Abstract
Secretion of virulence factors is a critical mechanism for the establishment of cryptococcosis, a disease caused by the yeast pathogen Cryptococcus neoformans. One key virulence strategy of C. neoformans is the release of glucuronoxylomannan (GXM), a capsule-associated immune-modulatory polysaccharide that reaches the extracellular space through secretory vesicles. Golgi reassembly and stacking protein (GRASP) is required for unconventional protein secretion mechanisms in different eukaryotic cells, but its role in polysaccharide secretion is unknown. This study demonstrates that a C. neoformans functional mutant of a GRASP ortholog had attenuated virulence in an animal model of cryptococcosis, in comparison to wild type (WT) and reconstituted cells. Mutant cells manifested altered Golgi morphology, failed to produce typical polysaccharide capsules and showed a reduced ability to secrete GXM both in vitro and during animal infection. Isolation of GXM from cultures of WT, reconstituted or mutant strains revealed that the GRASP ortholog mutant produced polysaccharides with reduced dimensions. The mutant was also more efficiently associated to and killed by macrophages than WT and reconstituted cells. These results demonstrate that GRASP, a protein involved in unconventional protein secretion, is also required for polysaccharide secretion and virulence in C. neoformans.
Introduction
Cryptococcus neoformans is a yeast-like pathogen associated with high mortality rates in immunosuppressed individuals (Prado et al., 2009, Park et al., 2009). C. neoformans virulence is dependent on the expression of a number of virulence factors, including enzymes, pigments, polysaccharides and lipids (Li & Mody, 2010). Like many bacterial pathogens, C. neoformans is surrounded by a polysaccharide capsule that is an important virulence factor (Zaragoza et al., 2009). Capsule formation in this fungal pathogen requires intracellular polysaccharide synthesis (Yoneda & Doering, 2006, Feldmesser et al., 2001), followed by secretion of capsular components to the extracellular space and their incorporation into the cell surface (Rodrigues et al., 2008b, Zaragoza et al., 2009). Capsule expression is purportedly the most important constraint for cryptococcal virulence (McClelland et al., 2005).
Glucuronoxylomannan (GXM), the major capsular component of C. neoformans, is presumably synthesized in the Golgi and targeted to the cell surface (Yoneda & Doering, 2006). The polysaccharide then traverses the cell wall in vesicles that reach the extracellular space (Rodrigues et al., 2007), where GXM is used for enlargement of the cryptococcal capsule (Zaragoza et al., 2006). Exposure of C. neoformans to brefeldin A, which affects the formation of Golgi-related transport vesicles, results in a strong inhibition of capsule assembly (Hu et al., 2007). Consequently, the Golgi apparatus is suggested to be required for GXM synthesis and secretion, based on results of two independent studies. Yoneda and Doering demonstrated that a C. neoformans mutant lacking expression of Sav1p, a putative secretory vesicle-associated Rab GTPase essential for exocytosis, accumulates intracellular, post-Golgi vesicles containing GXM (Yoneda & Doering, 2006). This is in agreement with results described by Panepinto and colleagues, who showed that C. neoformans cells with deficient expression of Sec6p, which mediates polarized targeting of secretory vesicles to active sites of exocytosis, had a decreased rate of GXM secretion (Panepinto et al., 2009). Although both studies clearly indicated an association of Golgi-derived pathways with GXM secretion, the fact that capsular expression was apparently normal in both sav1 and sec6 mutants suggested that other components of Golgi-associated secretory pathways could have a role in GXM traffic in C. neoformans.
Golgi reassembly and stacking proteins (GRASPs) have been implicated in the stacking of Golgi cisternae, vesicle tethering, and mitotic progression (Nickel & Rabouille, 2009, Nickel & Seedorf, 2008). GRASP is primarily attached peripherally to the cytoplasmic surface of Golgi membranes, but its distribution into other cellular compartments is also expected (Nickel & Rabouille, 2009). In Dictyostelium discoideum, the single GRASP orthologue (GrpA) is required for unconventional secretion of acyl-coenzyme A-binding protein (AcbA) during spore differentiation (Kinseth et al., 2007), in a process that requires secretory vesicles (Cabral et al., 2010). GRASP is also required for the delivery of integrin α subunits to the plasma membrane of Drosophila melanogaster in a Golgi-independent manner (Schotman et al., 2008). More recently, it has been demonstrated GRASP is also required for starvation-induced secretion of AcbA in Saccharomyces cerevisiae and Pichia pastoris (Duran et al., 2010, Manjithaya et al., 2010).
The fact that a number of the cryptococcal virulence factors are exocellular components (Li & Mody, 2010) implies that secretory activity is directly linked to virulence in C. neoformans. Most of the C. neoformans virulence factors are released to the extracellular space apparently through unconventional mechanisms of secretion (Rodrigues et al., 2007, Nosanchuk et al., 2008, Rodrigues et al., 2008a, Rodrigues et al., 2008b, Casadevall et al., 2009). The possible link between cryptococcal virulence and unconventional secretion led us to investigate the role of GRASP in an animal model of C. neoformans infection. Our results suggest that virulence is attenuated in a GRASP ortholog mutant of C. neoformans, which was associated with a defect in the ability of yeast cells to secrete GXM. To our knowledge, this is the first report showing a role for a GRASP ortholog in microbial virulence and in polysaccharide secretion in eukaryotic cells.
Results
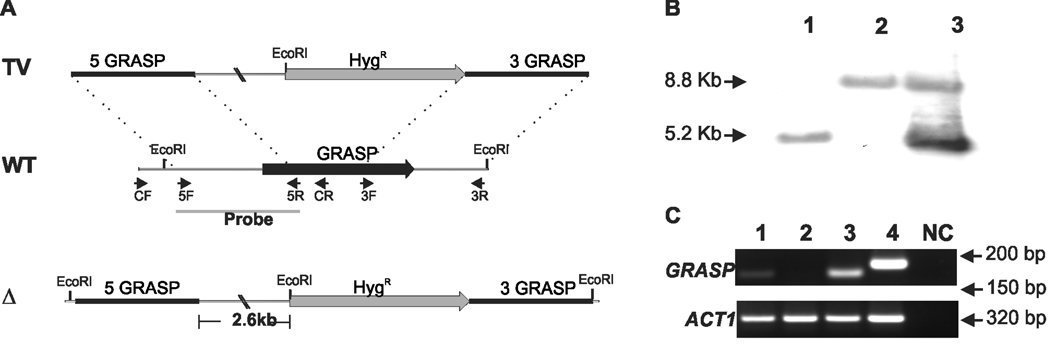
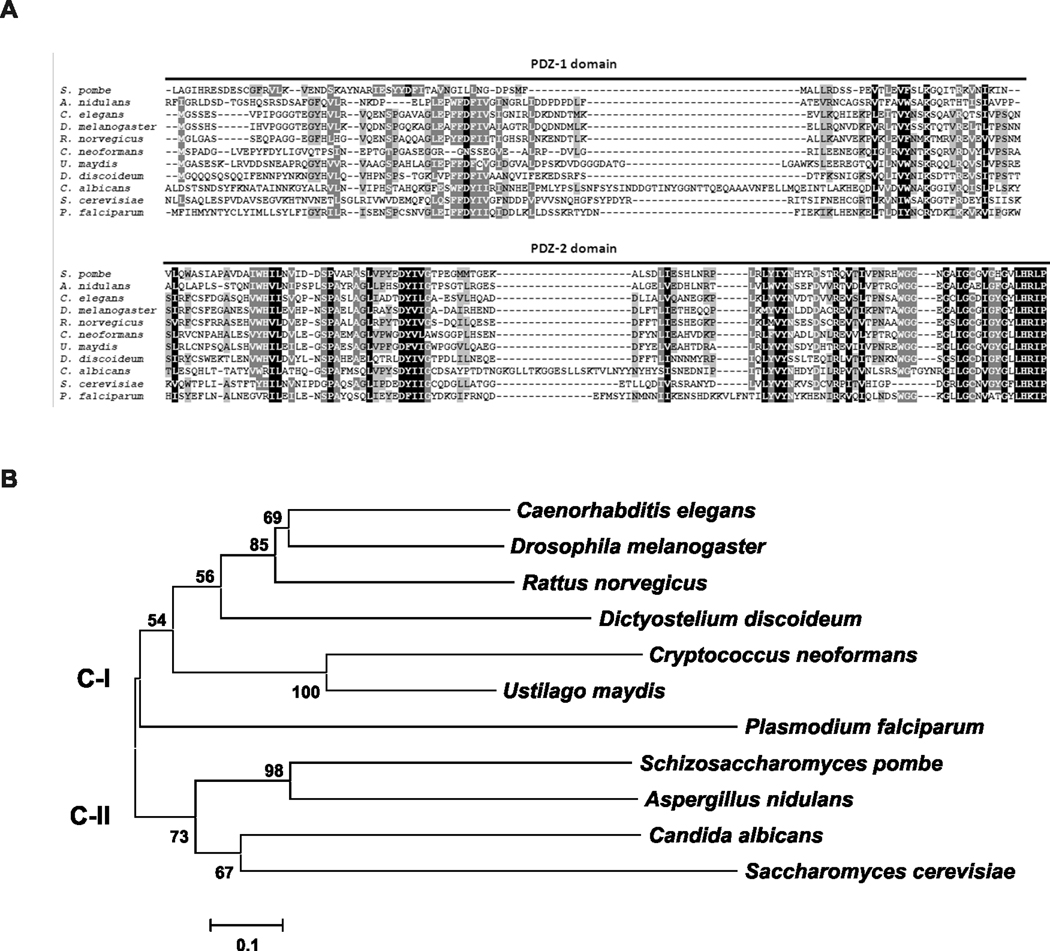
The construction of the GRASP ortholog mutant strain used in this study is summarized in Figure 1. GRASP 5’ and 3’ flanks were fused with the hygromicin resistant marker cassette by Delsgate methodology (Garcia-Pedrajas et al., 2008, Kmetzsch et al., 2010, Kmetzsch et al., 2011). The resulting targeting vector was used for C. neoformans transformation, which was monitored by Southern hybridization and semi-quantitative RT-PCR. We identified a putative GRASP ortholog in the C. neoformans var. grubii (serotype A) genomic database (Broad Institute, accession number CNAG_03291.2). The 1,051bp putative GRASP has four introns and predicts a 256-amino-acid protein. GRASPs are characterized by the presence of two PDZ-like domains in the N-terminal region (Kinseth et al., 2007). A BLAST search using the PDZ domains sequences from R. norvegicus GRASP revealed that these regions are also present in the C. neoformans GRASP ortholog. Sequence comparisons revealed that the identity range for the PDZ-1 and PDZ-2 domains were 40 and 48%, respectively (Figure 2A).
