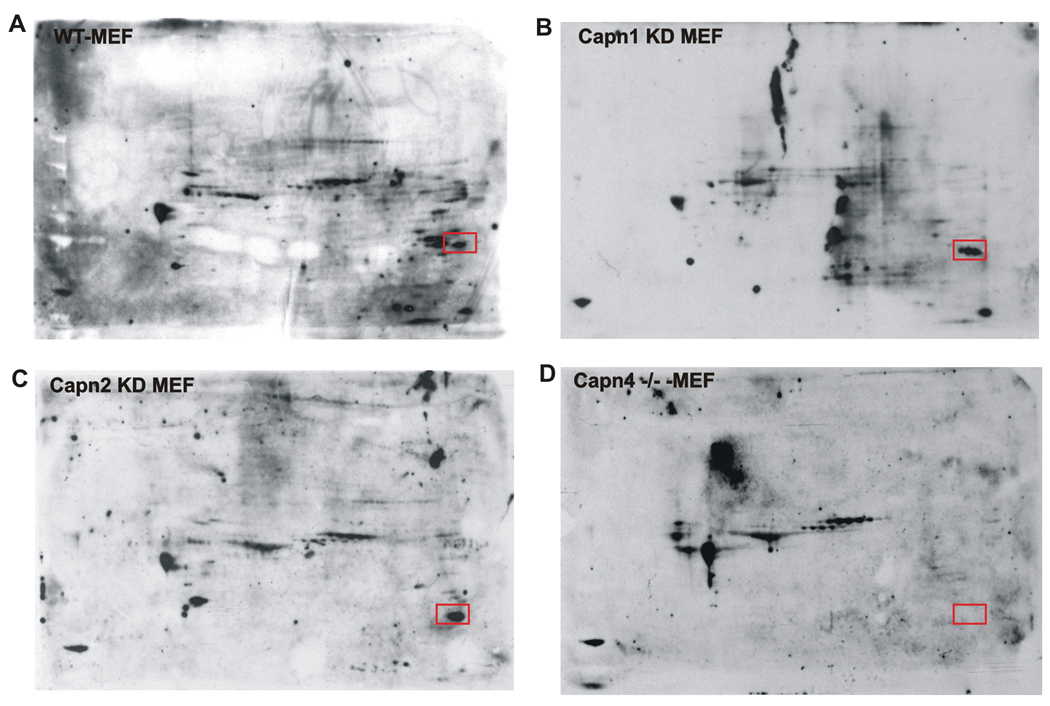
Figure 1. Galectin-3 is not tyrosine phosphorylated in the absence of the calpain small subunit, Capn4.
Total cellular protein from wildtype MEF cells (A), Capn1 silenced MEF cells (B), Capn2 silenced MEF cells (C) and Capn4 −/− MEF cells (D) were resolved by two-dimensional gel electrophoresis, transferred onto a PVDF membrane and probed for tyrosine phosphorylated proteins. Numerous protein spots are differentially phosphorylated in the four different cellular backgrounds. However, one prominent protein spot (red rectangular box) was phosphorylated in all cell types except in Capn4 silenced MEF cells. The protein spot was identified as the 30kDa protein Galectin-3 by mass spectrometric analysis.
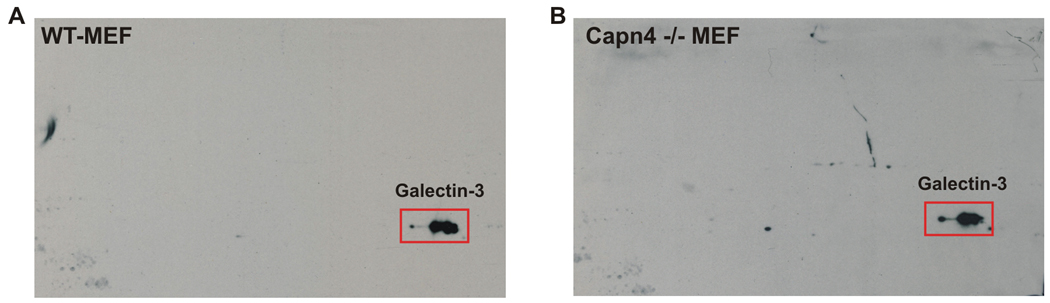
Figure 2. Galectin-3 expression is normal in the absence of Capn4.
