SUMMARY
The nature of follicular helper CD4+ T (Tfh) cell differentiation remains controversial, including the minimal signals required for Tfh differentiation, and the time at which Tfh differentiation occurs. Here we determine that Tfh development initiates immediately during dendritic cell (DC) priming in vivo. We demonstrate that inducible costimulator (ICOS) provides a critical early signal to induce the transcription factor Bcl6, and Bcl6 then induces CXCR5, the canonical feature of Tfh cells. Strikingly, a bifurcation between Tfh and effector Th cells was measurable by the second cell division of CD4+ T cells, at day 2 after an acute viral infection: IL2Rαint cells expressed Bcl6 and CXCR5 (Tfh cell program), whereas IL2Rαhi cells exhibited strong Blimp1 expression that repressed Bcl6 (effector Th cell program). Virtually complete polarization between Bcl6+ Tfh cells and Blimp1+ effector Th cell populations developed by 72 hours, even without B cells. Tfh cells were subsequently lost in the absence of B cells, demonstrating a B cell requirement for maintenance of Bcl6 and Tfh cell commitment via sequential ICOS signals.
Keywords: Follicular helper T (Tfh) cells, ICOS, CD25, Bcl6, Blimp-1, dendritic cells (DCs)
INTRODUCTION
Naïve CD4+ T cells differentiate into different effector cells and elicit various immunological effector functions, such as clearing viruses (Th1 cells), helminths (Th2 cells), and fungi (Th17 cells) or suppressing immune responses (iTreg cells) (Zhu et al., 2010). Follicular helper (Tfh) T cells are the CD4+ T cells specialized in B cell help (Crotty, 2011). Tfh cells express the B cell homing chemokine receptor, CXCR5, among other molecules important for their differentiation and function. CXCR5 surface expression not only phenotypically distinguishes Tfh cells from other effector CD4+ T cells but functionally drives Tfh cell migration into B cell follicles in a CXCL13 dependent manner. T-cell help to B cells is a pivotal process of adaptive immune responses. Tfh cells first interact with cognate B cells at the T-B border and subsequently induce germinal center B cell differentiation and germinal center formation within the follicle (Allen et al., 2007). Tfh cells have a gene expression profile that is distinct from Th1, Th2, Th17 and iTreg cells (Chtanova et al., 2004; Nurieva et al., 2008; Rasheed et al., 2006; Vinuesa et al., 2005; Yusuf et al., 2010), and is particularly enriched for cell surface molecules[JS1], reflecting the importance of cell-cell interactions between Tfh cells and B cells for Tfh cell functions. The Tfh cell program in both mice and humans is associated with high expression of CXCR5, PD-1, ICOS, BTLA, IL-21, SAP, and Bcl6 (Crotty, 2011).
Effector CD4+ T cell differentiation is controlled by specific transcription factors (Murphy and Stockinger, 2010; Zhu et al., 2010). We and others recently identified a transcriptional repressor, Bcl6, as a critical regulator of Tfh differentiation (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). Bcl6 is a BTB domain containing transcriptional repressor previously known to be required in B cells for germinal center B cell differentiation, and Bcl6 is an important oncogene in germinal center B cell derived lymphomas (Ci et al., 2008; Klein and Dalla-Favera, 2008). Bcl6-deficient CD4+ T cells are not able to differentiate into Tfh cells in vivo (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009), whereas constitutive Bcl6 expression induced CD4+ T cell differentiation into Tfh cells (Johnston et al., 2009). These results demonstrated that not only is Bcl6 expression required in B cells for germinal center development, it is also simultaneously essential in CD4+ T cells, and thus Bcl6 must regulate complementary gene expression programs in two different lymphocyte cell types differentiating in parallel. One of the functions of Bcl6 in Tfh cell differentiation is to regulate miRNA expression. Bcl6 downregulated miRNA clusters that were shown to partially inhibit CXCR5 in B cells (Yu et al., 2009). Bcl6 can repress expression or function of transcription factors necessary for other CD4+ T cell differentiation pathways, such as RORγt (Nurieva et al., 2009). Bcl6 also directly represses the transcription factor Blimp1 (encoded by Prdm1) (Johnston et al., 2009; Tunyaplin et al., 2004). All of these data demonstrate that Bcl6 is necessary and sufficient for Tfh cell differentiation by CD4+ T cells. Therefore, a deeper understanding of the regulation of Bcl6 expression is essential to understand Tfh cell differentiation.
Different models have been proposed to explain Tfh cell differentiation, which hinge on different Bcl6 expression kinetics and mechanisms of induction. One model proposed is cytokine-dependent Tfh cell differentiation. Th1 and Th2 CD4+ T cell differentiation can be achieved in vitro by providing naïve CD4+ T cells with activation signals through the TCR and appropriate cytokine receptors (Zhu et al., 2010). Analogously, it was proposed that Tfh cells can be differentiated in vitro by IL-6 or IL-21. Both Bcl6 mRNA and CXCR5 mRNA were upregulated in the presence of IL-6 or IL-21 (Nurieva et al., 2008; Nurieva et al., 2009). However, this simple model has been challenged by studies that found normal Tfh cell differentiation in vivo in the absence of IL-6 or IL-21 (Poholek et al., 2010; Zotos et al., 2010), though partial defects were found in other studies (Linterman et al., 2010; Vogelzang et al., 2008), possibly due to reduced maintenance of Tfh cells (Linterman et al., 2010). There have been conflicting reports regarding cytokine induction of Bcl6 and CXCR5 in vitro (Dienz et al., 2009; Eddahri et al., 2009). In our studies, limited Bcl6 and CXCR5 mRNA was induced by in vitro purified CD4+ T cells cultured in the presence of TCR stimulation plus IL-6 or IL-21, and no substantial expression of Bcl6 protein or CXCR5 protein was found (Eto et al., 2011). Cytokines alone appear to be insufficient for Tfh cell differentiation.
Other models have focused on the importance of B cell dependent Tfh cell differentiation (Crotty et al., 2010). This model is supported by the observation that Tfh cell differentiation was severely defective in B cell deficient mice (µMT) or cognate B cell deficient mice (MD4-µMT) after protein immunization, viral infection, or parasite infection (Haynes et al., 2007; Johnston et al., 2009; Zaretsky et al., 2009). B cell-dependent Bcl6 induction for Tfh cell differentiation was further evidenced by rescuing Tfh cell differentiation in µMT mice by constitutive expression of Bcl6 in antigen-specific CD4+ T cells (Johnston et al., 2009). B cell dependent Tfh cell differentiation is, however, challenged by a recent study that found Tfh cells could develop in mice deficient for MHCII only on B cells if mice were given repeated Ag injection (Deenick et al., 2010). The interdependence of Tfh cell and GC B cell differentiation, both of which require Bcl6, adds an additional layer of complexity to assessing the cell autonomy of Tfh cell defects.
An alternative model has proposed that Tfh cell differentiation is not an independent developmental pathway, but instead Tfh cells are possibly a subsequent state of Th1, Th2 and Th17 cells (Awasthi and Kuchroo, 2009; Bauquet et al., 2009; Murphy and Stockinger, 2010; Zaretsky et al., 2009). Several studies have shown that Tfh cells can exhibit features of Th1, Th2 and Th17 cells (Bauquet et al., 2009; Fazilleau et al., 2009; Johnston et al., 2009; Reinhardt et al., 2009; Zaretsky et al., 2009). Indeed, such cytokine production is important for appropriate induction of B cell class switch recombination. Nevertheless, Bcl6 is capable of repressing Th1, Th2, and Th17 cells programs (Nurieva et al., 2009), and Tfh cells generally express low amounts of Th1, Th2, or Th17 cells associated cytokines and transcription factors, particularly in humans (Breitfeld et al., 2000).
Given that Bcl6 is a critical regulator for Tfh differentiation, it is crucial to understand the regulation of Bcl6 induction in CD4+ T cells. Therefore, in this study, we probed the differentiation of CD4+ T cells in vivo to determine molecular and cellular cues for Bcl6 expression, and the kinetics of Tfh cell differentiation. We demonstrate that Tfh cell differentiation occurs early after viral infection, as an independent differentiation program that is dependent on ICOS signals during DC priming, independent of B cells. We further show that an APC transition then occurs from DC to B cells and ICOS signaling is again required a second time for maintenance of Bcl6 and Tfh cells. These results allow for the development of an integrated model of Tfh cell differentiation.
RESULTS
Early development of Bcl6+CXCR5+ Tfh cells in vivo
Tfh cells are readily identifiable at the peak of the CD4+ T cell response to an acute LCMV infection as CXCR5hiSLAMloBTLAhiPD1hiBcl6+ virus-specific CD4+ T cells. Equivalent results are observed with either LCMV gp-specific TCR transgenic CD4+ T cells (“SM” SMARTA) (Figure 1A) or polyclonal CD4+ T cells (Figure S1). Tfh (CXCR5+) antiviral CD4+ T cells at the peak of the immune response express Bcl6 (p = 0.0002, Figure 1B).