Figure 1. Deletion and complementation of the C. neoformans GRASP ortholog.
A. Scheme for the construction of the mutant strain. GRASP gene was replaced with the hygromicin resistant marker (HygR) cassette (gray box). GRASP 5’ and 3’ flanks (5 GRASP and 3 GRASP, respectively) were fused with HygR cassette by Delsgate methodology (Garcia-Pedrajas et al., 2008). The resulting targeting vector (TV) was used for C. neoformans transformation. The wild type locus of GRASP (WT) and the position of primers used for GRASP gene disruption are also indicated. The black bar scale corresponds to 500 base pairs (bp). The cleavage sites of EcoRI restriction enzyme are indicated in the deletion scheme. B. Southern blot analysis. Genomic DNA (10 µg) from WT (lane 1), grasp mutant (lane 2) and grasp::GRASP reconstituted (lane 3) strains were digested with EcoRI restriction enzyme. The 5’ gene flank was used as probe in Southern hybridization. Numbers at left indicate the hybridization signal sizes based upon the position of molecular size marker. C. Semi-quantitative RT-PCR with cDNA from WT (lane 1), grasp mutant (lane 2) and grasp::GRASP reconstituted (lane 3) strains as template. Numbers at rigth indicate the length of the transcript amplification for GRASP (upper panel) and ACT1 (lower panel) genes. Lane 4: positive control with genomic DNA as template. NC: negative control of the PCR reaction.
Figure 2. Phylogenetic analysis of the C. neoformans GRASP ortholog.
A. Alignment of the GRASP PDZ domains (PDZ-1 and PDZ-2) from R. norvegicus (AAB81355.2), C. elegans (NP_501354), D. melanogaster (AAF49092), S. cerevisiae (NP_010805), D. discoideum (EAL60823), S. pombe (NP_593015.1), P. falciparum (AAN35366), A. nidulans (Broad Institute accession number ANID_11248), U. maydis (Broad Institute accession number UM01076), C. albicans (Broad Institute accession number CAWG_01021) and C. neoformans (Broad Institute accession number CNAG_03291) using ClustalX2. B. Phylogenetic analysis applying the Neighbor-Joining method including GRASP sequences from distinct eukaryotic organisms listed above. The phylogeny tree splits into two major clades. C-I and C-II represents clades I and II, respectively. The bar marker indicates the genetic distance, which is proportional to the number of amino acid substitutions.
A broad phylogenetic analysis including GRASP sequences from distinct eukaryotic organisms rendered a phylogeny tree split into two major clades (Figure 2B). Clade C-II encompasses the GRASPs from the majority of the fungi analyzed, all belonging to the ascomycetes. Clade C-I harbored the GRASP from the basidiomicetous fungi C. neoformans and U. maydis, as well as those from mammalian (R. norvegicus), fly (D. melanogaster), worm (C. elegans), protist (P. falciparum), and amoeba (D. discoideum).
GRASP is primarily localized to the cytoplasmic surface of Golgi membranes, where it is apparently involved in the stacking of Golgi cisternae in eukaryotic cells (Vinke et al., 2011). Based on this observation, we evaluated whether lack of GRASP would affect Golgi morphology in C. neoformans. The Golgi marker N-[7-(4-nitrobenzo-2-oxa-1, 3-diazole)]-6-aminocaproyl-D-erythro-sphingosine (C6-NBD-ceramide) stained WT cells producing a pattern that was very similar to that described for S. cerevisiae (Levine et al., 2000) (Figure 3). Mutant cells, however, showed altered Golgi morphology. In mutant cells, the Golgi apparatus appeared to be compacted and limited to a smaller area of the cytoplasm.
Figure 3.
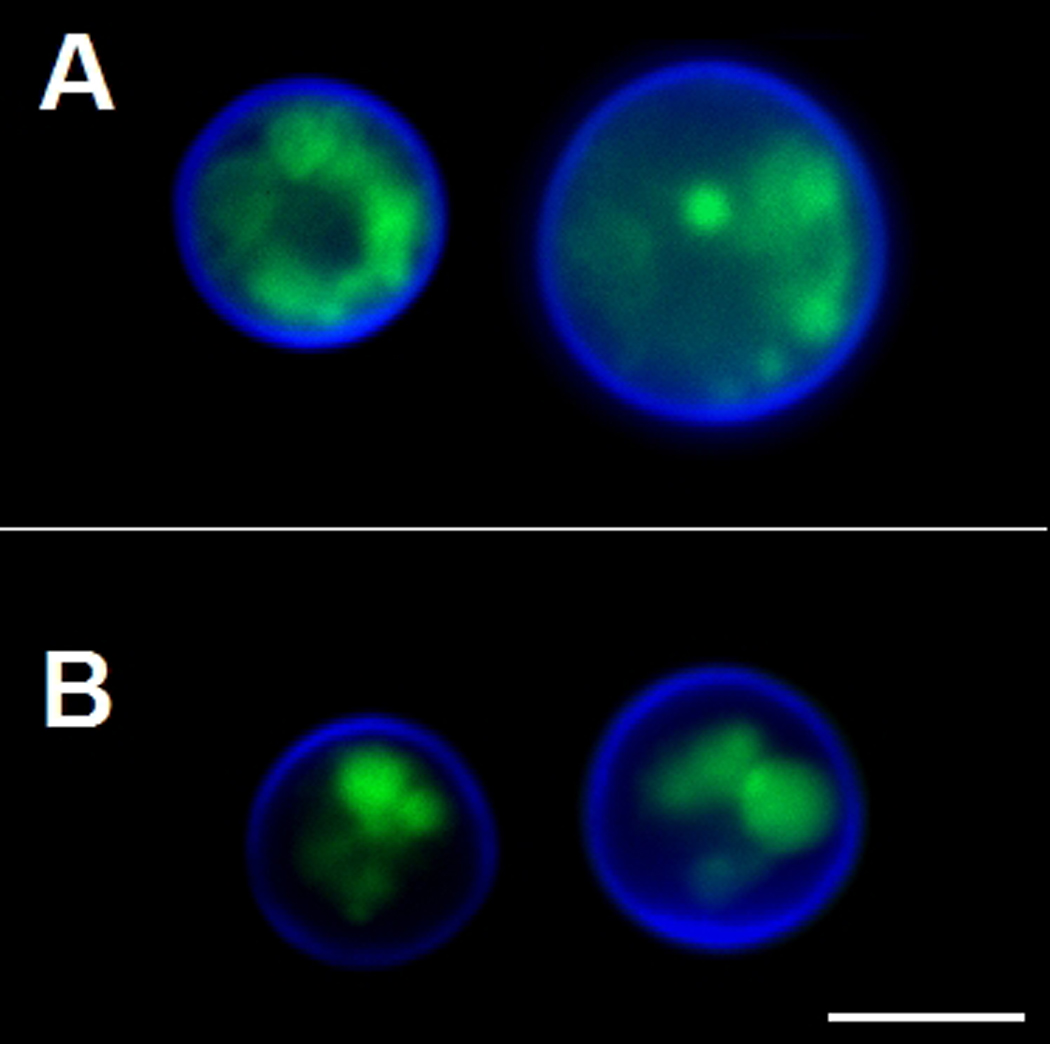
Modified morphology of the Golgi apparatus in C. neoformans GRASP ortholog mutant cells. WT cells (A) and the GRASP ortholog mutant (B) were sequentially incubated with C6-NBD-ceramide for Golgi visualization (green) and Uvitex 2B for staining of the cell wall (blue). Scale bar, 3 µm.