Total cellular protein from wildtype MEF cells (A) and Capn4 −/− MEF cells (B) were resolved by two-dimensional gel electrophoresis, transferred onto a PVDF membrane and probed using an anti-Galectin-3 antibody. A distinct Galectin-3 spot (red rectangular box) was obtained in both cell types, indicating that Galectin-3 expression is normal in the absence of Capn4.
3.2 The Non-Tyrosine Phosphorylated form of Galectin-3 Fails to Localize to the Cell Periphery
Post-translational modifications of proteins are known to affect numerous facets of a protein including its function, interacting partners, localization and stability. We tested if the intracellular localization of the non-tryosine phosphorylated form of galectin-3 differed in the Calpain 4 deficient cells. In our immunofluorescence studies we used four different antibodies each recognizing a distinct region of the protein. We observed that the non-tyrosine phosphorylated form of Galectin-3 does not localize to the periphery of the Capn 4−/− cell (Fig 3). However, when tyrosine phosphorylated, as is the case in wildtype MEF cells and cells deficient in the catalytic heavy chains, galectin-3 is ubiquitously expressed in the cell and is not excluded from the cell periphery. This prompted us to ask if secretion of galectin-3 was disrupted in the Calpain 4 deficient cells.
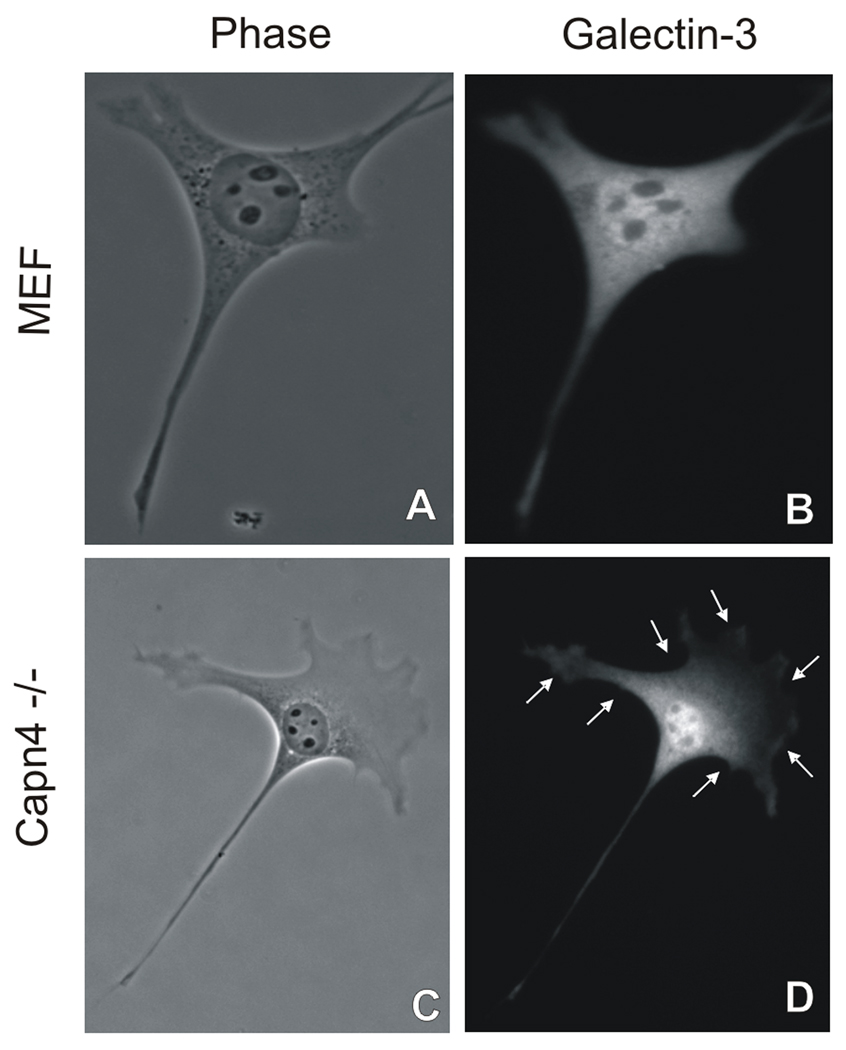
Figure 3. Non- tyrosine phosphorylation Galectin-3 affects its localization in Capn4 −/− cells.
Wildtype MEF cells and Capn4 −/− MEF cells were fixed and immunofluorescence was performed using a monoclonal anti-Galectin-3. Galectin-3 is present uniformly throughout the wildtype cell. However, in a Capn4 −/− MEF cell, galectin-3 was localized within the nucleus and in the peri-nuclear region, but absent from the cell periphery as indicated by white arrows around the cell periphery.