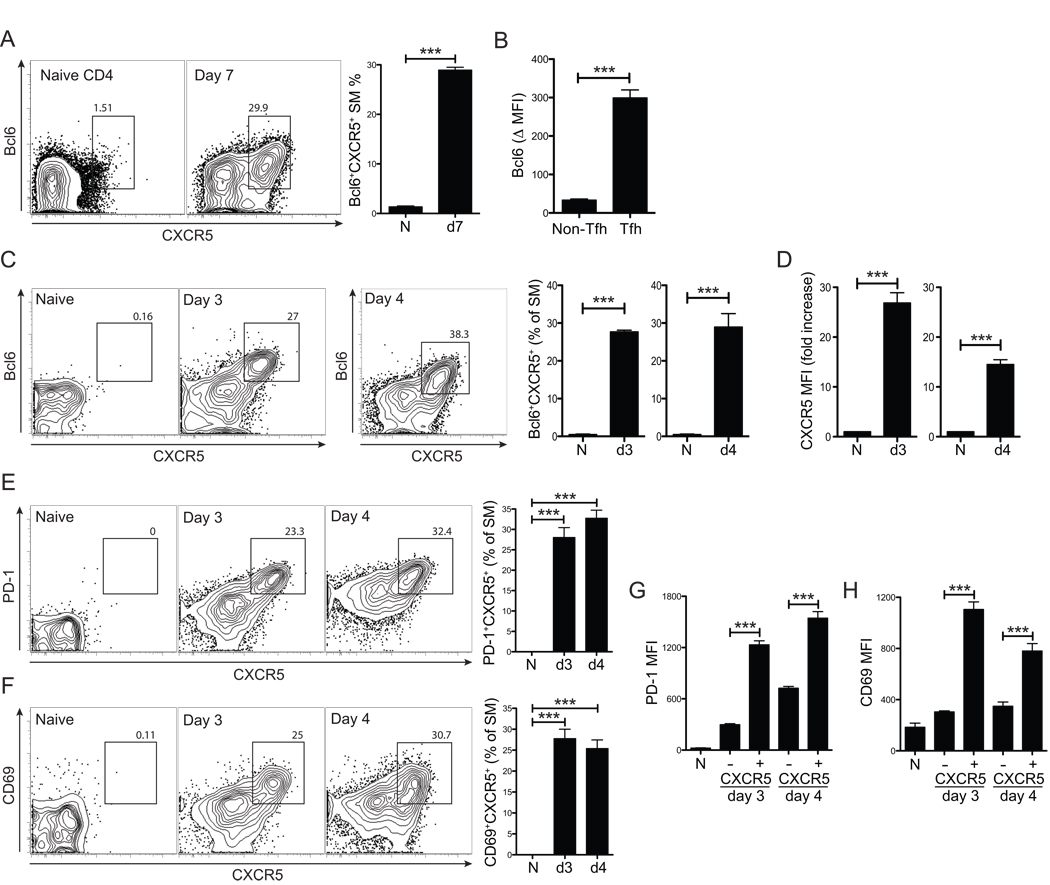
Figure 1. Bcl6+CXCR5+ Tfh cell differentiation occurs within 72 hours in vivo.
(A–D) LCMV-specific SM CD4+ T cells were transferred into B6 mice that were subsequently infected with LCMV. (A) Bcl6+CXCR5+ Tfh cells analyzed 7 days post-infection (p.i.) (“d7”) vs. naïve (“N”, CD44loCD62hi) CD4+ T cells of uninfected control mice. (B) Bcl6 protein expression in d7 SM as Δ MFI (e.g. Bcl6 MFITfh - Bcl6 MFInaiveCD4). (C) Bcl6+CXCR5+ Tfh cells, 3 and 4 days p.i. (“d3”, “d4”). “N”, uninfected mice. FACS plot shows SM CD4+ T cells. (D) Increase in SM CXCR5 MFI compared to that of naive (CD44loCD62hi) CD4+ T cells of uninfected mice. (E–H) Day 3 and 4 after LCMV infection, PD-1 (E) and CD69 (F) co-expression with CXCR5 on SM CD4+ T cells. (G) PD-1 and (H) CD69 MFIs of CXCR5+ SM vs. CXCR5− SM. Data are representative of three or more independent experiments; n = 4 per time point for all panels. *** P<0.001.
To probe the molecular and cellular requirements of Tfh cell differentiation, we examined the kinetics of Tfh cell development in vivo, with a particular focus on when Bcl6 protein expression was induced, as Bcl6 is required for Tfh cell differentiation and expression of Bcl6 is sufficient to induce Tfh cell differentiation. SM CD4+ T cells were transferred into B6 mice and analyzed 3 and 4 days after LCMV infection. Strikingly, robust numbers of Bcl6+CXCR5+ CD4+ T cells were present at day 3 (Figure 1C). Surface CXCR5 expression was induced 15 to 30-fold higher on Bcl6+ cells than naïve CD4+ T cells (p = 0.0001, day 3. p = 0.0005, day 4. Figure 1D). Tfh cells are Bcl6+CXCR5+ CD4+ T cells. As PD-1 is a third canonical Tfh cell marker (Chtanova et al., 2004; Haynes et al., 2007; Linterman et al., 2009), we examined PD-1 expression. Day 3 Bcl6+CXCR5+ SM expressed highly elevated PD-1 compared to the CXCR5− SM population (CXCR5+ vs. CXCR5− PD-1 MFI, p = 4.0×10−5. Figure 1E and 1G). Tfh cell differentiation was further evidenced by analysis of ICOS expression, which is highly expressed on Tfh cells in humans and mice (Breitfeld et al., 2000; Vinuesa et al., 2005). Indeed, day 3 CXCR5+ SM expressed significantly more ICOS (p = 0.0005 vs. CXCR5− SM, Figure S1D). Interestingly, early Tfh cells also expressed more CD69 than effector Th antiviral CD4+ T cells (CXCR5+ vs. CXCR5− CD69 MFI, p = 0.0001. Figure 1F and 1H). CD69 inhibits S1P1-dependent egress of T cells from lymphoid tissues (Pham et al., 2008) and likely enhances retention of Tfh cells in and near B cell follicles (Fazilleau et al., 2007). Taken together, our Bcl6 data indicated that the Tfh developmental program commences early during CD4+ T cell priming in vivo.
DC priming: SAP, CD40, and B cell-independent Tfh cell commitment
Tfh cells are crucial for the development of germinal centers, from which long-lived plasma cells and memory B cells are predominantly generated (Allen et al., 2007; Good-Jacobson et al., 2010). Germinal center formation requires SLAM-associated protein (SAP, encoded by Sh2d1a) expression in CD4+ T cells (Crotty et al., 2003; Qi et al., 2008). SAP is an intracellular signaling molecule that binds to members of the SLAM family of receptors, which are prominently expressed on T and B cells (Cannons et al., 2011). SAP deficiency in CD4+ T cells severely impairs the duration of cognate interactions with B cells, whereas the absence of SAP does not affect CD4+ T cell-dendritic cell (DC) interactions (Cannons et al., 2010; Qi et al., 2008). We therefore examined whether SAP has a role in early Tfh cell differentiation in vivo. Sh2d1a−/− SM CD4+ T cells were transferred into C57BL/6 mice (B6) and analyzed for Tfh cell differentiation after acute LCMV infection. Both CXCR5 induction and Bcl6+CXCR5+ Tfh cell development (Figure 2A) by Sh2d1a−/− SM CD4+ T cells were normal at day 3 and day 4 in vivo. Thus, Bcl6 expression and Tfh cell differentiation is induced in CD4+ T cells prior to sustained B cell - T cell interactions.
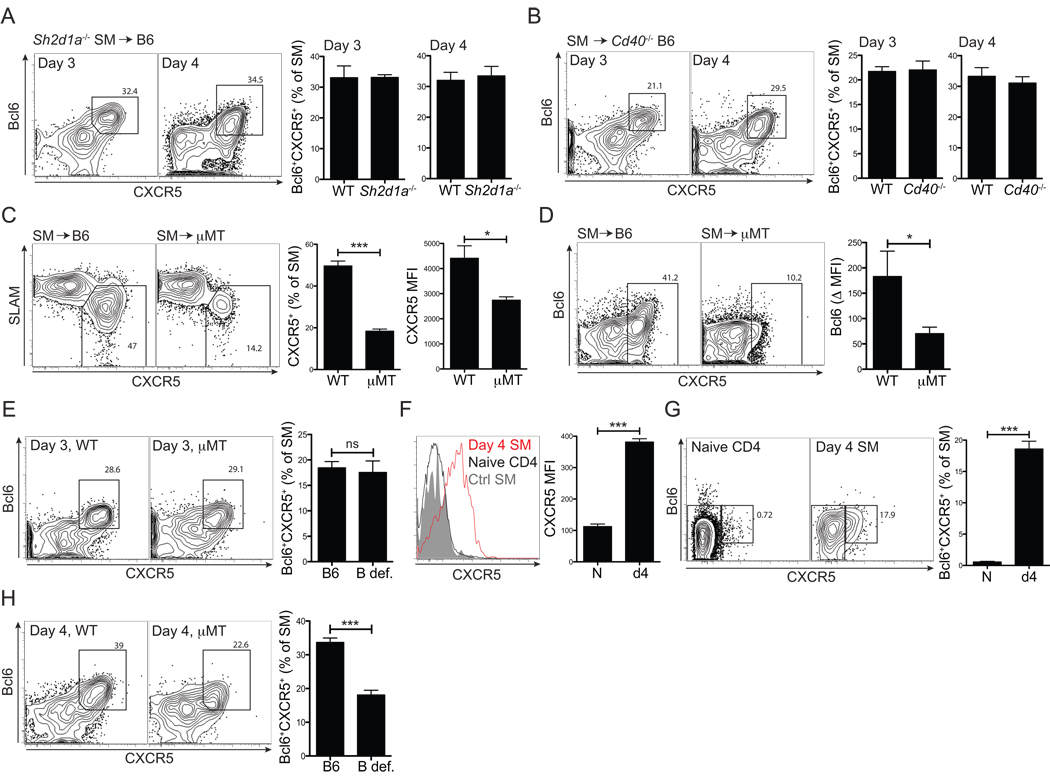
Figure 2. Early Bcl6+CXCR5+ Tfh cell differentiation is B cell-independent and does not require SAP- or CD40L–mediated signals.