Extracellular molecules are crucial to the cryptococcal pathogenesis (Li & Mody, 2010). Post-Golgi secretion is also involved in the release of C. neoformans virulence factors (Yoneda & Doering, 2006, Panepinto et al., 2009, Chayakulkeeree et al., 2011). These observations and the recently described roles of GRASP in unconventional secretion (Schotman et al., 2008, Kinseth et al., 2007, Levi & Glick, 2007, Manjithaya et al., 2010, Duran et al., 2010) led us to hypothesize that Golgi integrity would be required for the pathogenic mechanisms of C. neoformans. Therefore, we evaluated some virulence determinants in the GRASP ortholog mutant in comparison to both WT and complemented cells.
The GRASP ortholog mutant showed normal growth rates at 37°C (Figure 4A). In an intranasal model of animal infection, this mutant caused death of all animals in 18 days (Figure 4B). In contrast, when animals were infected with either WT or complemented cells, 100% killing was observed within 11 and 12 days post-infection, respectively (P<0.01, in comparison to mutant cells). These results revealed that the GRASP ortholog mutant had attenuated virulence in an animal model of cryptococcosis.
Figure 4. Virulence phenotype of the GRASP ortholog mutant.
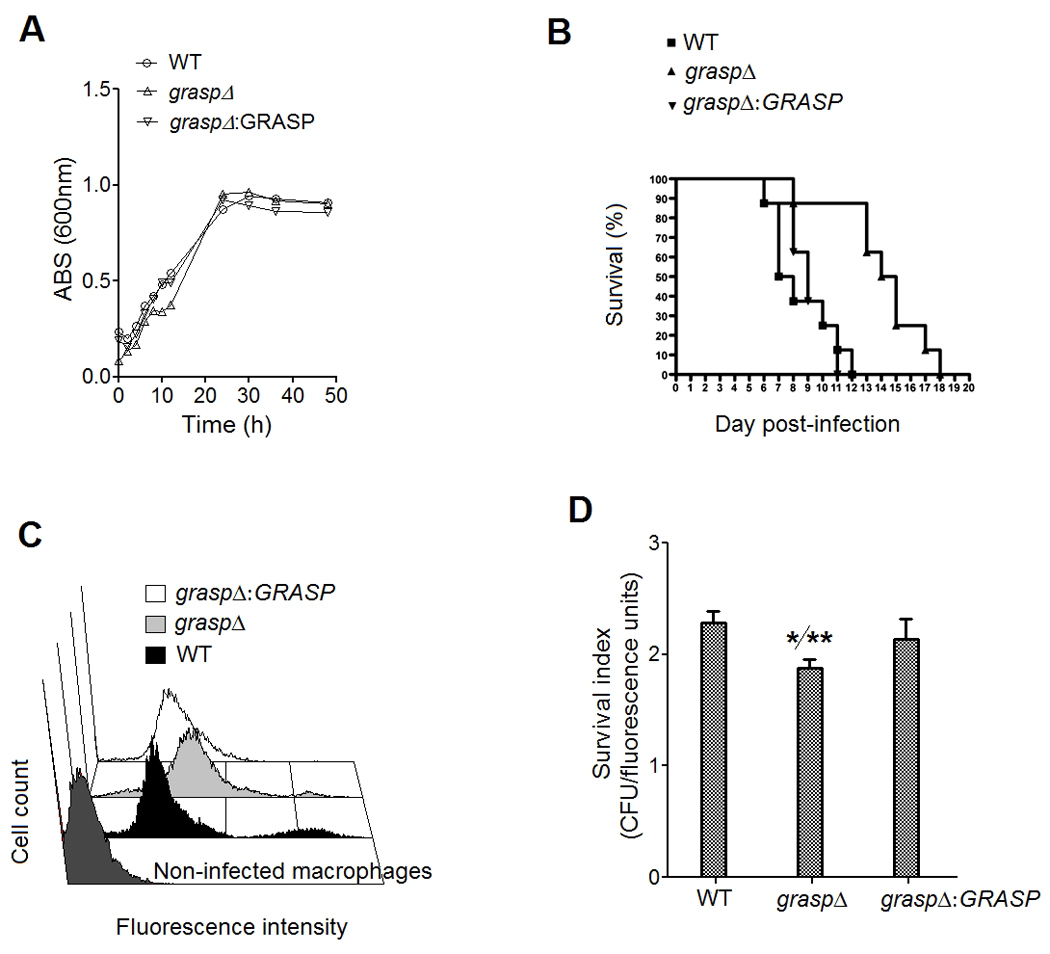
A. Growth rates of WT, mutant and complemented cells. B. The GRASP ortholog mutant exhibited attenuated virulence in an animal model of cryptococcosis. Mice were lethally infected with C. neoformans for daily monitoring of survival. Animals infected with the mutant strain survived significantly longer (P<0.01). C. Association of FITC-labeled C. neoformans cells with murine phagocytes. The similarity in the fluorescence levels of macrophages after infection with non-opsonized WT and reconstituted cells is indicative of similar indices of association between fungal and host cells. Higher fluorescence levels were observed for the mutant, suggesting increased phagocytosis. D. Survival of cryptococci after interaction with the phagocytes. The GRASP ortholog mutant was significantly more susceptible to killing by macrophages than WT (*) and complemented (**) cells (P<0.01).
Since the interaction of C. neoformans with macrophages is considered to be determinant in a number fungal infections (Seider et al., 2010), we have also evaluated whether the attenuated virulence of the GRASP ortholog mutant was related to decreased levels of association of C. neoformans with macrophages. For this purpose, we incubated phagocytes with FITC-labeled yeast cells, and measured phagocytosis in an assay where the index of fluorescence of each macrophage in flow cytometry analysis was proportional to the efficacy of the fungi-host cell interaction. Our results indicate that WT and complemented strains showed similar levels of association with murine phagocytes (Figure 4C). The mutant, however, was more efficiently associated to macrophages, suggesting increased phagocytic indices. Treatment of the macrophage-fungi complexes with trypan blue, which quenches the fluorescence of extracellularly-associated yeast cells, resulted in a decreased of fluorescence levels corresponding to 8, 12 and 7% for WT, mutant and reconstituted cells, respectively (data not shown). The relatively high resistance of infected macrophages to lose fluorescence after exposure to trypan blue indicates that, in all systems, C. neoformans cells were internalized by the macrophages in high rates. WT and complemented cells were significantly more resistant than the GRASP ortholog mutant against the microbicidal activity of macrophages (P<0.01). Similar experiments were performed after opsonization of yeast cells with monoclonal antibody (mAb) 18B7, which recognizes GXM (Casadevall et al., 1998) (data not shown). Antibody treatment did not influence phagocytosis of both WT and GRASP ortholog mutant cells (not shown), which can be linked to the high efficacy of fungal ingestion by the phagocytes after the 18 h-incubation.
Pigmentation, urease activity and synthesis and release of capsular components are associated with the survival of C. neoformans during infection of host cells (reviewed in (Zaragoza et al., 2009)). We therefore analyzed the ability of the GRASP ortholog mutant to pigment, to produce extracellular urease activity, to secrete GXM and to form a polysaccharide capsule. Levels of pigmentation and urease activity in cultures of WT, mutant and reconstituted cells were similar (Figure 5). After cultivation under regular (non-inducing) conditions of growth and capsule synthesis, the GRASP ortholog mutant manifested a hypocapsular phenotype, in comparison to WT and reconstituted cells (Figure 6A–C). Fluorescence microscopy revealed that GXM was detected at the cell surface of both WT and reconstituted cells, as well as in the mutant. In the latter, some of the cells appeared to lack a detectable capsule, while other cells showed the hypocapsular phenotype as observed by India ink counterstaining.
Figure 5. Absence of GRASP does not affect pigmentation (A–B) or urease activity (C) in cryptococcal cultures.