3.3 Calpain 4 is Essential for Secretion of Galectin-3
Galectin-3 can be found on the surface of the cell and is also known to be secreted, although the mechanism is currently unknown. Galectin-3’s extracellular activities are known to impact cell adhesion and migration in both normal and tumor cells. Since we were seeing an absence in the localization of galectin-3 to the cell periphery in Calpain 4 deficient cells, we tested for its secretion into the media of these cells. Conditioned media was collected on day 2 from cultures of all four cell lines; wildtype MEF, Capn1 and 2 deficient cultures and Calpain 4 deficient cell cultures. Consistent with our immunofluorescence results, we found that galectin-3 was not being secreted from the Capn4−/− cells, cells in which galectin-3 was not tyrosine phosphorylated (Fig 4a). In addition to the four cell lines, we also tested the conditioned media of cultured cells in which an endogenous inhibitor (calpastatin) of the calpain holoenzymes was overexpressed, thereby ensuring that the catalytic activity of both the large subunits would be abrogated. As expected, conditioned media from these cell cultures also showed an abundance of secreted Galectin-3 (Fig 4b). To further support our observation, we rescued Capn4 −/− cells using a recombinant rat Capn4 sequence. Conditioned media from these cells showed that galectin-3 was now being secreted by these cells indicating that the calpain small subunit (calpain 4), but not the catalytic activities of calpain 1 and calpain 2, is essential for galectin-3 secretion (Fig 4b).
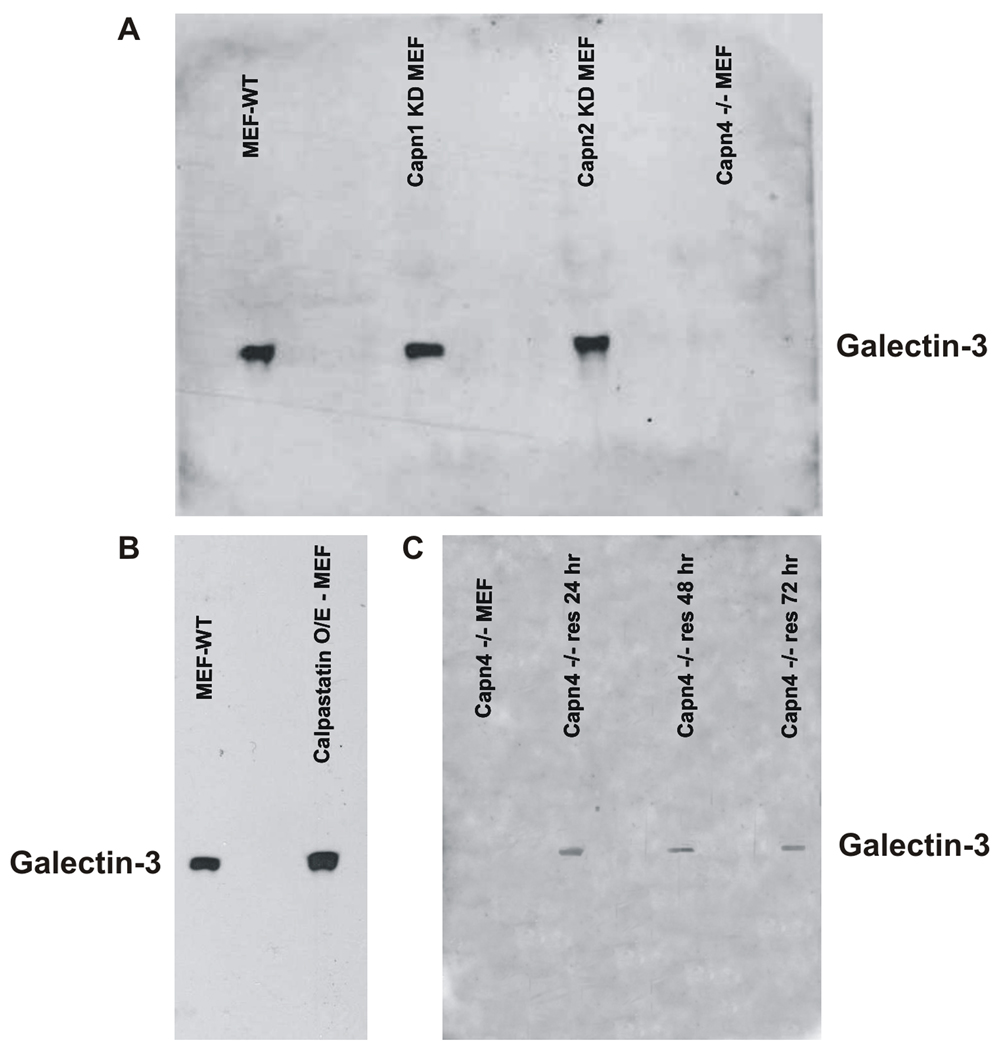
Figure 4. Capn4 is essential for Galectin-3 secretion.