(A) Naïve Sh2d1a−/− or WT SM CD4+ T cells were transferred into B6 mice subsequently infected with LCMV. Bcl6+CXCR5+ Tfh cell differentiation analyzed at 3 and 4 days p.i. FACS plots show Sh2d1a−/− SM and gates identify Bcl6+CXCR5+ Tfh cells. (B) WT SM CD4+ T cells were transferred into either B6 or Cd40−/− mice subsequently infected with LCMV. Tfh cell differentiation was analyzed as shown in (A). (C, D) SM CD4+ T cells were transferred into B6 or B cell-deficient (µMT) mice, infected with LCMV, and the CD4+ T cell response was analyzed 7 days later. (C) SM CD4+ T cells are shown. Gate indicates SLAMloCXCR5hi Tfh cells. (D) SM cells are shown and gates identify Bcl6+CXCR5+ Tfh cells. Bcl6 protein calculated Δ MFI (e.g., Bcl6 MFITfh - Bcl6 MFInaiveCD4). (E, H) SM CD4+ T cells were transferred into B6 and µMT (or MD4-µMT. B def.) mice. Mice were then infected with LCMV and analyzed 3 (E) and 4 (H) days p.i. FACS plots show total SM cells, and gates identify Bcl6+CXCR5+ Tfh cells. Data are a composite of 3 or more independent experiments (n=15–16 per group for day 3, n=17–20 per group for day 4). (F, G) LCMV Gp61 peptide pulsed DCs were activated in vitro with LPS and transferred into B6 mice that had received naïve SM CD4+ T cells. SM were analyzed 4 days after DC immunization. (F)Left, SM CD4+ T cells in DC immunized (red line) vs. unimmunized mice (gray filled) compared to that of endogenous naïve (CD44lo) CD4+ T cells in DC immunized mice (black line). Right, CXCR5 MFIs calculated. Day 4 SM (“day4”) vs. SM in unimmunized mice (“N”). (G) Bcl6+CXCR5+ Tfh cells. (A-D, F and G) Data are representative of two independent experiments. n = 4 per group. n.s., no statistically significant difference (P>>0.05). *P<0.05, ***P<0.001.
CD40 is also required for germinal center development (MacLennan, 1994) and is prominently expressed on B cells. Therefore we analyzed whether CD40L signals direct early Tfh development. We tested the early Tfh cell commitment of SM CD4+ T cells in Cd40−/− mice. Robust Bcl6 induction was observed in the absence of CD40, and the frequency of Bcl6+CXCR5+ CD4+ T cells was normal (Figure 2B). However, lack of CD40 results in a severe truncation of the B cell response by day 8 (Figure S2A) and resulted in a loss of Tfh cells (Figure S2B).
The finding of early Bcl6 induction revealed a conundrum, as Tfh cells were previously demonstrated to require the presence of cognate B cells in the work of our lab (Johnston et al., 2009) and others (Haynes et al., 2007; Zaretsky et al., 2009). We have confirmed those earlier results (WT vs. µMT Tfh = 50% vs. 18%. P = 1.8×10−5, Figure 2C), and determined that Bcl6 protein expression by CD4+ T cells was severely reduced at the peak of the antiviral CD4+ T cell response in the absence of B cells (p = 0.014, Figure 2D). This led us to directly examine whether B cells were required for early Tfh cell differentiation. Clear Bcl6 induction and Bcl6+CXCR5+ Tfh cell differentiation was present in the absence of total (µMT) or cognate B cells (MD4-µMT) at day 3 in vivo (B def., Figure 2E). Thus, early Tfh cell differentiation is B cell independent.
Given that Tfh cell differentiation initiates early and is B cell independent, DCs were implicated. To determine whether DCs could directly induce Tfh cell differentiation, we conducted in vivo experiments using peptide pulsed DCs. Indeed, DC immunization was sufficient to induce CXCR5 expression (p = 3.2×10−5, Figure 2F) and Bcl6 expression (p = 0.0001, Figure 2G) by CD4+ T cells. This was not due to transfer of antigen from DCs to B cells, as comparable CXCR5 induction was observed in DC-immunized H2−/− recipients (Figure S2C–D). These results strongly indicated that DC priming is sufficient to induce Bcl6 expression and initial Tfh cell differentiation.
B cell-independent Tfh cell differentiation led us to analyze whether Tfh cells require sequential antigen presentation by DCs and B cells, with early Tfh cell commitment being instructed by DCs and Tfh cell maintenance determined by B cells. Whereas normal Bcl6+CXCR5+ Tfh cell differentiation was present in the absence of cognate B cells (µMT or MD4-µMT mice) at day 3 p.i. (Figure 2E), the frequency of Bcl6+CXCR5+ CD4+ T cells was significantly reduced at day 4 (p = 1.79×10−9, Figure 2H), which implied that a major APC transition from DCs to B cells occurs for CD4+ T cells approximately three to four days after an acute infection. This is consistent with a recent report that Tfh cells were present in the absence of B cells when mice were given continuous antigen via peptide injection (Deenick et al., 2010). Our data thus far indicate that early induction of Bcl6 and Tfh cell development occurs during interaction with DCs and is independent of SAP- or CD40-mediated signals, whereas maintenance of Bcl6 expression and stable commitment to Tfh cell differentiation normally requires additional interactions with cognate B cells.
ICOS instructs Tfh cell differentiation
These observations led us to explore DC signals that could initiate Bcl6 induction and instruct Tfh cell differentiation. Interest in early signals controlling Tfh cell differentiation led us to examine ICOS signaling. Reduced numbers of Tfh cells are observed in the absence of ICOS signals (Akiba et al., 2005; Gigoux et al., 2009). However, it has not been addressed whether ICOS-mediated signals are required for Bcl6 induction and initiation of Tfh cell differentiation. To determine this, we analyzed whether Icos−/− SM CD4+ T cells undergo early Tfh cell differentiation in vivo. Strikingly, Bcl6 induction was severely reduced in the absence of ICOS (p = 7.8×10−5 at day 3, p = 0.00093 at day 4. Figure 3A – C). CD4+ T cell activation and proliferation were not affected by the absence of ICOS (Figure S3A–D).
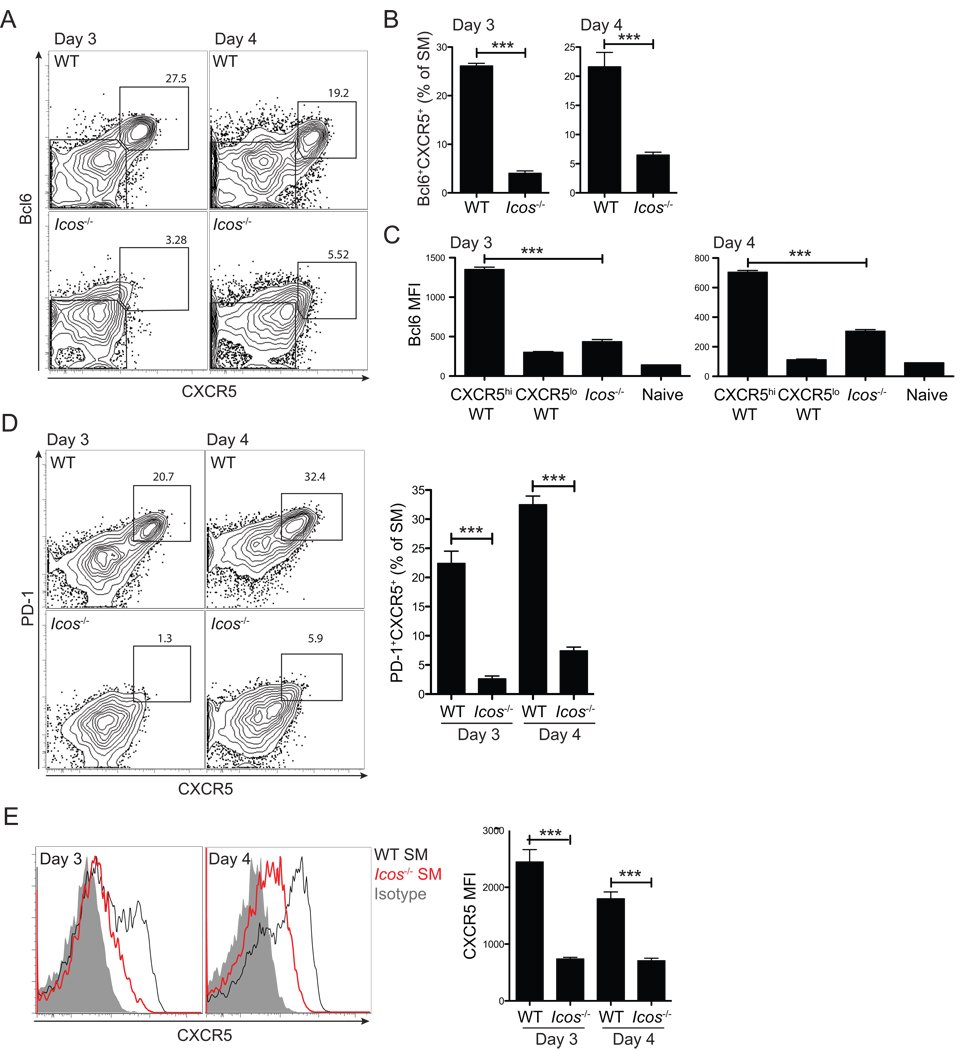
Figure 3. Early Tfh cell commitment requires ICOS signals for Bcl6 expression.
WT or Icos−/− SM CD4+ T cells were transferred into B6 mice subsequently infected with LCMV. (A, B) Bcl6+CXCR5+ Tfh cell development was analyzed 3 and 4 days p.i. by FACS (A) and quantified (B). (C) Bcl6 protein expression was quantified in WT CXCR5+ SM and WT CXCR5 SM vs. Icos−/− SM CD4+ T cells. (D) PD-1 expression on WT and Icos−/− SM CD4+ T cells. (E)Left, CXCR5 expression on WT (black) and Icos−/− (red) SM CD4+ T cells, compared to isotype control (gray filled). Right, CXCR5 MFIs. Data are representative of three independent experiments; n = 4 per group. ***P<0.001.
Icos−/− SM failed to differentiate to Tfh cells, with near background Tfh cell frequencies at day 3 (p = 0.0001, Figure 3D). Bcl6hi Icos−/− SM CD4+ T cells were absent (Figure S4F). At day 4, a similarly severe loss of Icos−/− Tfh cells was seen (p = 4.65×10−6, Figure 3D). The rare Icos−/− Tfh cells remaining had aberrantly low levels of Bcl6 (p = 3.95×10−7, Figure 3C) and CXCR5 (p = 7.4×10−7, Figure 3E and Figure S3E). Thus, ICOS-mediated signals are crucial for Bcl6 induction during CD4+ T cell priming for initiation of Tfh cell differentiation.