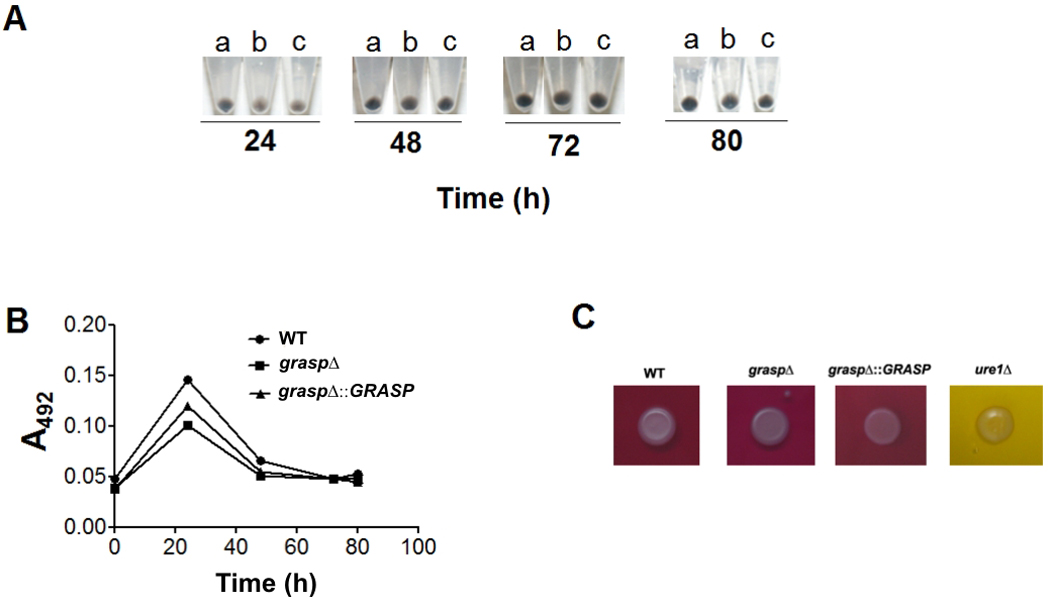
A. Pigmentation of C. neoformans cells after growth in the presence of L-DOPA. Pigmented pellets of WT (a), mutant (b) and reconstituted cells (c) are shown. B. Release of pigment-like molecules into C. neoformans cultures. C. Urease activity was detected (pink color) in cultures of WT, mutant and reconstituted cells, but not in cultures of a urease deletion mutant of C. neoformans (ure1Δ, yellow).
Figure 6. GRASP is required for normal GXM secretion and capsule assembly.
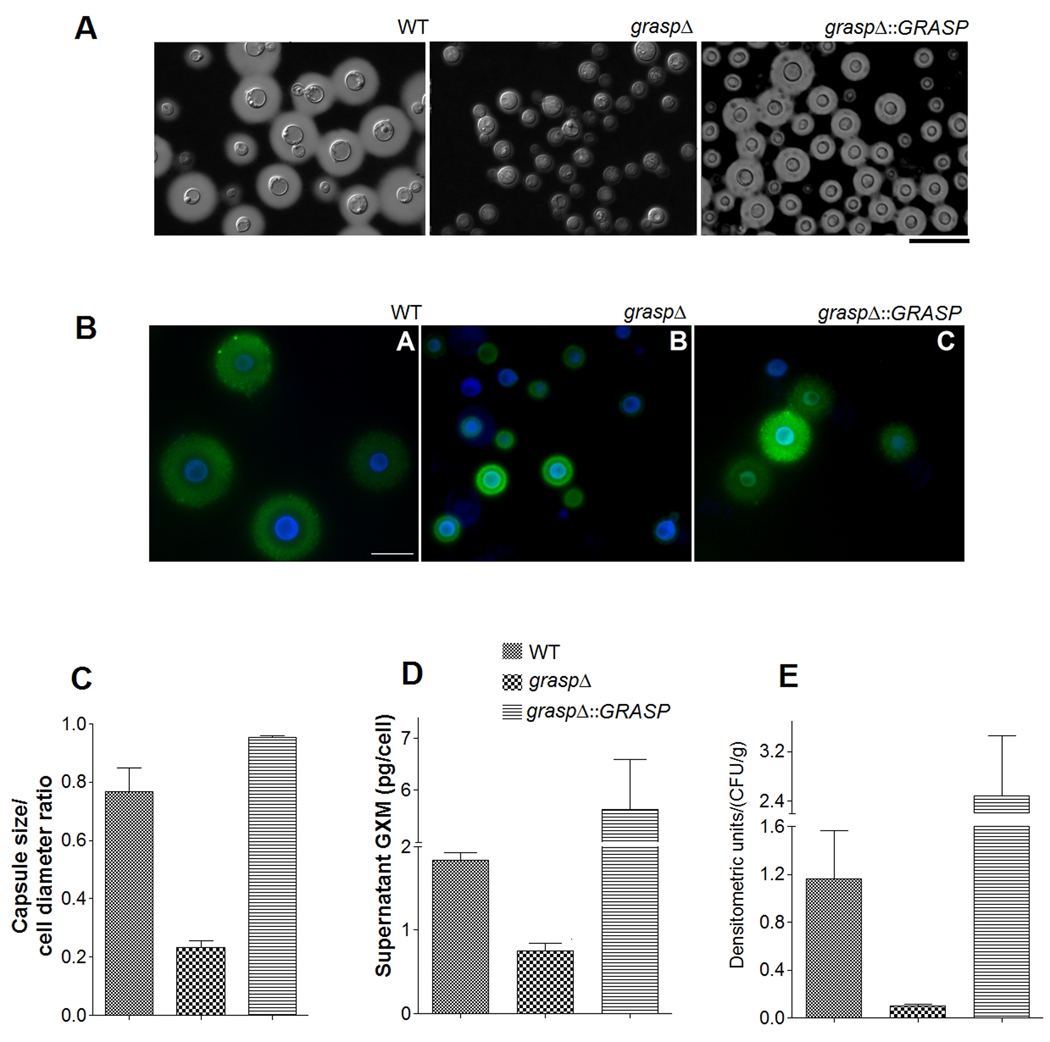
A. India ink counterstaining of C. neoformans cells. Yeast strains are indicated on the top of each panel. B. Reactivity of C. neoformans cells with calcofluor white (blue fluorescence) and a monoclonal antibody raised against GXM (green fluorescence). Scale bars in A and B represent 20 and 10 µm, respectively. C. Determination of capsule size of the C. neoformans cells illustrated in A and B. GXM determination in culture supernatants (D) and infected brains (E) are shown, indicating that C. neoformans GRASP ortholog mutant shows a reduced content of extracellular GXM. Statistical analysis of the results shown in C, D and E indicates that values obtained for the GRASP ortholog mutant are significantly smaller than those found for WT and complemented systems (P<0.05 in all cases).
The hypocapsular phenotype of the GRASP mutant was apparently related to a defective ability to secrete GXM, since the concentration of this major capsular component in culture supernatants of the GRASP ortholog mutant was significantly lower than in supernatants of WT and reconstituted cultures (Figure 6D). Analysis of infected organs revealed that the concentration of lung GXM was not significantly affected by lack of GRASP (data not shown). In the brain, however, deletion of GRASP resulted in a marked decrease in the ability of cryptococci to release GXM (Figure 6E).
Although secretion of capsular components is required for capsule assembly, it was recently demonstrated that capsule enlargement also requires polysaccharide molecules with higher effective diameters (Frases et al., 2009). We then analyzed this parameter in GXM fractions from WT and mutant cells (Figure 7). Profiles of diameter distribution of GXM isolated from culture supernatants of WT and complemented cells were very similar. However, values for the effective diameter for polysaccharide fractions obtained from cultures of the mutant were below the detection limit under the conditions used in this study. Since interaction with divalent cations is known to promote GXM aggregation and to increase the dimensions of polysaccharide molecules (Nimrichter et al., 2007, Frases et al., 2008) we incubated the extracellular GXM samples with 1 mM CaCl2 for 1 h to favor detection of smaller molecules. Under these conditions, extracellular GXM fractions from the GRASP ortholog mutant reached dimensions that were within the limit of detection of the method.
Figure 7. Effective diameter determination of GXM obtained from cultures of WT, mutant, and reconstituted cells.