A) Western blot of galectin-3 from media of wildtype MEF (MEF-WT), Capn1 silenced MEF (Capn1 KD MEF), Capn2 silenced MEF (Capn2 KD MEF) and Capn4 −/− MEF cell (Capn4 −/− MEF). B) Calpastatin overexpressing MEF cells secrete Galectin-3 at levels similar to mock-nucleofected MEF cells (lanes labeled as MEF-WT and Calpastatin O/E - MEF). C) Capn4 −/− MEF cells rescued by exogenous expression of Calpain 4 secrete galectin-3 as compared to Capn4 −/− cells indicated as lane Capn4−/− MEF. Lanes indicated as Capn4−/− res 24 hr, Capn4−/− res 48 hr, Capn4−/− res 72 hr shows results from conditioned media collected from Calpain 4 rescued cells 24, 48 and 72 hours post nucleofection. The amount of secreted galectin-3 decreases as the proportion of Calpain 4 rescued cells is overgrown by Capn4−/− cells.
4. Discussion
Cell migration is a complex process and both biochemical and mechanical components of the environment impact how a cell migrates. Environmental information is transmitted into the cell through transmembrane receptors, such as GPCRs, hormone receptors, and integrins to name a few. Activation of a complex system of overlapping signaling cascades ultimately lead to altered cytoskeletal and focal adhesion dynamics necessary for cell spreading or migration.
As a cell migrates it generates its strongest traction forces at the leading edge of a migrating cell resulting in the maturation of focal complexes into focal adhesions [30]. Much of how traction forces are generated in a migrating cell remains to be elucidated though previous studies have identified proteolytic and phosphorylation activities as significant events. As part of identifying the role of calcium-dependent proteases in mechanical signaling, our group has previously established that the calpain small subunit (calpain 4) regulates traction forces and strengthening of adhesions, independent of the catalytic activity of the large subunits [20]. Furthermore, various studies have identified Src family kinases, focal adhesion kinase, the SH2 domain-containing phosphates and receptor-like protein tyrosine phophatases as important components of the force-dependent signal transduction pathways [22,23]. Based on this evidence we compared the tyrosine phosphorylation levels of proteins extracted from wildtype MEF cells and MEF cells in which the expression of each of the three subunits of the Calpain 1 and 2 holoenzymes were silenced independently. From this screen galectin-3 was found to be differentially phosphorylated.
Until recently, phosphorylation of galectin-3 was believed to occur only on Serine residues 6 and 12 at the amino terminus of galectin-3. A single study suggested that galectin-3 is was phosphorylated on tyrosine residues at the N-terminal PGAY or PXXY motifs [10]. It was recently confirmed that galectin-3 is also phosphorylated on tyrosine residues 79, 107 and 118 and suggested that c-Abl kinase is the responsible kinase [31,32]. However, the functional significance of tyrosine phosphorylation of galectin-3 residues has not yet been established until now.
Many studies have confirmed the importance of this protein in the extracellular environment of cells and its impact on cell adhesion and migration under both normal and disease conditions. It has been shown in-vivo that circulating galectin-3 promotes tumor progression [33]. However the literature is conflicting on how extracellular galectin-3 is influencing migration. For example, one study finds that in-vitro galectin-3, along with caveolin-1, bind to N-glycans that have been modified by Mgat5 and recruit conformationally active α5β1 integrin to adhesions, resulting in the activation of FAK and PI3K, hence enhancing the formation of adhesions [13,14]. Conversely, a second study found that galectin-3 is involved in internalization of β1 integrin, thereby working against the formation of adhesions [12]. Despite these different roles attributed to extracellular galectin-3 in cell adhesion and migration, a potential mechanism for the secretion of galectin-3 has not been identified.