Sequential ICOS requirements for Tfh cells and germinal centers
Given the early Tfh defect of Icos−/− CD4+ T cells, we then analyzed the impact of the Tfh cell defect on the magnitude of the germinal center B cell response in Icos−/− mice after an acute VACV infection. Germinal centers were impaired at day 8 in Icos−/− mice compared to WT controls (p = 0.0004, Figure 4A). This was consistent with severe germinal center defects reported in ICOS-deficient mice in response to non-replicating antigens (Akiba et al., 2005; McAdam et al., 2001; Tafuri et al., 2001). CD4+ T cell activation was normal (CD44hiCD62Llo CD4+ T cell frequency; 24.0 ± 2.3 % WT vs. 26.3 ± 7.3% ICOS−/−). The defective germinal centers after VACV infection were likely a direct consequence of the reduced availability of T cell help, as Tfh cell numbers were significantly reduced in Icos−/− mice at day 8 after VACV infection (p = 0.002, Figure 4B). Remaining Icos−/− Tfh cells had significantly reduced CXCR5 expression (p = 0.0015, Figure 4B). Two populations of Tfh cells can be distinguished phenotypically in vivo (Haynes et al., 2007; Yusuf et al., 2010). Tfh cells physically within germinal centers (GC Tfh) have the highest PD-1 expression (Haynes et al., 2007) and can be distinguished on the basis of GL7 expression in mice with an acute viral infection (Yusuf et al., 2010). Generation of GC Tfh cells was impaired in the absence of ICOS-mediated signals in mice 8 days post-infection (p = 0.042, Figure 4C). Defective Tfh cell differentiation and CXCR5 induction were well correlated with a loss of Bcl6 expression by the Icos−/− CD4+ T cells (p = 0.0004, Figure 4D). Icos−/− Tfh cell and GC Tfh cell defects were even more severe by day 12 after VACV infection (Figure 4E–G). Icos−/− Tfh cell frequencies were merely 27% of normal (p = 4.2×10−6, Figure 4E) and Icos−/− GC Tfh cells were barely detectable (p = 0.0001, Figure 4F). As a result, Icos−/− germinal centers decayed rapidly, with a 95% loss of GC B cells at day 12 after VACV infection (p = 0.0005, Figure 4G and Figure S4A). Similar defects were found after LCMV infection of Icos−/− mice (Figure S4B–D).
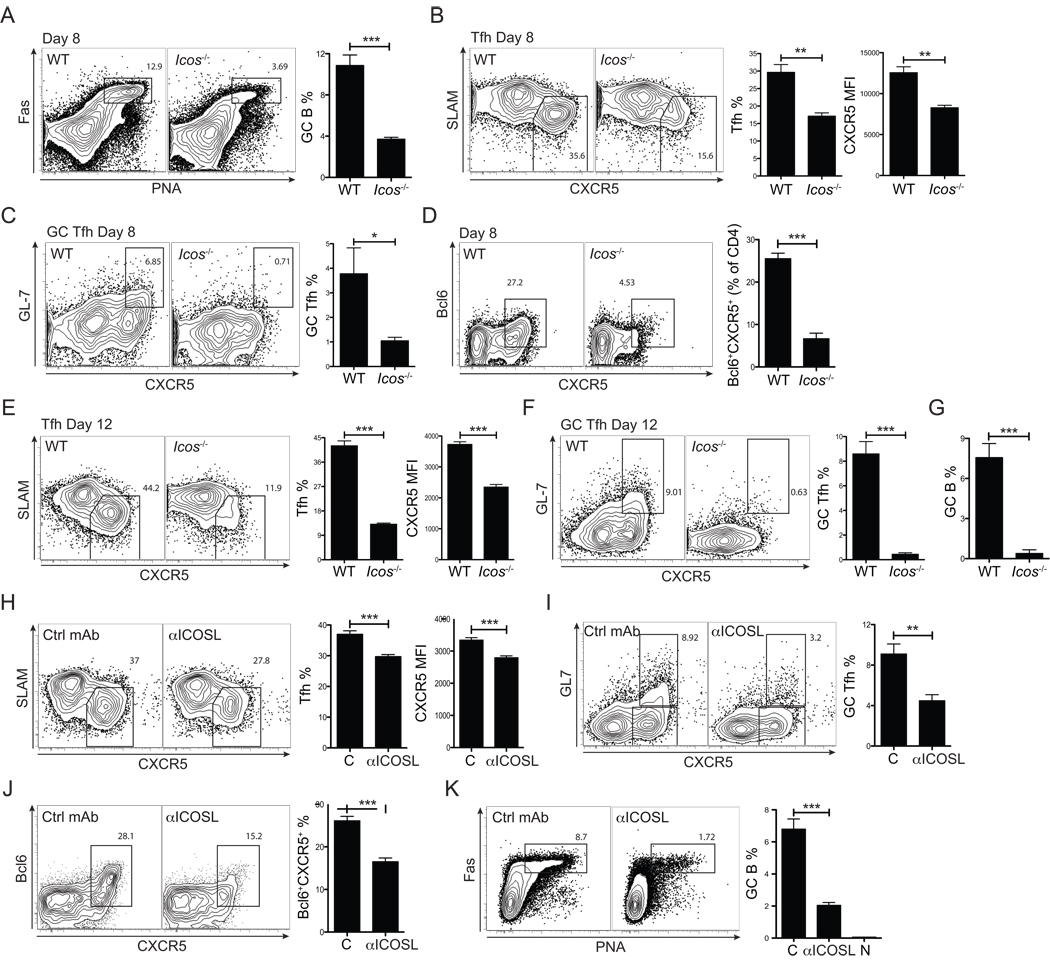
Figure 4. ICOS-dependent Tfh cell maintenance and germinal center formation.
B6 and Icos−/− mice were infected with vaccinia virus (VACV). Mice were analyzed 8 days p.i. (A–D) and 12 days p.i. (E–G). (A) Representative FACS plots of germinal center B cells (Fas+PNA+). Total B220+ B cells are shown. Right, GC B cells were quantified as % of total B cells. (B) Representative FACS plots of WT and Icos−/− polyclonal Tfh cells. Activated CD4+ T cells (CD44hiCD62Llo) are shown and gates identify SLAMloCXCR5+ Tfh cells. Tfh cell frequencies (% of activated CD4+ T cells (CD44hiCD62Llo)) and CXCR5 MFIs were quantified. (C) Representative FACS plots of WT and Icos−/− polyclonal GC Tfh cells. Activated CD4+ T cells (CD44hiCD62Llo) are shown and gates identify GL7+CXCR5hi GC Tfh cells. GC Tfh cell frequencies were quantified (% of activated CD4+ T cells (CD44hiCD62Llo)). (D) Representative FACS plots of Bcl6 expression by CD4+ T cells in B6 and Icos−/− mice. Bcl6+CXCR5+ Tfh cells were quantified (% of activated CD4+ T cells). Tfh cells (E), GC Tfh cells (F), and germinal center B cells (G) were analyzed 12 days p.i., as for day 8 (A–C). Data are representative of two independent experiments for day 8 and day 12; n = 4 per group. (H–K) B6 mice were infected with VACV and treated with anti-ICOSL (αICOSL) or isotype control mAb at day 3, 5, and 7 as schematically shown in Figure S4E. Tfh cells (H), GC Tfh cells (I), Bcl6 expression (J), and GC B cells (K) were analyzed 8 days p.i. “C” = isotype control mAb treated mice. “αICOSL” = anti-ICOSL treated mice. “N” = naïve uninfected mice. n = 5–6 per group. Data are representative of two independent experiments. *P<0.05, **P<0.01, ***P<0.001.
Experiments above show a requirement for ICOS early during DC priming. DCs and B cells both express ICOSL, the ligand for ICOS. Icosl−/− mice were unable to support Tfh cell differentiation, as wild type SM cells transferred into Icosl−/− recipients failed to develop a Bcl6hi population (data not shown). A major APC transition from DCs to B cells occurs at 3–4 days p.i. (Figure 2). Therefore, we explored whether ICOSL signals were required both early and late during Tfh cell differentiation. ICOS signals after CD4+ cells priming were blocked by in vivo treatment with anti-ICOSL (αICOSL) starting at day 3 p.i. (Figure S4E). ICOSL blockade after priming resulted in a significant loss of Tfh cells (~ 25%, p = 0.0004. Figure 4H), and GC Tfh cells (~ 50%, p = 0.003. Figure 4I) by day 8 p.i. Importantly, Bcl6 expressing CD4+ T cells were substantially reduced (p = 0.0001, Figure 4J). Germinal centers were severely reduced (p = 0.005, Figure 4K), whereas overall B cells and activated CD4+ T cell numbers were largely unaffected (data not shown). Taken together, these data demonstrate there are two independent Tfh cell requirements for ICOS during sequential interactions with different APCs. ICOSL is first required on DCs for Tfh cell induction and ICOSL is then required again during T - B interactions for maintenance of Bcl6 expression.