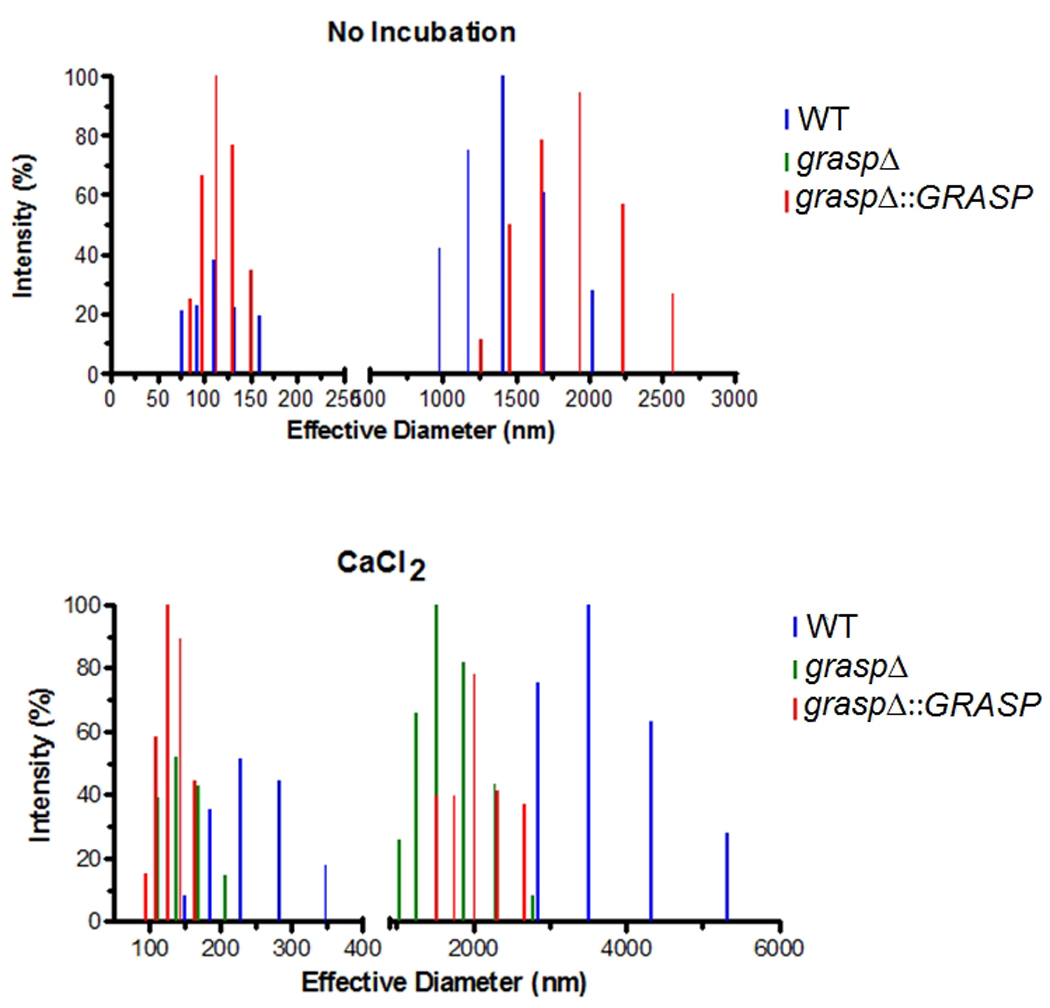
The upper panel shows diameter determination under regular conditions of GXM analysis. The lower panel illustrates diameter determination after incubation of polysaccharide fractions with 1 mM CaCl2.
In the presence of CaCl2, the complemented strain and the GRASP ortholog mutant exhibited a similar pattern of GXM diameter, in contrast to the apparently ordered structures formed by WT cells. The explanation for these findings is still unclear. GXM aggregation is dependent on the availability of glucuronic acid residues, which is favored when larger polysaccharide fractions are tested (Frases et al., 2008, Frases et al., 2009, Nimrichter et al., 2007). Therefore, the pattern in size observed for each sample after addition of Ca2+ is likely influenced by the availability of glucuronic acid residues. However, this remains to be determined. In fact, properties of capsule/GXM were modified in some of the experiments in which the complemented strain was used. These modified characteristics included exacerbated GXM secretion (Figure 6) and aggregation in the presence of Ca2+ (Figure 7). The complemented graspΔ::GRASP strain used in this study was generated by ectopic integration of the WT gene in the GRASP ortholog mutant, which is likely responsible for over expression of the GRASP-coding gene.
Discussion
Fungal cells evolved a number of unconventional secretion mechanisms to release proteins to the extracellular milieu (Panepinto et al., 2009, Rodrigues et al., 2008a, Duran et al., 2010, Manjithaya et al., 2010, Albuquerque et al., 2008, Oliveira et al., 2010a, Oliveira et al., 2010b). In addition to the well characterized unconventional secretory pathways, fungi have been proposed to exploit a vesicular pathway for the trans-cell wall passage of molecules to the extracellular milieu (Oliveira et al., 2009, Rodrigues et al., 2008a, Nosanchuk et al., 2008, Rodrigues et al., 2007, Rodrigues et al., 2008b, Casadevall et al., 2009, Vallejo et al., 2010, Oliveira et al., 2010b, Eisenman et al., 2009). The genes and cognate proteins involved in such mechanisms, however, are only partially characterized. Polysaccharides, lipids and pigments can also be extracellularly secreted by fungi (Eisenman et al., 2009, Rodrigues et al., 2007), but the pathways required for these processes remain largely unknown. GXM biosynthesis in C. neoformans, in fact, illustrates this scenario. Many of the enzymes required for synthesis of this polysaccharide have been described (reviewed in (Doering, 2009)), but several aspects related to its cellular distribution and traffic remain unknown. Intracellular GXM is associated to the membranes of still uncharacterized organelles (Oliveira et al., 2009). Extracellular GXM is found either free as a soluble polysaccharide (reviewed in (Zaragoza et al., 2009)), or associated to exosome-like structures (Albuquerque et al., 2008, Rodrigues et al., 2007). Defects in GXM production and capsule assembly led to avirulent phenotypes in C. neoformans (reviewed in (Zaragoza et al., 2009)). Since GXM production and extracellular release are crucial for cryptococcal pathogenesis, this is clearly an area of active study.
A few examples of cryptococcal genes implicated in GXM traffic and secretion are available in the literature, including CAP59, SAV1 and SEC6. CAP59 gene produces a 458-amino-acid protein of unknown function that has identity to CMT1, whose product has α-1,3-mannosyltransferase activity (Chang et al., 1995, Sommer et al., 2003). Deletion of CAP59 resulted in acapsular cells with increased cell body diameters (Garcia-Rivera et al., 2004), which was attributed to intracellular polysaccharide accumulation. In fact, a missense mutation of CAP59 partially hampered the trafficking of GXM, but not of proteins (Garcia-Rivera et al., 2004). The existence of cellular pathways required for GXM export was further confirmed in studies focused on the role of post-Golgi secretion events in C. neoformans. The products of SAV1 and SEC6 genes, which correspond to a putative vesicle-associated Rab GTPase and to a member of the post-Golgi exocytic complex, respectively, were demonstrated to be involved in vesicle-mediated export of GXM to the surface of C. neoformans (Yoneda & Doering, 2006, Panepinto et al., 2009). GXM is then released to the extracellular space in vesicles that traverse the cell wall (Rodrigues et al., 2007). Therefore, extracellular GXM release is linked to elements of the conventional, post-Golgi secretory pathway (Hu et al., 2007, Panepinto et al., 2009) and to exosome-like structures (Rodrigues et al., 2007). Nevertheless, the involvement of other cellular pathways in the traffic of the polysaccharide is largely unknown.
In S. cerevisiae and P. pastoris, GRASP is involved in unconventional secretory mechanisms that require the participation of genes related to autophagy, early endosomal compartments, and MVBs (Duran et al., 2010, Manjithaya et al., 2010). The possibility raised by many authors that GXM secretion could involve such organelles (Yoneda & Doering, 2006, Casadevall et al., 2009, Takeo et al., 1973b, Takeo et al., 1973a, Rodrigues et al., 2008b, Nosanchuk et al., 2008, Albuquerque et al., 2008, Oliveira et al., 2009) combined to the emerging roles of GRASP in unconventional secretory pathways, (Cabral et al., 2010, Duran et al., 2010, Kinseth et al., 2007, Levi & Glick, 2007, Nickel & Rabouille, 2009, Schotman et al., 2008) prompted us to ask whether GRASP would also regulate GXM secretion and virulence in C. neoformans.
In comparison to WT and reconstituted cells, the GRASP ortholog mutant of C. neoformans was less efficient in killing lethally infected mice and more effectively phagocytized by macrophages. A possible explanation for this finding would be its reduced ability to secrete GXM and/or to form regular capsules. Since the polysaccharide is believed to cause many deleterious effects to the host (reviewed in (Zaragoza et al., 2009)), the reduction of the extracellular concentration of GXM would favor host defense resulting in infection control. Although the absence of GRASP was correlated with attenuated virulence, the C. neoformans mutant lacking this protein was still able to kill all the mice. Hence, it would be reasonable to infer that GRASP deletion generated a partial phenotype in that capsule assembly, GXM secretion and virulence were affected. This scenario, therefore, would reflect quantitative and not qualitative alterations, suggesting that other molecules in addition to GRASP may play redundant roles in polysaccharide secretion and capsule assembly. Remarkably, GXM obtained from the GRASP ortholog mutant had altered physical chemical properties (reduced dimensions), although still recognized by an antibody raised to the polysaccharide. The fact that the mutant produced smaller GXM molecules is consistent with the observation of a reduced capsule. Capsule enlargement was demonstrated to be linked to the availability of polysaccharide molecules of increased dimensions (Frases et al., 2009).