Galectin-3 is not secreted by the classical secretion pathway and adopts a non-classical mechanism [6,7]. It has been reported, that the first 11 amino acids of galectin-3 act to regulate of the localization of galectin-3, as truncation of this region eliminates secretion and nuclear localization [8]. However little else is known about how this protein is secreted. In this study we have found a previously unknown link between calpain-4 and the secretion of galectin-3. More specifically, we have made the novel observation that the tyrosine phosphorylation status of galectin-3, indirectly modulated by calpain-4, influences its secretion. We speculate that phosphorylation of tyrosine residues 79, 107 and 118 [32] alters the quaternary structure of galectin-3, making the N-terminal 11 amino acids unavailable for mediating galectin-3 secretion. Nonetheless, the lack of galectin-3 secretion serves to explain the defects observed in traction forces and migration found in calpain 4 deficient cells and further experimentation is currently underway to establish this fact.
Highlights.
-
-Tyrosine phosphorylation of galectin-3 is absent in calpain 4 deficient cells.
-
-Galectin-3 does not localize normally in calpain4 deficient cells
-
-Galectin-3 is not secreted from calpain 4 deficient cells.
-
-Galectin-3 secretion does not require the proteolytic activities of Calpain1 and Calpain2.
Acknowledgements
We greatly appreciate the assistance of Mr. Vishnu Undyala on this project. This work was supported by NIH R01GM084248 to K.A.B. and WSU Graduate Enhancement GRA to S.M..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakahara S, Raz A. On the role of galectins in signal transduction. Methods Enzymol. 2006;417:273–289. doi: 10.1016/S0076-6879(06)17019-6. [DOI] [PubMed] [Google Scholar]
- 2.Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572:285–293. doi: 10.1016/s0304-4165(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 3.van den Brule F, Califice S, Castronovo V. Expression of galectins in cancer: a critical review. Glycoconj J. 2004;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- 4.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673:75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993;268:11750–11757. [PubMed] [Google Scholar]
- 7.Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res. 1993;207:8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- 8.Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, Raz A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239–6245. [PubMed] [Google Scholar]
- 9.Mehul B, Hughes RC. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997;110(Pt 10):1169–1178. doi: 10.1242/jcs.110.10.1169. [DOI] [PubMed] [Google Scholar]
- 10.Menon RP, Hughes RC. Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur J Biochem. 1999;264:569–576. doi: 10.1046/j.1432-1327.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 11.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 12.Furtak V, Hatcher F, Ochieng J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem Biophys Res Commun. 2001;289:845–850. doi: 10.1006/bbrc.2001.6064. [DOI] [PubMed] [Google Scholar]
- 13.Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol. 2006;26:3181–3193. doi: 10.1128/MCB.26.8.3181-3193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz JG, Joshi B, Lajoie P, Strugnell SS, Scudamore T, Kojic LD, Nabi IR. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol. 2008;180:1261–1275. doi: 10.1083/jcb.200709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53 Suppl 1:S12–S18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 16.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 17.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 18.Huttenlocher A. Cell polarization mechanisms during directed cell migration. Nat Cell Biol. 2005;7:336–337. doi: 10.1038/ncb0405-336. [DOI] [PubMed] [Google Scholar]
- 19.Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 20.Undyala VV, Dembo M, Cembrola K, Perrin BJ, Huttenlocher A, Elce JS, Greer PA, Wang YL, Beningo KA. The calpain small subunit regulates cell-substrate mechanical interactions during fibroblast migration. J Cell Sci. 2008;121:3581–3588. doi: 10.1242/jcs.036152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 26.Franco S, Perrin B, Huttenlocher A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp Cell Res. 2004;299:179–187. doi: 10.1016/j.yexcr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115:3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 28.Kang CM, Abbott DW, Park ST, Dascher CC, Cantley LC, Husson RN. The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 2005;19:1692–1704. doi: 10.1101/gad.1311105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canelle L, Bousquet J, Pionneau C, Deneux L, Imam-Sghiouar N, Caron M, Joubert-Caron R. An efficient proteomics-based approach for the screening of autoantibodies. J Immunol Methods. 2005;299:77–89. doi: 10.1016/j.jim.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Ma Q, Wang J, Liu X, Yang Y, Zhao H, Wang Y, Jin Y, Zeng J, Li J, Song L, Li P, Qian X, Cao C. c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3. Cell Death Differ. 2010;17:1277–1287. doi: 10.1038/cdd.2010.8. [DOI] [PubMed] [Google Scholar]
- 32.Balan V, Nangia-Makker P, Jung YS, Wang Y, Raz A. Galectin-3: A novel substrate for c-Abl kinase. Biochim Biophys Acta. 2010;1803:1198–1205. doi: 10.1016/j.bbamcr.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–1393. [PubMed] [Google Scholar]