ICOS induction of Bcl6 leads to Bcl6 induction of CXCR5
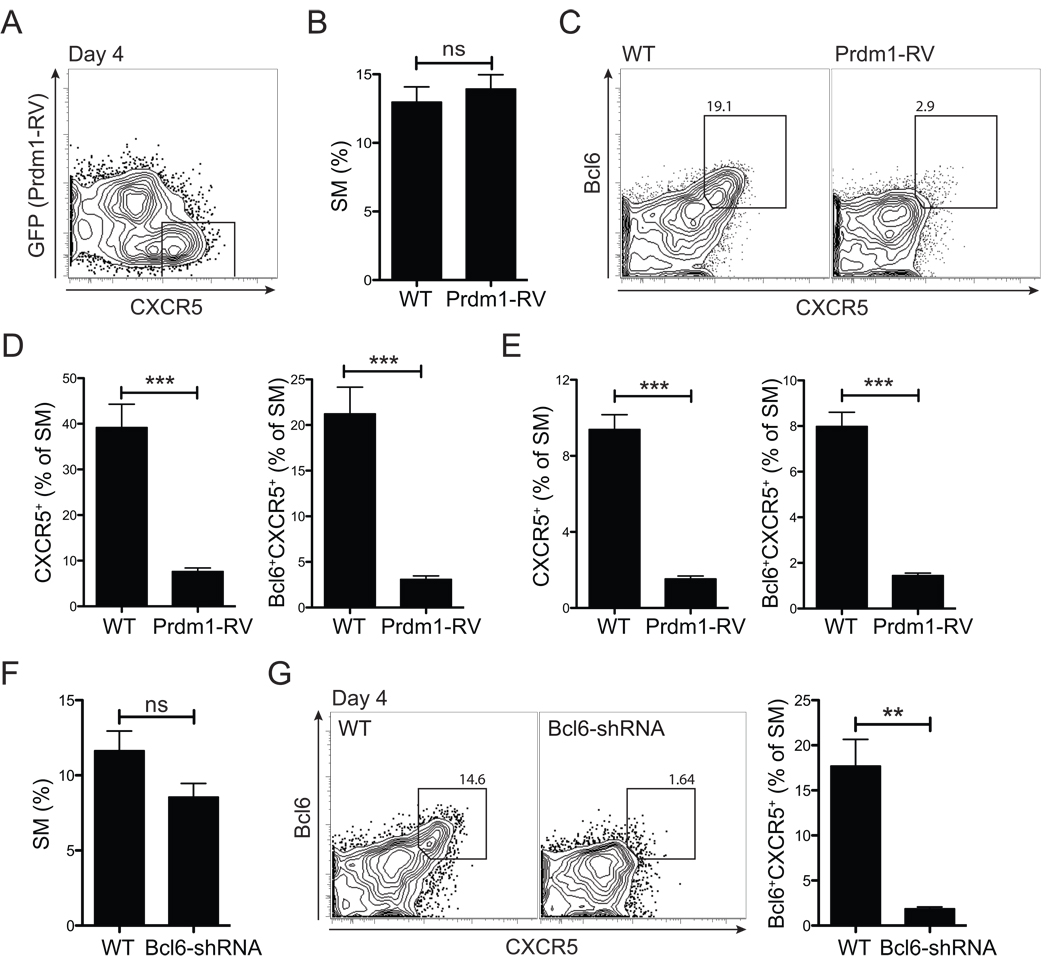
Overall, the experiments elaborated above showed that Tfh cell development is present by day 3 in vivo and ICOS-mediated signals are essential. However, it was not known why ICOS is required. Is the signaling hierarchy such that ICOS induces Bcl6 first and then Bcl6 induces CXCR5, or is Bcl6 induction dependent on CXCR5 expression? Or does ICOS directly induce both CXCR5 and Bcl6 independently? To address these questions, we determined whether ICOS could induce CXCR5 expression on CD4+ T cells in the absence of Bcl6. Bcl6 expression in SM CD4+ T cells was prevented by expression of Blimp1 (Prdm1-RV. Figure S5A). Mice received a 1:1 mixture of control and Prdm1-RV+ SM cells (Figure 5A). Although SM CD4+ T cell activation (CD44hi, Figure S5D) and proliferation (Figure 5B) was normal at day 4 after LCMV infection, Bcl6 expression was efficiently blocked (p = 4.79×10−5 at day 3, Figure S5C and Figure 5E, right panel. p = 0.0008 at day 4, Figure 5C and 5D, right panel). Importantly, CD4+ T cells were unable to upregulate CXCR5 expression in the absence of Bcl6 (p = 6.0×10−5 at day 3, Figure S5B and Figure 5E, left panel. p = 0.0008 at day 4, Figure 5C and 5D, left panel).
Figure 5. ICOS → Bcl6 → CXCR5.
(A–E) Blimp1 expressing (Prdm1-RV+, GFP+) and non-transduced (GFP) SM CD4+ T cells were transferred into the same (A) or separate (B–E) B6 recipient mice. (A) SM CD4+ T cells at day 4 after LCMV infection. (B) WT SM vs. Prdm1-RV+ SM CD4+ T cell expansion (% of total CD4+ T cells). (C, D) CXCR5 expression by Prdm1-RV+ SM and WT SM, day 4 p.i. (C) Representative FACS plots showing WT (GFP) and Prdm1-RV+ (GFP+). Gates identify CXCR5+Bcl6+ SM. (D) Quantitation of CXCR5+ and CXCR5+Bcl6+ SM. (E) CXCR5+ SM and Bcl6+CXCR5+ Tfh cell frequencies day 3 p.i. (F, G) Bcl6 shRNA-RV+ (GFP+Ametrine+) and non-transduced (GFP−Ametrine) SM CD4+ T cells were transferred into separate B6 recipient mice subsequently infected with LCMV. (F) SM CD4+ T cell expansion, day 4 p.i. (% of total CD4+ T cells). (G) Representative FACS plots of CXCR5 and Bcl6 expression by WT SM and Bcl6 shRNA+ SM CD4+ T cells. Total SM cells are shown and gates identify Bcl6+CXCR5+ SM. Data are representative of three (A–E) and two (F–G) independent experiments; n = 4 per group per time point. **P<0.01, ***P<0.001.
In another set of experiments, Bcl6 expression was directly quenched in SM CD4+ T cells by expressing Bcl6 shRNA in the context of a natural micro RNA backbone for optimal processing (shRNAmir). Whereas both expansion (Figure 5F) and activation (CD44hi, Figure S5E) were normal for Bcl6-shRNA transduced SM CD4+ T cells, CXCR5 was not expressed when Bcl6 was suppressed (p = 0.001, Figure 5G). Collectively, our data define a molecular hierarchy in Tfh cell commitment initiated at T cell priming, wherein ICOS signals to induce Bcl6, which subsequently induces high CXCR5 expression.
Tfh vs. effector Th cell commitment: High affinity IL-2 receptor vs. ICOS signals
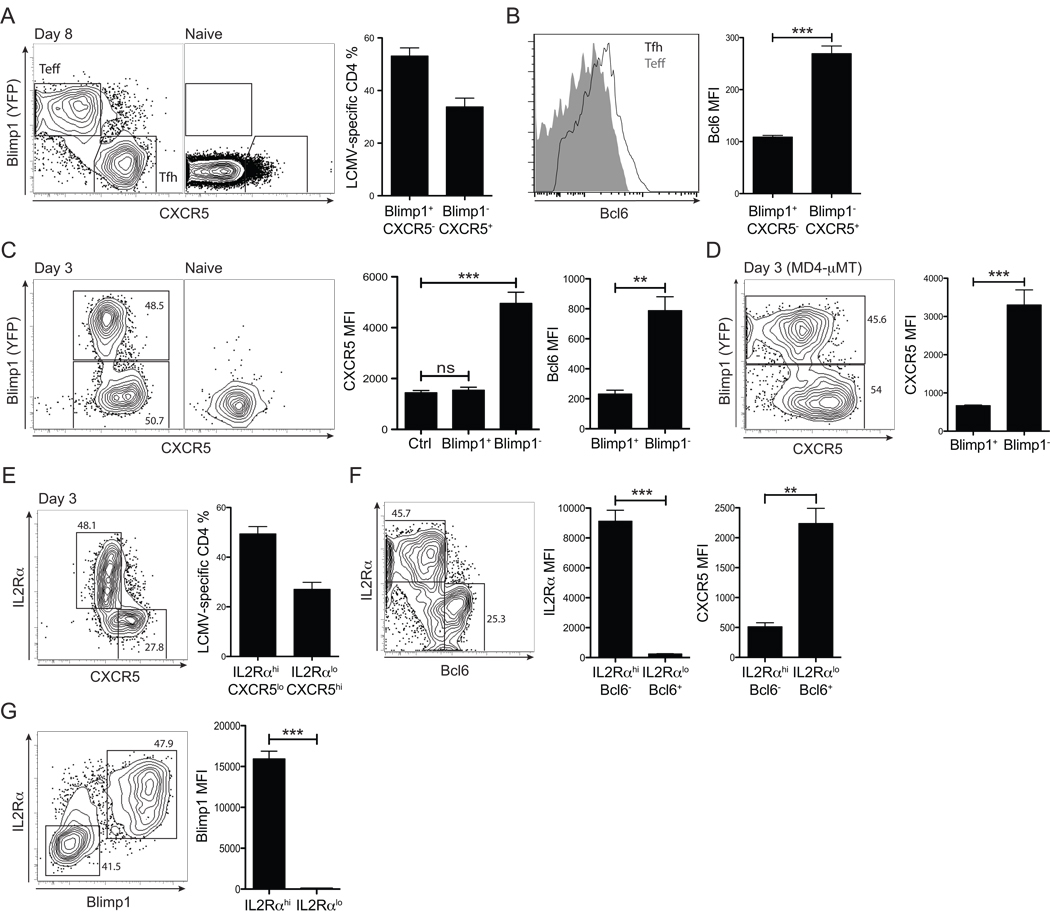
Given the rapid in vivo differentiation of Bcl6+CXCR5+ CD4+ T cells, we next examined when the earliest developmental features of Tfh cells versus effector Th cells can be distinguished. We previously reported that Bcl6 and Blimp1 are antagonistic transcription factors differentially expressed in Tfh and effector Th cells, respectively (Johnston et al., 2009). By using a Blimp1-YFP reporter we now show that this bifurcation is dramatically polarized. Day 8 effector SM cells fall into two clear populations: Blimp1+ effector Th cells and Blimp1−CXCR5+ Tfh cells (Figure 6A). The Blimp1− Tfh cells express more Bcl6 than Blimp1+ effector Th cell counterparts (p = 9.0×10−7, Figure 6B). This reinforces the concept that Blimp1 and Bcl6 serve as a bimodal transcription factor switch in CD4+ T cell differentiation (Fazilleau et al., 2009; Johnston et al., 2009).
Figure 6. Rapid Bcl6 versus Blimp-1 bifurcation of CD4+ T cell differentiation in vivo.