Based on studies with monoclonal antibodies, it is generally accepted that the first steps of GXM synthesis occur intracellularly (Oliveira et al., 2009, Yoneda & Doering, 2006, Garcia-Rivera et al., 2004, Feldmesser et al., 2001). However, it is not known whether polysaccharide molecules distributed to intracellular organelles show the same structural features observed in extracellular fractions. For instance, antibody-binding GXM small precursors could be synthesized in intracellular compartments to be then transferred to the extracellular space, where polymerization would occur by aggregative mechanisms (Frases et al., 2009, Nimrichter et al., 2007). In this case, the reduced secretion of GXM by the GRASP ortholog mutant would be simply related to impaired cellular traffic, as described for other systems (Kinseth et al., 2007, Manjithaya et al., 2010, Duran et al., 2010). GXM polymerization would be favored in conditions where the polysaccharide is more abundant (Nimrichter et al., 2007), and this might explain why the effective diameter of GXM is reduced in supernatants of the mutant.
GRASP is required for unconventional secretion in D. discoideum, S. cerevisiae, P. pastoris and D. melanogaster (Kinseth et al., 2007, Manjithaya et al., 2010, Duran et al., 2010). It is well accepted that GRASP is a Golgi-associated protein, which is in agreement with our current results. However, it has also been proposed that this protein could mediate vesicle fusion events at the plasma membrane (Nickel & Rabouille, 2009). This proposal is in agreement with the fact that GRASP is localized at the plasma membrane during epithelial cell remodeling in D. melanogaster (Schotman et al., 2008). Remarkably, D. discoideum cells lacking GRASP show defects in the final stage of fusion of vesicles required for unconventional secretion with the plasma membrane (Cabral et al., 2010). In contrast to what has been demonstrated for a S. cerevisiae grasp mutant (Oliveira et al., 2010b), C. neoformans cells lacking GRASP showed normal extracellular release of vesicles (L. Sobrino, unpublished data). This could suggest that, in C. neoformans, GRASP is required for GXM loading into secretory vesicles rather than in the release of these structures to the extracellular space, although we still do not have any evidence that GXM and GRASP interact. Alternatively, the role played by GRASP in polysaccharide traffic could be related to its presence in Golgi cisternae, which also requires experimental confirmation.
Protein secretion has been extensively explored in bacterial and eukaryotic cells. Although polysaccharide secretion in fungi was described many decades ago, the regulatory mechanisms are largely unexplored. To our knowledge, this is the first study to demonstrate a role for GRASP in polysaccharide secretion, as well as in microbial virulence. The observation that GRASP regulates a process required for the pathogenesis of C. neoformans adds a new function to the list of the important roles played by this protein in the biology of eukaryotic organisms.
Experimental procedures
Fungal strains, plasmids and media
C. neoformans H99 strain was employed as a recipient for creating target gene deletion. Plasmid pJAF15, which contains the hygromycin marker cassette, was a generous gift of Joseph Heitman (Duke University, Durham, NC USA). Plasmid pAI4, which contains the nourseothricin marker cassette, was kindly provided by Alexander Idnurm (University of Missouri-Kansas City Kansas City, MO USA). The strains were maintained on YPD medium (1% yeast extract, 2% peptone, 2 % dextrose, and 1,5% agar). YPD plates containing hygromycin (200 µg/ml) were used to select C. neoformans grasp deletion transformants (graspΔ strain). YPD plates containing nourseothricin (100 µg/ml) were used to select C. neoformans grasp reconstituted transformants (graspΔ::GRASP strain).
In silico analysis of the C. neoformans GRASP ortholog
The putative C. neoformans GRASP gene sequence was identified by a BLAST search of the C. neoformans var. grubii strain H99 genomic database at the Broad Institute using GRASP sequences of S. cerevisiae (GenBank accession number NP_593015) and D. discoideum (GenBank accession number EAL60823). The aminoacid sequences of GRASP orthologs from Rattus norvegicus, Caenorhabditis elegans, Drosophila melanogaster, Saccharomyces cerevisiae, Dictyostelium discoideum, Schizosaccharomyces pombe, Plasmodium falciparum, Aspergillus nidulans, Ustilago maydis, Candida albicans and C. neoformans were aligned using ClustalX2 (Larkin et al., 2007). Mega4 were used for phylogenetic analysis applying the Neighbor-Joining method and the tree architecture was inferred from 10,000 bootstraps (Tamura et al., 2007). The identification of the PDZ domains in the sequences was performed by BLAST search of the previous sequences with the R. norvegicus PDZ domains as previously described (Kinseth et al., 2007).
Disruption and complementation of GRASP
Disruption of GRASP was achieved employing the Delsgate methodology (Garcia-Pedrajas et al., 2008, Kmetzsch et al., 2010, Kmetzsch et al. 2011). A Gateway cloning system donor vector (Invitrogen, Carlsbad, Ca) containing the hygromycin selectable marker for C. neoformans transformation was constructed. A 2.2 Kb PCR product spanning the hygromycin marker cassette fragment was amplified from pJAF15 and cloned into the EcoRV site of pDONR201 (Invitrogen, Carlsbad, Ca). The resulting vector was named pDONRHYG. The 5’ and 3’ GRASP flanks (702 bp and 700 bp, respectively) were PCR amplified and purified from agarose gels (Illustra GFX PCR DNA and Gel Band Purification kit, GE Healthcare, Buckinghamshire, UK). Approximately 300 ng of pDONRHYG and 30 ng of each PCR product were submitted to BP clonase reaction, according to manufacturer’s instructions (Invitrogen, Carlsbad, Ca). The product of this reaction was transformed into Escherichia coli OmniMAX 2-T1. After confirmation of the correct deletion construct, the plasmid was linearized with I-SceI prior to C. neoformans biolistic transformation (Toffaletti et al., 1993). The mutants were screened by colony PCR, and the deletion was confirmed by Southern blot and semi-quantitative RT-PCR analyses. For complementation, a 3 Kb genomic PCR fragment containing the wild-type GRASP gene was cloned into the SmaI site of pAI4. The resulting plasmid was used for transformation of the GRASP ortholog mutant strain. Genomic insertion of the complemented gene was confirmed by Southern blot and semi-quantitative RT-PCR analyses. The primers used in these plasmid constructions are listed in Table 1. The strategy used for generation of C. neoformans grasp mutant strain is summarized in Figure 1.
Table 1.
List of primers used in this study.
| Primer name | Sequence (5’-3’) | Purpose |
|---|---|---|
| CnGRASPF | AAAATAGGGATAACAGGGTAATGAGATACCAGATGGACTGAA | Disruption construct for GRASP1, 5’ flank |
| CnGRASP5R | GGGGACAAGTTTGTACAAAAAAGCAGGCTATATATTCTGCCCAGCACATCT | Disruption construct for GRASP1, 5’ flank |
| CnGRASP3F | GGGGACCACTTTGTACAAGAAAGCTGGGTAATGCTAATGTGAAACGCAAT | Disruption construct for GRASP1, 3’ flank |
| CnGRASP3R | AAAAATTACCCTGTTATCCCTAGTAACGAGAAGTGCTGTCTC | Disruption construct for GRASP1, 3’ flank |
| GRASPcompF | ATAATGCAATGCGATGGCTGC | Amplification of GRASP for complementation |
| GRASPcompR | AATCCCCTCAAGAGCTCACGG | Amplification of GRASP for complementation |
| RTGRASPF | AGTTCTTTACCCTACTAGACAG | Amplification of GRASP for RT-PCR |
| RTGRASPR | TCTCCTCACATTGTCAGATTC | Amplification of GRASP for RT-PCR |
| RTACTF | CCTTCTACGTCTCTATCCAG | Amplification of ACT1 for RT-PCR |
| RTACTR | TTTCAAGCTGAGAAGACTGG | Amplification of ACT1 for RT-PCR |
Staining of the Golgi apparatus
Staining of the Golgi apparatus was based on the protocols described by Pagano and colleagues (Pagano, 1989, Pagano et al., 1989). The Golgi staining reagent was C6-NBD-ceramide, which accumulates at the Golgi apparatus of either living or fixed cells (Pagano, 1989). C6-NBD-ceramide has been successfully used to stain S. cerevisiae Golgi-related structures (Levine et al., 2000). Yeast cells were fixed with paraformaldehyde 4% in PBS, followed by washing with PBS and incubation with C6-NBD-ceramide (5 µM) for 16 h at 4°C. The cells were then incubated with fetal calf serum (10%) at 4°C for 1h to remove the excess of C6-NBD-ceramide (Pagano, 1989). For staining of the cell wall, the cells were then incubated for 15 minutes with Uvitex 2B (0.1 mg/ml; Polysciences, Warrington, PA), followed by washing with PBS and analysis by fluorescence microscopy. Images were generated in an ApoTome 2 epifluorescence microscope (Carl Zeiss, Germany) and processed with the AxioVision software (Carl Zeiss, Germany).
Virulence assay
Virulence studies were conducted according to a previously described intranasal inhalation infection model (Cox et al., 2000) using eight female BALB/C mice (approximately 6 weeks old) for each strain. Mice were infected with 107 yeast cells suspended in 50 µl of saline and monitored daily. Animal studies were approved by the Federal University of Rio Grande do Sul Ethics Committee. Kaplan–Meier analysis of survival was performed using GraphPad Software.
Phagocytosis of C. neoformans cells by murine macrophages
Murine macrophage-like cells (RAW 264.7 lineage) were obtained from the American Type Culture Collection (ATCC). Cultures were maintained and grown to confluence in 25 cm2 culture flasks containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS) at 37 °C and 5% CO2. The culture medium was replaced with fresh media for further incubation with fluorescein isothiocyanate (FITC, Sigma Aldrich Corp, St Louis, Mo)-labeled C. neoformans yeast cells (Barbosa et al., 2006). Fluorescent yeast cells were prepared by staining with 0.5 mg/ml FITC in PBS (25°C) for 10 min. FITC-labeled C. neoformans were then suspended in DMEM to generate a ratio of 10 fungal cells per macrophage for further incubation at 37°C and 5 % CO2 for 18 h. In some systems, yeast cells were opsonized by treatment with mAb 18B7 (10 µg/ml) for 1 h at 37°C. For negative control we used an isotype-matched irrelevant IgG at the same concentrations used for mAb 18B7. Non-adherent yeast cells were removed by washing with PBS. Fungi-host cell complexes were then treated for 10 min at 25 °C with trypan blue (200 µg/ml) to discriminate between surface-associated and intracellular yeast cells. After removal from the plastic surface with a cell scrapper, the cells were analyzed by flow cytometry as described previously (Barbosa et al., 2006). Control preparations were developed as described above using uninfected cells and non-stained yeast. Alternatively, infected macrophages were lysed with cold water and the remaining suspension was plated onto Sabouraud solid agar for counting of colony forming units (CFU). These values were used for calculation of the survival indices of C. neoformans after interaction with the phagocytes. The survival index was defined as the number of CFUs divided by the fluorescence index of macrophages in each experimental system.
GXM and capsule determination
C. neoformans cells (WT, mutant and reconstituted strains) were placed onto glass slides and mixed with similar volumes of India ink. The suspensions were covered with glass coverslips and analyzed with an Axioplan 2 (Zeiss, Germany) microscope. Images were acquired using a Color View SX digital camera and processed with the software system analySIS (Soft Image System). Capsule sizes, defined as the distances between the cell wall and the outer border of the capsule in India ink stained yeast cells, were determined by using the ImageJ Software (version 1.33), elaborated and provided by National Institutes of Health (NIH, http://rsb.info.nih.gov/ij/). Cell diameters were determined using the same software. Final measurements were presented as ratio of capsule size / cell diameter. Cellular suspensions were analyzed by fluorescence microscopy. The staining reagents used in fluorescence microscopy included calcofluor white, which has been extensively used to stain chitin in fungal cell walls, and the monoclonal antibody (mAb) 18B7, a mouse IgG1 with high affinity for GXM of different cryptococcal serotypes (Casadevall et al., 1998). Yeast cells (106) were suspended in 4% paraformaldehyde cacodylate buffer (0.1 M, pH 7.2) and incubated for 30 min at room temperature. Fixed yeast cells were washed twice in PBS and incubated in 1% bovine serum albumin in PBS (PBS-BSA) for 1 h. The cells were then suspended in 100 µl of a 25 µM calcofluor white solution (Invitrogen, Carlsbad, Ca) for 30 min at 37°C. After washing in PBS, the cells were incubated for 1 h in the presence of mAb 18B7 (1 µg/ml). The cells were finally incubated with a fluorescein isothiocyanate (FITC) labeled goat anti-mouse IgG (Fc specific) antibody (Sigma Aldrich Corp, St Louis, Mo). For negative control we used an isotype-matched irrelevant IgG at the same concentrations used for mAb 18B7. Cell suspensions were mounted over glass slides as described above and analyzed under an Axioplan 2 (Zeiss, Germany) fluorescence microscope. Images were acquired and processed as described above.
Determination of GXM concentration in fungal supernatants and infected tissues
Culture supernatants were obtained as recently described (Fonseca et al., 2010). GXM concentration in fungal supernatants was determined by ELISA with mAb 18B7, using modifications of a previously described protocol for GXM detection (Casadevall et al., 1992, Fonseca et al., 2009). Briefly, 96-Well polystyrene plates were coated with standard GXM, supernatant samples for further blocking with bovine serum albumin. The plates were sequentially incubated with mAb 18B7 and an alkaline phosphatase-conjugated goat anti-mouse IgG1 for 1 h. Reactions were developed after the addition of p-nitrophenyl phosphate disodium hexahydrate, followed by measuring absorbance at 405 nm with a microplate reader (TP-reader, Thermo Plate). Antibody concentration in this assay corresponded to 1 µg/ml. For in vivo determinations, tissue samples were obtained after intranasal infection female BALB/c (n = 10) with 106 yeast cells of WT, grasp or reconstituted C. neoformans cells (Cox et al., 2000). At day 7 post-infection, animals were euthanized and their lungs and brains were aseptically excised. Tissues were then homogenized by maceration in PBS. These suspensions were plated on Sabouraud agar for CFU counting or were processed for GXM determination. For this purpose, suspensions were supplemented with proteinase K (0.2 mg/ml, final concentration) and incubated overnight at 37°C. Samples were then heated for 20 min at 100°C, placed on ice, and centrifuged at 10.000 g. Supernatants were then used in for GXM determination. For the in vivo systems, high backgrounds were observed when samples were analyzed by ELISA (data not shown). We therefore analyzed in vivo samples by dot blotting. Samples were loaded onto nitrocellulose membranes, which were allowed to dry for 1 h at 37°C and then were blocked with PBS containing 5% bovine serum albumin. Blocked membranes were incubated with mAb 18B7 at a starting dilution of 1 µg/ml. After being washed extensively, membranes were sequentially incubated with peroxidase-conjugated goat anti-mouse IgG1 and ortho-phenylene diamine solutions. Reactions were quantified by densitometry with the Scion Image software (version 4.03) and normalized to CFU values and weight of infected tissues.
Analysis of virulence factors
Urease activity and melanin formation, two well defined C. neoformans virulence factors that are related to vesicular secretion (Rodrigues et al., 2008a, Eisenman et al., 2009), were used as prototypes in assays aiming at the evaluation of virulence determinants that are not associated to capsule expression. Urease activity was assayed after growth of WT, mutant and reconstituted strains in Christensen’s agar medium at 30°C for 48h (Cox et al., 2000). This medium contains 300 mM urea and phenol red as a pH indicator. The urease activity is expected to convert urea into ammonia, resulting in an increase in the pH of the medium. This feature is reflected by a color change from yellow to bright pink. An ure1 mutant was used as a negative control. Analysis of melanin production followed the methodology described by Baker and colleagues (Baker et al., 2007). WT, mutant and complemented cells were inoculated in a chemically defined medium containing L-asparagine (1g/L), MgSO4·7H2O (0.5 g/l), KH2PO4 (3g/l) and thiamine (1 mg/l), supplemented with 10 mM L-3,4-dihydroxyphenylalanine (L-DOPA). C. neoformans cells were then cultivated at 30°C. After 24 h intervals, the cultures were centrifuged 1,000 g for 10 min; pellets were photographed for visual analysis of pigmentation and the supernatants were spectrophotometrically analyzed (absorbance 492 nm). Shorter times were also analyzed, but pigmentation was only observed after 24 h in the culture medium (data not shown).
GXM effective diameter
For diameter determination, extracellular GXM was isolated from culture supernatants as previously described by our group (Nimrichter et al., 2007). Yeast cells were cultivated in a minimal medium composed of glucose (15 mM), MgSO4 (10 mM), KH2PO4 (29.4 mM), glycine (13 mM), and thiamine-HCl (3 µM), pH 5.5, for two days at room temperature with shaking and separated from culture supernatants by centrifugation at 4,000 g (15 min, 4°C). The supernatant fluids were collected and again centrifuged at 15,000 g (15 min, 4°C), to remove smaller debris. The pellets were discarded and the resulting supernatant was concentrated approximately 20-fold using an Amicon (Millipore, Danvers, MA) ultrafiltration cell (Nimrichter et al., 2007). After supernatant concentration, the viscous layer formed was collected with a cell scraper and transferred to graduated plastic tubes for GXM determinations. The effective diameter of GXM in these samples was determined by Quasi elastic light scattering in a 90Plus/BI-MAS Multi Angle Particle Sizing analyzer (Brookhaven Instruments Corp., Holtsville, NY), using minor modifications of the method described by Frases and colleagues (Frases et al., 2009). Polysaccharide diameter was modulated by incubation of dialyzed fractions with 1 mM CaCl2 for 1 h at room temperature.