(A) Blimp1 and CXCR5 expression by Blimp1-YFP BAC tg SM CD4+ T cells was analyzed at 8 days p.i. in B6 recipient mice. Gates identify Blimp1+CXCR5− effector Th cells and Blimp1−CXCR5+ Tfh cells. (B)Left, Bcl6 expression in Blimp1+CXCR5− (gray filled) vs. Blimp1CXCR5+ (black line) SM CD4+ T cells. Right, Bcl6 MFIs. (C) Day 3 p.i., analysis of Blimp1, Bcl6, and CXCR5 expression by Blimp1-YFP BAC Tg SM CD4+ T cells in B6 recipients. Representative FACS plots of SM cells from LCMV infected (“Day3”) and uninfected (“Naïve”) recipients are shown. CXCR5 and Bcl6 expression by Blimp1+ vs. Blimp1− SM CD4+ T cells were quantified. “Ctrl” = isotype control stain. (D) MD4-µMT recipient mice of Blimp1-YFP BAC tg SM CD4+ T cells, day 3 p.i. Representative FACS plot of SM cells and CXCR5 quantitation. (E–G) Blimp1-YFP SM CD4+ T cells were transferred into B6 recipients and analyzed for IL2Rα expression 3 days after LCMV infection. IL2Rαhi vs. IL2Rαlo SM CD4+ T cells are identified in co-stains with CXCR5 (E), Bcl6 (F), and Blimp1 (G). Data in all panels are representative of three or more independent experiments; n = 3–4 per group. **P<0.01, ***P<0.001.
Blimp1 expression is commonly considered a late event in effector T cell differentiation (Martins and Calame, 2008; Rutishauser et al., 2009). However, given that Bcl6+ CD4+ T cells could be identified at day 3 in vivo, we examined whether Blimp1+ effector CD4+ T cells might be also identified early in the immune response. Indeed, already by day 3 antiviral CD4+ T cells segregated into two distinct cell types: Blimp1+ or Blimp1−, with significant CXCR5 induction only on Blimp1− populations (p = 1.09×10−5, Figure 6C). Importantly, day 3 Blimp1+ and Blimp1− populations (Blimp1 MFI difference, p = 8.1×10−5) showed reciprocal Bcl6 expression (Bcl6 MFI difference, p = 0.004, Figure 6C). The early bifurcation in Blimp 1+CXCR5lo/− effector Th cell versus Bcl6+CXCR5hi Tfh cell differentiation occurred via DC priming, as it was able to occur in the absence of cognate B cells (46% Blimp 1+ and 53% Blimp 1− SM in B6, Figure 6C. 46% Blimp1+ and 54% Blimp1− SM in MD4-µMT, Figure 6D). Thus, DC priming instructs a bifurcation between Blimp1 and Bcl6 expressing CD4+ cells by day 3 in vivo.
Blimp1 can be induced by IL-2 (Malek and Castro, 2010; Martins and Calame, 2008). Therefore we examined expression of the high affinity IL-2 receptor alpha subunit (IL2Rα, CD25) at day 3 p.i. Two clear SM cell populations were distinguishable: L2Rαhi CXCR5lo and L2Rαlo CXCR5hi (Figure 6E). Strikingly, 98% less L2Rα was expressed on Bcl6+ CD4+ cells compared to Bcl6lo CD4+ cells (p = 0.0002, Figure 6F). Conversely, Blimp1 was strictly expressed in the cells with high IL2Rα expression (p = 0.00008, Figure 6G). In contrast to ICOS-mediated Bcl6 induction (Figure 3), these data demonstrate a competing pathway, via which IL-2-mediated Blimp1 upregulation occurs through IL2Rα signaling.
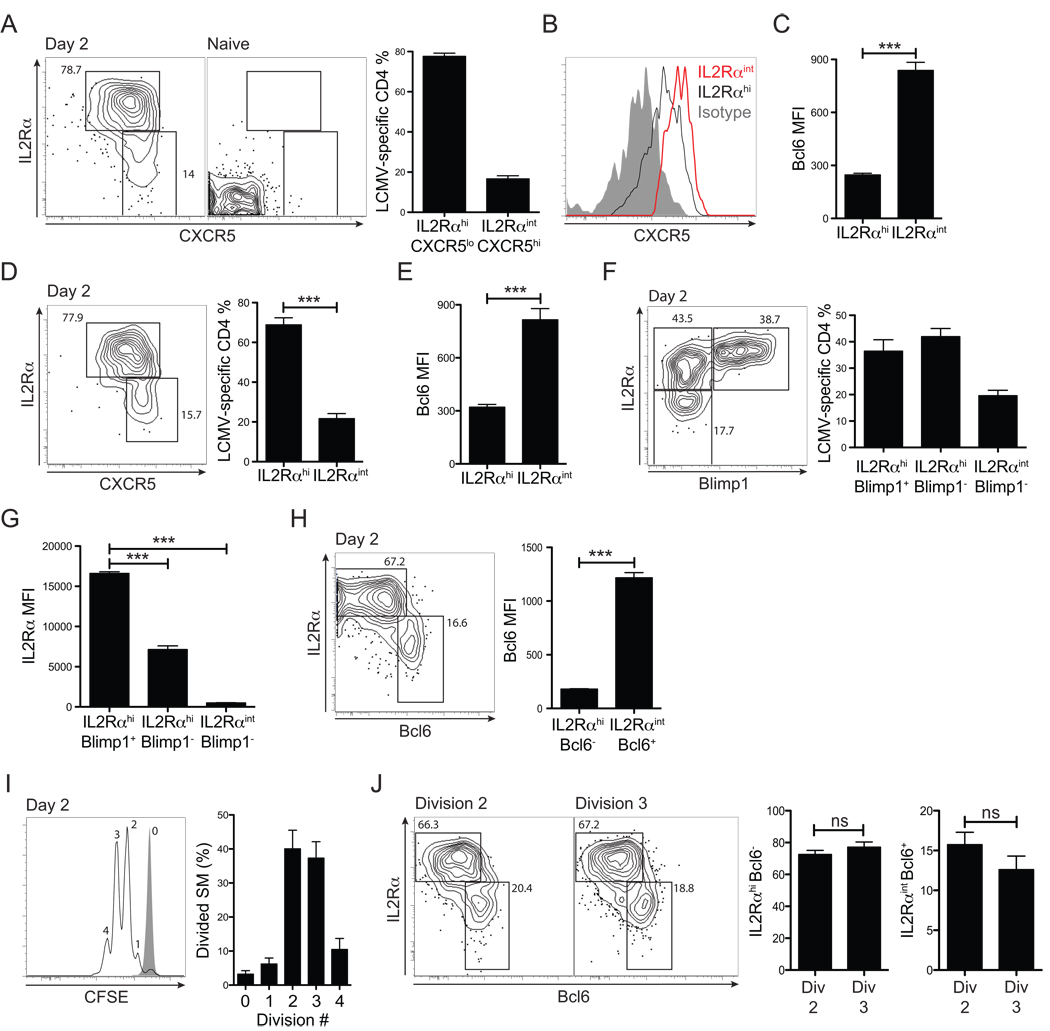
The dramatic segregation in CD4+ cell differentiation observed by day 3 led us to examine an even earlier in vivo time point. Surprisingly, at 48 hours p.i., Tfh versus effector Th cell differentiation was already distinguishable. First we determined that IL2Rα expression is not homogeneous at this time point (Figure 7A). Although a large percentage of antiviral CD4+ cells were L2Rαhi , a significant percentage was L2Rαint (p = 1.5×10−7 , Figure 7A). Importantly, L2Rαint cells exhibited the highest CXCR5 MFI (p = 4.9×10−6 , Figure 7B) and, indeed, expressed the highest amounts of Bcl6 (p = 0.00001, Figure 7C). The early L2Rαhi CXCR5lo versus L2Rαint CXCR5hi SM cell polarization was also observed in the absence of cognate B cells (MD4-µMT, p = 0.00004. Figure 7D). Again, the L2Rαint cells expressed the highest amounts of Bcl6 (p = 0.0002, Figure 7E). Therefore, early differential IL2Rα expression occurs during CD4+ cell priming by DCs, and is associated with distinct differentiation fates of the CD4+ cells. Given that Bcl6 induction was already identifiable by 48 hours in vivo, and was segregated in the L2Rαint SM population, we examined whether Blimp1 was expressed at this very early time point. Indeed, at 48 hours p.i., strong Blimp1 expression was already occurring in 35% of antiviral CD4+ T cells (approximately half of the IL2Rαhi cells, Figure 7F). Notably, Blimp1 expression specifically occurred in the CD4+ T cells that expressed the highest amount of IL2Rα (p = 1.5×10−6 IL2Rαint vs. IL2RαhiBlimp1−. p = 2.7×10−10 IL2Rαint vs. IL2RαintBlimp1−. Figure 7G), which is the opposite of the Bcl6 expression pattern (p = 6.2×10−7 IL2Rαint vs. IL2Rαhi. Figure 7H). Virus-specific CD4+ T cells at this 48 hr time point had predominantly undergone only two (40%) or three (37%) cell divisions (Figure 7I). IL2RαintBcl6+ cells were equally present in both the two and three cell division populations (Figure 7J). Collectively, our data indicate that differential ICOS versus IL2Rα signaling within the first two cell divisions is a major decision point directing Bcl6 or Blimp1 expression and Tfh versus effector Th cell differentiation during T cell priming.
Figure 7. Tfh versus effector Th cells are distinguishable by the second cell division, in association with differential early IL-2Rα expression levels.