Acknowledgements
MLR, MHV, LN and AS are supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Brazil), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil) and Financiadora de Estudos e Projetos (FINEP, Brazil). AC is supported by NIH grants AI033142, AI033774, AI052733, and HL059842. We thank Priscila C. Albuquerque and Ana Claudia Zimbres for help with handling cultures of fungal mutants. We are thankful to the reviewers for their insightful comments that helped strengthen this article. We are also thankful to Prof. Vivek Malhotra for initial suggestions on the role of GRASP in polysaccharide secretion.
References
- Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, Almeida IC, Nosanchuk JD. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LG, Specht CA, Donlin MJ, Lodge JK. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryotic cell. 2007;6:855–867. doi: 10.1128/EC.00399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FM, Fonseca FL, Holandino C, Alviano CS, Nimrichter L, Rodrigues ML. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 2006;8:493–502. doi: 10.1016/j.micinf.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Cabral M, Anjard C, Malhotra V, Loomis WF, Kuspa A. Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot Cell. 2010;9:1009–1017. doi: 10.1128/EC.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR, Lendvai N, Mukherjee J, Pirofski LA, Rivera J, Rosas AL, Scharff MD, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Mukherjee J, Scharff MD. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17:158–162. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Wickes BL, Kwon-Chung KJ. Further analysis of the CAP59 locus of Cryptococcus neoformans: structure defined by forced expression and description of a new ribosomal protein-encoding gene. Gene. 1995;167:179–183. doi: 10.1016/0378-1119(95)00640-0. [DOI] [PubMed] [Google Scholar]
- Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, Zuo X, Leal AL, Vainstein MH, Meyer W, Sorrell TC, May RC, Djordjevic JT. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol. 2011;80:1088–1101. doi: 10.1111/j.1365-2958.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–448. doi: 10.1128/iai.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TL. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol. 2009;63:223–247. doi: 10.1146/annurev.micro.62.081307.162753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol. 2010;188:527–536. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155:3860–3867. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- Fonseca FL, Frases S, Casadevall A, Fischman-Gompertz O, Nimrichter L, Rodrigues ML. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet Biol. 2009;46:496–505. doi: 10.1016/j.fgb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca FL, Nohara LL, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. Immunomodulatory effects of serotype B glucuronoxylomannan from Cryptococcus gattii correlate with polysaccharide diameter. Infect Immun. 2010;78:3861–3870. doi: 10.1128/IAI.00111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frases S, Nimrichter L, Viana NB, Nakouzi A, Casadevall A. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell. 2008;7:319–327. doi: 10.1128/EC.00378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frases S, Pontes B, Nimrichter L, Viana NB, Rodrigues ML, Casadevall A. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1228–1233. doi: 10.1073/pnas.0808995106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pedrajas MD, Nadal M, Kapa LB, Perlin MH, Andrews DL, Gold SE. DelsGate, a robust and rapid gene deletion construction method. Fungal Genet Biol. 2008;45:379–388. doi: 10.1016/j.fgb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Rivera J, Chang YC, Kwon-Chung KJ, Casadevall A. Cryptococcus neoformans CAP59 (or Cap59p) is involved in the extracellular trafficking of capsular glucuronoxylomannan. Eukaryot Cell. 2004;3:385–392. doi: 10.1128/EC.3.2.385-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Steen BR, Lian T, Sham AP, Tam N, Tangen KL, Kronstad JW. Transcriptional regulation by protein kinase A in Cryptococcus neoformans. PLoS Pathog. 2007;3:e42. doi: 10.1371/journal.ppat.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 2007;130:524–534. doi: 10.1016/j.cell.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Kmetzsch L, Staats CC, Simon E, Fonseca FL, de Oliveira DL, zSobrino L, Rodrigues J, Leal AL, Nimrichter L, Rodrigues ML, Schrank A, Vainstein MH. The vacuolar Ca(2)(+) exchanger Vcx1 is involved in calcineurin-dependent Ca(2)(+) tolerance and virulence in Cryptococcus neoformans. Eukaryot Cell. 2010;9:1798–1805. doi: 10.1128/EC.00114-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L, Staats CC, Simon E, Fonseca FL, Oliveira DL, Joffe LS, Rodrigues J, Lourenco RF, Gomes SL, Nimrichter L, Rodrigues ML, Schrank A, Vainstein MH. The GATA-type transcriptional activator Gat1 regulates nitrogen uptake and metabolism in the human pathogen Cryptococcus neoformans. Fungal Genet Biol. 2011;48:192–199. doi: 10.1016/j.fgb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Levi SK, Glick BS. GRASPing unconventional secretion. Cell. 2007;130:407–409. doi: 10.1016/j.cell.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Levine TP, Wiggins CA, Munro S. Inositol phosphorylceramide synthase is located in the Golgi apparatus of Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:2267–2281. doi: 10.1091/mbc.11.7.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Mody CH. Cryptococcus. Proc Am Thorac Soc. 2010;7:186–196. doi: 10.1513/pats.200907-063AL. [DOI] [PubMed] [Google Scholar]
- Manjithaya R, Anjard C, Loomis WF, Subramani S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol. 2010;188:537–546. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland EE, Bernhardt P, Casadevall A. Coping with multiple virulence factors: which is most important? PLoS Pathog. 2005;1:e40. doi: 10.1371/journal.ppat.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- Nickel W, Seedorf M. Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol. 2008;24:287–308. doi: 10.1146/annurev.cellbio.24.110707.175320. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Frases S, Cinelli LP, Viana NB, Nakouzi A, Travassos LR, Casadevall A, Rodrigues ML. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell. 2007;6:1400–1410. doi: 10.1128/EC.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk JD, Nimrichter L, Casadevall A, Rodrigues ML. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun Integr Biol. 2008;1:37–39. doi: 10.4161/cib.1.1.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. Biogenesis of extracellular vesicles in yeast: Many questions with few answers. Commun Integr Biol. 2010a;3:533–535. doi: 10.4161/cib.3.6.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One. 2010b;5:e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Nimrichter L, Miranda K, Frases S, Faull KF, Casadevall A, Rodrigues ML. Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan. Fungal Genet Biol. 2009;46:956–963. doi: 10.1016/j.fgb.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano RE. A fluorescent derivative of ceramide: physical properties and use in studying the Golgi apparatus of animal cells. Methods Cell Biol. 1989;29:75–85. doi: 10.1016/s0091-679x(08)60188-0. [DOI] [PubMed] [Google Scholar]
- Pagano RE, Sepanski MA, Martin OC. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, Williamson PR. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol Microbiol. 2009;71:1165–1176. doi: 10.1111/j.1365-2958.2008.06588.x. [DOI] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz. 2009;104:513–521. doi: 10.1590/s0074-02762009000300019. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008a;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. Vesicular Trans-Cell Wall Transport in Fungi: A Mechanism for the Delivery of Virulence-Associated Macromolecules? Lipid Insights. 2008b;2008:27. doi: 10.4137/lpi.s1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotman H, Karhinen L, Rabouille C. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev Cell. 2008;14:171–182. doi: 10.1016/j.devcel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Seider K, Heyken A, Luttich A, Miramon P, Hube B. Interaction of pathogenic yeasts with phagocytes: survival, persistence and escape. Curr Opin Microbiol. 2010;13:392–400. doi: 10.1016/j.mib.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Sommer U, Liu H, Doering TL. An alpha-1,3-mannosyltransferase of Cryptococcus neoformans. J Biol Chem. 2003;278:47724–47730. doi: 10.1074/jbc.M307223200. [DOI] [PubMed] [Google Scholar]
- Takeo K, Uesaka I, Uehira K, Nishiura M. Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. J Bacteriol. 1973a;113:1442–1448. doi: 10.1128/jb.113.3.1442-1448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo K, Uesaka I, Uehira K, Nishiura M. Fine structure of Cryptococcus neoformans grown in vivo as observed by freeze-etching. J Bacteriol. 1973b;113:1449–1454. doi: 10.1128/jb.113.3.1449-1454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, Freymuller-Haapalainen E, Sinigaglia-Coimbra R, Almeida IC, Puccia R. The Pathogenic Fungus Paracoccidioides brasiliensis Exports Extracellular Vesicles Containing Highly Immunogenic {alpha}-Galactosyl Epitopes. Eukaryot Cell. 2010;10:343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinke FP, Grieve AG, Rabouille C. The multiple facets of the Golgi reassembly stacking proteins. Biochem J. 2011;433:423–433. doi: 10.1042/BJ20101540. [DOI] [PubMed] [Google Scholar]
- Yoneda A, Doering TL. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol Biol Cell. 2006;17:5131–5140. doi: 10.1091/mbc.E06-08-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Telzak A, Bryan RA, Dadachova E, Casadevall A. The polysaccharide capsule of the pathogenic fungus Cryptococcus neoformans enlarges by distal growth and is rearranged during budding. Mol Microbiol. 2006;59:67–83. doi: 10.1111/j.1365-2958.2005.04928.x. [DOI] [PubMed] [Google Scholar]