Early virus-specific Tfh vs. effector Th cell differentiation was analyzed at 48 hrs after acute LCMV infection. (A) Representative FACS plots of SM CD4+ T cells from LCMV infected (“Day 2”) and uninfected (“Naïve”) mice. Gates identify IL2RαhiCXCR5lo vs. IL2RαintCXCR5hi SM CD4+ T cells. (B) CXCR5 expression by IL2Rαhi (black) vs. IL2Rαint (red) SM CD4+ T cells. Isotype control = gray filled histogram. (C) Bcl6 expression by IL2Rαhi vs. IL2Rαint SM CD4+ T cells. (D) Expression of IL2Rα and CXCR5 by SM CD4+ T cells in MD4-µMT recipients, day 2 p.i. (E) Bcl6 expression by SM CD4+ T cells in MD4-µMT recipients, day 2 p.i. (F) IL2Rα and Blimp1 expression by Blimp1-YFP SM CD4+ T cells in B6 recipients, day 2 p.i. Representative SM CD4+ T cell FACS plot and quantitation are shown. (G) IL2R α expression (MFI) by SM CD4+ T cell populations gated in (F). (H) Bcl6 expression by SM CD4+ T cells in B6 recipients at day 2 p.i. Gates identify IL2Rαhi vs. IL2Rαint SM CD4+ T cells. Bcl6 protein expression (MFI) by each population was calculated. (I, J) CFSE-labeled SM CD4+ T cells were transferred into B6 recipient mice subsequently infected with LCMV. Cell divisions and differentiation were analyzed at day 2 p.i. (I) Cell divisions. Gray filled histogram = SM CD4+ T cells in uninfected mice. (J) Representative FACS plots are shown of SM CD4+ T cells that have undergone 2 (left) and 3 (right) cell divisions. Gates identify IL2RαhiBcl6− and IL2RαintBcl6+ SM CD4+ T cells. Data in all panels are representative of three or more independent experiments; n = 3–4 per group. **P<0.01, ***P<0.001.
DISCUSSION
Accumulating evidence has determined that Bcl6 is a critical determinant for the development and function of Tfh cells; however, the mechanisms by which Bcl6 is induced and Tfh cells develop have remained mostly unclear. Here we have used a variety of genetic and in vivo cellular approaches to show that Bcl6 induction and Tfh cell differentiation is initiated upon DC priming in vivo and requires ICOS. Notably, Bcl6+CXCR5+ CD4+ T cells can be identified within the first two cell divisions in vivo, with strong polarization between Bcl6-expressing and Blimp1-expressing Th cells by three days p.i. These findings revise our understanding of both the mechanism and kinetics of Tfh cell differentiation.
Previous studies have shown that Tfh cells are a distinguishable cell type in vivo required for germinal center development, and that the emergence of Tfh cells is fully dependent on Bcl6 expression (Crotty, 2011). Nevertheless, there has been little consensus regarding the nature of Tfh cell differentiation, in part due to lack of information about the earliest events in Tfh cell differentiation in vivo. One commonly held model of Tfh cell differentiation was that the Tfh cell program is a secondary program acquired later by some Th1, Th2, or Th17 cells, and Tfh cells do not exist as an independent cell type. Our data do not support that model. A detailed examination of the kinetics of development of Tfh cell in vivo revealed that Bcl6 expression occurs in a subset of CD4+ T cells within two cell divisions following infection. These findings support models of Tfh differentiation whereby Tfh development is not contingent on a CD4+ T cell first acquiring Th1, Th2, or Th17 cells characteristics.
Our findings also clarify the relationship between Tfh cells and APCs, particularly B cells and dendritic cells. Previous studies found that Tfh cells were largely dependent on B cells. We confirmed that the presence of Tfh cells at time points later than one week post infection was dependent on B cells, specifically cognate B cells. In addition, B cell interaction with Tfh cells is required for maintenance of Bcl6 expression in Tfh cells. Nevertheless, Tfh cell differentiation initiates independently of B cells, as evidenced by normal Bcl6 induction in B cell deficient mice at day three p.i. In parallel experiments, we reasoned that presence of Bcl6+ CD4+ T cells within two divisions in vivo implicated DCs as the responsible APCs for the CD4+ T cells, given the rarity of cognate B cells early. Indeed, DC immunization experiments revealed that DC priming can induce Bcl6 and CXCR5 in CD4+ T cells. B cells become the primary APCs after approximately day three to four p.i., likely due to the increased availability of antigen-specific B cells as APCs when they undergo extensive clonal expansion, as well as the co-localization of the antigen-specific B cells and Tfh cells. This APC shift is likely also due to death of the majority of the antigen-presenting mature DC by four days after an acute viral infection or protein immunization, limiting MHCII presented antigen in the lymphoid tissue predominantly to B cells after this time. B cells can influence T cell responses in a variety of situations (Lund and Randall, 2010). This phenomenon of distinct stimulation requirements for initiation of differentiation and then maintenance of differentiation is a common feature of many developmental processes in biology, including CD4+ T cell differentiation, as observed by the instability of iTreg or Th17 cells differentiation in the absence of co-factors or sustained signals. A distinct attribute of Tfh cell differentiation is the requirement for two different APC types at two stages of differentiation.
Each simple model of Tfh cell differentiation (cytokine program, B cell dependent program, and secondary program) has had strengths and weaknesses. Our studies here now allow for proposal of an integrated model of Tfh cell differentiation, focused on the central role of Bcl6 in this process. We have found that Bcl6 expression occurs early during DC priming and Tfh cell maturation occurs quickly thereafter. The cytokine only model does not suffice, on the basis of the requirement for ICOS signaling during DC priming for Bcl6 expression, as well as the limited impact of IL6, IL21 double-deficiency (Eto et al., 2011). The B cell dependence model does not suffice, on the basis of the early Tfh cell differentiation in the absence of B cells. The secondary program model fails on the basis of Bcl6 induction and Tfh cell differentiation occurring immediately upon DC priming. Nevertheless, aspects of each of these available models were accurate, supported by quality data from multiple published studies. Therefore key attributes of each are incorporated into our integrated model based on their biological relevance (Figure S6). The secondary program model is inaccurate, but it is nevertheless true that Tfh cells may possess attributes of Th1, Th2, or Th17 cells in some conditions. IL-4, IFN-γ, and IL-17 can be important class switch factors produced by Tfh cells to control B cell antibody isotypes. We interpret these findings as evidence that Tfh cell differentiation that occurs at the time of DC priming is done in the context of a cytokine milieu that, depending on the conditions, can allow partial Th1 (or Th2 or Th17) cell features that co-exist with the Tfh cell program. This may relate to the recently appreciated functional plasticity of CD4+ T cells (Murphy and Stockinger, 2010).
ICOS is the only homolog of CD28 found in humans or mice. ICOS has functions distinct from CD28 due to two unique characteristics. Unlike CD28, ICOS is not expressed on resting T cells and is rapidly upregulated after TCR stimulation (Hutloff et al., 1999). Second, the signaling motifs in the cytoplasmic tail of ICOS are distinct from those of CD28 (Yong et al., 2009). The importance of ICOS for germinal centers and T-dependent antibody responses was rapidly recognized with Icos−/− mice (McAdam et al., 2001; Tafuri et al., 2001). Rare ICOS-deficient humans have a severe loss of antibody responses and memory B cells (Warnatz et al., 2006). In the absence of ICOS, reduced Tfh cell numbers are observed in both mice and humans (Akiba et al., 2005; Bossaller et al., 2006; Grimbacher et al., 2003). Conversely, overexpression of ICOS in mice due to a loss of function mutation in Roquin (sanroque) causes severe autoimmunity via unchecked germinal center development and autoantibody production (Vinuesa et al., 2005). Sanroque mice have dramatic increases in Tfh cell numbers (Linterman et al., 2009; Vinuesa et al., 2005). Although ICOS is clearly important for germinal centers and Tfh cell development, it has been unknown why ICOS is so central to this process. We reasoned that ICOS may be required for initiation of Tfh cell differentiation by induction of Bcl6 in CD4+ T cells during DC priming. Consistent with that idea, a profound defect in early Tfh cell differentiation was demonstrated in the absence of ICOS. Notably, Bcl6 expression was substantially reduced, indicating that ICOS is required for Bcl6 upregulation and subsequent Bcl6 control of the Tfh differentiation program. Not only is ICOS required for Bcl6 induction for Tfh cells during DC priming, ICOS signals are again required a second time for Tfh cell maintenance of Bcl6, this time from B cells during T - B cognate interactions. Relevant to our studies, CXCR5+ CD4+ T cells were reduced in mice with B cell specific deletion of ICOSL (ICOSLfl/fl CD19-Cre) (Nurieva et al., 2008). Our experiments indicate that the mechanism by which ICOS initiates Tfh differentiation during DC priming is through Bcl6 expression. We found that Bcl6 controls the downstream expression of CXCR5 in CD4+ T cells, thereby establishing a molecular hierarchy of Tfh differentiation from ICOS to Bcl6 to CXCR5. This is consistent with evidence that miR17–92 can reduce CXCR5 expression in B cells (Yu et al., 2009). ICOS signals can promote IL-21 production (Bauquet et al., 2009; Hiramatsu et al., 2010), and IL-21 can enhance Bcl6 mRNA expression (Nurieva et al., 2009). However, several studies showed that Tfh cells develop in Il21−/− or Il21r−/− mice (Crotty, 2011). Nevertheless, IL-6 and IL-21 doubly deficient mice do exhibit reduced Tfh cell frequencies and reduced Bcl6 expression by Tfh cells, confirming that these cytokines do contribute to Tfh cell differentiation (Eto et al., 2011). Intracellular signals from ICOS to induce Bcl6 expression are likely augmented by cytokines to control Tfh versus effector Th cell differentiation.
An important function of Bcl6 is repression of Blimp1. Tfh cells express Bcl6 and effector Th cells express Blimp1 (Fazilleau et al., 2009; Johnston et al., 2009). Blimp1 is capable of blocking Bcl6 expression and thereby extinguishing the Tfh cell differentiation program (Johnston et al., 2009). Our observation of the dramatic bimodal polarization of CD4+ T cells to almost exclusive Blimp1+ effector Th and Bcl6+ Tfh cell types at the peak of the CD4+ T cell response highlighted the stringency of the antagonism between these two transcription factors. Blimp1 is known to be expressed late in effector CD8+ T cell differentiation and is considered a late marker of T cell differentiation. Surprisingly, polarized Bcl6 versus Blimp1 expression was found as early as 48 hours p.i., after only two cell divisions. We infer from these results that Bcl6 and Blimp1 are important early orchestrators of CD4+ T cell differentiation, thereby driving antagonistic and self-reinforcing differentiation processes. Given our finding that ICOS signaling instructs Bcl6 expression and Tfh cell differentiation, the knowledge that an early bifurcation occurs between Tfh and effector Th cell developments during priming led us to ask what signaling molecule may instruct Blimp1 in association with canonical effector Th cell differentiation. One molecule known to affect Blimp1 expression is IL-2 (Malek and Castro, 2010). The high affinity IL2Rα subunit is crucial for IL-2-mediated signaling and is expressed after TCR activation. Our study shows that Blimp1 is regulated by CD4+ T cells according to differential IL2Rα expression during the priming phase of the immune response. At 48 hours p.i., the virus-specific CD4+ T cell population possessed marked heterogeneity in IL2Rα expression, covering a 100 to 500 fold range of variation in expression. A large proportion of CD4+ T cells highly expressed IL2Rα and had already upregulated Blimp1 expression. In contrast, cells possessing more moderate amounts of IL2Rα expression (IL2Rαint) instead expressed Bcl6.
IL-2 can induce Blimp1 in both CD4+ T cells and CD8+ T cells (Malek and Castro, 2010). Interestingly, terminal effector CD8+ T cells (short-lived effector cells, SLECs) express Blimp1 and require Blimp1 for their full functionality (Kallies et al., 2009; Rutishauser et al., 2009). The question of how CD8+ T cell memory is generated has been a topic of intensive interest in cellular immunology. Recently it was determined that differential IL2Rα expression on virus-specific CD8+ T cells as early as 3.5 days after LCMV infection was associated with two separate cell fates. IL2Rαhi CD8+ T cells express Blimp1 more than their IL2Rαint counterparts, and consequentially are biased to differentiate into terminal effector CD8+ T cells (SLEC) (Kalia et al., 2010; Pipkin et al., 2010). Notably, CD8+ T cells that are instead IL2Rαint are destined to become memory precursor CD8+ T cells (MPEC), which express elevated Bcl6 (Rutishauser et al., 2009), in contrast to the Blimp1 expression by terminal effector CD8+ T cells. Therefore CD8+ T cells undergo a fate choice decision process during priming surprisingly analogous to the mechanism of CD4+ T cell Tfh differentiation we demonstrate here. These parallels indicate that mechanisms limiting early IL2Rα expression on T cells may enhance both Tfh cell differentiation and the development of T cell memory.
Our study illuminates the involvement of ICOS versus IL2Rα signals during priming for induction of Bcl6 expression and Tfh cell differentiation and highlights the importance of further examination of the intersections and interplay between these antagonizing pathways for determining T cell fates. How are these two upstream pathways regulated? ICOS-mediated signals for Tfh require the phosphoinositide 3-kinase (PI3K) pathway (Gigoux et al., 2009). Unlike CD28, ICOS fails to bind both Grb2 and Gads and therefore does not induce IL-2 production (Harada et al., 2003; Watanabe et al., 2006). It is plausible that higher TCR affinity results in prolonged T cell - DC interactions during the extended periods of CD4+ T cell - DC interaction in the first 24 hours of priming in vivo (Celli et al., 2007; Mempel et al., 2004). Higher TCR affinity is associated with a preference for Tfh cell differentiation over effector Th cell differentiation (Fazilleau et al., 2009). This may well be due to higher TCR affinity causing extended T -DC interactions, resulting in prolonged ICOS - ICOSL exposure at the time of DC priming, with CD4+ T cell integration of total ICOS signal strength over the duration of the APC interaction resulting in initiation of Tfh differentiation. We hypothesize that repeated but less prolonged early exposure of CD4+ T cells to antigen preferentially induces IL2Rα expression. Each of these processes can be an amplifying and self-reinforcing loop that would be capable of leading to rapidly polarized Bcl6-expressing Tfh cells and Blimp1 expressing effector Th cells. It is appealing to consider that a comparable ICOS signaling process could be occurring during T - B interactions. The higher amount of ICOS expression on Tfh cells combines with the duration of the T - B interaction determines the overall amount of ICOS signaling received by the Tfh cells. The duration of T-B interaction is dependent on the magnitude of SAP-dependent SLAM family receptor adhesion. Such an idea is consistent with the requirement for SAP to obtain GC Tfh cells and germinal centers. This is in keeping with the concept that Tfh cell differentiation is a multi-stage, multi-component process (Crotty, 2011).
Collectively, our findings provide mechanistic insights for signaling pathways in regulation of two antagonizing transcription factors that balance Tfh versus effector Th cell differentiation. Given that most successful vaccines are dependent on CD4+ T cell provision of help to B cells to produce protective antibodies and B cell memory, further understanding of Tfh versus effector Th cells fate determination may be useful in rational vaccine design.
EXPERIMENTAL PROCEDURES
Mice and viral infections
Mice were either purchased from the Jackson Laboratory or obtained from in-house breeders (details in Supplemental Information). LCMV Armstrong and VACVWR (Western Reserve strain) stocks were prepared and quantified as previously described (McCausland et al., 2007; Sette et al., 2008). All animal experiments were conducted in accordance with approved animal protocols at LIAI.
Retrovirus production and cell transfers
Prdm1 (Blimp 1) expressing retroviral vector (pMIG-GFP) was reported previously (Johnston et al., 2009). Bcl6 shRNA sequences were cloned into pLMP-GFP (Open Biosystem) and a custom pLMP-Ametrine, with additional modifications. Virions were produced from the Plat-E cell line as previously described (Johnston et al., 2009) with some modifications. VSV-g expressing plasmid (pHDM-VSV-G) was co-transfected for each RV production, for increased virion stability. Culture supernatants were obtained 2 days after transfections, filtered through 0.45 µm syringe filters, and ultracentrifuged at 24,000 rpm for 90 mins. VSV-g expressing chimeric retroviral stocks were saved at −80°C until use.
Naïve or retrovirally transduced (details in Supplemental Information) SM CD4+ T cells were transferred into recipient mice by intravenous injections via the retro-orbital sinus. Cell transfer numbers for each time point were as follows: 1 × 106, 4–5 × 105, 2 × 105, and 5 × 103 SM CD4+ T cells for day 2, 3, 4 and 8 (or 7) experiments, respectively.
Anti-ICOSL treatments
Anti-ICOSL (rat IgG2a, clone HK5.3) and isotype control (rat IgG2a) mAbs were purchased from BioXcell. C57BL/6 mice were given 100 µg of anti-ICOSL or isotype control mAb via both i.p. and retro-orbitally 3 days after VACVWR infection. Subsequent doses of 100 µg of mAbs were injected retro-orbitally at 5 and 7 days after infection.
Flow Cytometry
Single cell suspensions were obtained by a gentle mechanical disruption of spleens. After ACK lysis, cell suspensions were surface stained in FACS buffer (PBS + 0.5% BSA) with monoclonal antibodies: SLAM (CD150, Biolegend); CD4, CD8, Fas, GL7 (BD Biosciences); CD44, CD45.1/2, IL2Rα (CD25), CD62L, CD69, PD-1 (J43), ICOS, B220 (eBioscience); PNA (Vector Labs). CXCR5 stains and intracellular Bcl6 stains are described in Supplemental Information. All FACS samples were washed twice with FACS buffer, acquired using an LSRII (BD Biosciences), and then analyzed using FlowJo (Tree Star).
Statistics
Statistical analyses were done using Prism 5.0 (GraphPad). P-values were calculated using two-tailed unpaired Student’s t tests with a 95% confidence interval. Error bars on bar graphs depict the standard error of mean (SEM).
Supplementary Material
ACKNOWLEDGEMENTS
We thank C. Kim, K. Van Gunst, and A. Jose for cell sorting, and L. Crickard for animal care. We thank BD for generously providing αBcl6 mAb (clone K112-91). We thank M. Pipkin and R. Rickert for advice and discussions. This study was supported by grants from the NIH, as well as a Pew Scholars in Biomedical Research award, and a Cancer Research Institute Young Investigator award, and LIAI internal funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERNCES
- Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- Allen CDC, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Kuchroo VK. Immunology. The yin and yang of follicular helper T cells. Science. 2009;325:953–955. doi: 10.1126/science.1178752. [DOI] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho I-C, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Tangye SG, Schwartzberg PL. SLAM Family Receptors and SAP Adaptors in Immunity. Annu Rev Immunol. 2011;29 doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- Celli S, Lemaître F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Ci W, Polo JM, Melnick A. B-cell lymphoma 6 and the molecular pathogenesis of diffuse large B-cell lymphoma. Current Opinion in Hematology. 2008;15:381–390. doi: 10.1097/MOH.0b013e328302c7df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (T(FH)) Annu Rev Immunol. 2011;29 doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. Journal of Experimental Medicine. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426–2433. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto D, DiToro D, Lao C, Barnett B, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 collaborate in the differentiation of follicular helper CD4 T cells (Tfh) and B cell immunity. PLos One. 2011 doi: 10.1371/journal.pone.0017739. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, Mcheyzer-Williams LJ, Mcheyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, Mcheyzer-Williams LJ, Rosen H, Mcheyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh W-K. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Dräger R, Eibel H, Fischer B, Schäffer AA, Mages HW, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K, Toma H, Altman A, Abe R. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S-i, Ikeda K, Hirose K, Watanabe N, Grusby MJ, Iwamoto I, Nakajima H. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, Ditoro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 Are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, Verma NK, Brink R, Hutloff A, Goodnow CC, Vinuesa CG. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007;178:817–828. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang Y-h, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Y-H, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham THM, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed A-U, Rahn H-P, Sallusto F, Lipp M, Müller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur. J. Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Liang H-E, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, Peters B, Rafii-El-Idrissi Benhnia M, Hoffmann J, Su H-P, et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28:847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, van Dongen JJM, Orlowska-Volk M, Knoth R, Durandy A, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107:3045–3052. doi: 10.1182/blood-2005-07-2955. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Harada Y, Takeda K, Takahashi J, Ohnuki K, Ogawa S, Ohgai D, Kaibara N, Koiwai O, Tanabe K, et al. Grb2 and Gads exhibit different interactions with CD28 and play distinct roles in CD28-mediated costimulation. J Immunol. 2006;177:1085–1091. doi: 10.4049/jimmunol.177.2.1085. [DOI] [PubMed] [Google Scholar]
- Yong PFK, Salzer U, Grimbacher B. The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol Rev. 2009;229:101–113. doi: 10.1111/j.1600-065X.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner K-M, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.