Abstract
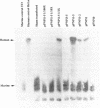
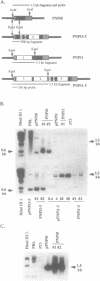
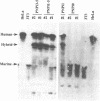
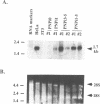
Abbreviated purine nucleoside phosphorylase (PNP) genes were engineered to determine the effect of introns on human PNP gene expression. PNP minigenes containing the first intron (complete or shortened from 2.9 kb down to 855 bp), the first two introns or all five PNP introns resulted in substantial human PNP isozyme expression after transient transfection of murine NIH 3T3 cells. Low level human PNP activity was observed after transfection with a PNP minigene containing the last three introns. An intronless PNP minigene construct containing the PNP cDNA fused to genomic flanking sequences resulted in undetectable human PNP activity. Heterogeneous, stable NIH 3T3 transfectants of intron-containing PNP minigenes (verified by Southern analysis), expressed high levels of PNP activity and contained appropriately processed 1.7 kb message visualized by northern analysis. Stable transfectants of the intronless PNP minigene (40-45 copies per haploid genome) contained no detectable human PNP isozyme or mRNA. Insertion of the 855 bp shortened intron 1 sequence in either orientation upstream or downstream of a chimeric PNP promoter-bacterial chloramphenicol acetyltransferase (CAT) gene resulted in a several-fold increase in CAT expression in comparison with the parental PNP-CAT construct. We conclude that human PNP gene expression at the mRNA and protein level is dependent on the presence of intronic sequences and that the level of PNP expression varies directly with the number of introns included. The disproportionately greatest effect of intron 1 can be explained by the presence of an enhancer-like element retained in the shortened 855 bp intron 1 sequence.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M. A., Ramesh N., Miller A. D., Osborne W. R. Internal initiation of translation in retroviral vectors carrying picornavirus 5' nontranslated regions. J Virol. 1991 Sep;65(9):4985–4990. doi: 10.1128/jvi.65.9.4985-4990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow B., Lattier D., Silbiger R., Dusing M., Hutton J., Jones G., Stock J., McNeish J., Potter S., Witte D. Evidence for a complex regulatory array in the first intron of the human adenosine deaminase gene. Genes Dev. 1989 Sep;3(9):1384–1400. doi: 10.1101/gad.3.9.1384. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Gelinas R. E., Miller A. D. A majority of mice show long-term expression of a human beta-globin gene after retrovirus transfer into hematopoietic stem cells. Mol Cell Biol. 1989 Apr;9(4):1426–1434. doi: 10.1128/mcb.9.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Miller A. D., Gelinas R. E. Expression of the human beta-globin gene after retroviral transfer into murine erythroleukemia cells and human BFU-E cells. Mol Cell Biol. 1988 Apr;8(4):1725–1735. doi: 10.1128/mcb.8.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Liska D. J., Apone S., Devarayalu S. Interactions between the promoter and first intron are involved in transcriptional control of alpha 1(I) collagen gene expression. Mol Cell Biol. 1988 Nov;8(11):4851–4857. doi: 10.1128/mcb.8.11.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Berg P. Comparison of intron-dependent and intron-independent gene expression. Mol Cell Biol. 1988 Oct;8(10):4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987 Dec;1(10):1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee P. P., Klassy R. C., Slightom J. L. Expression of a bean storage protein 'phaseolin minigene' in foreign plant tissues. Gene. 1986;41(1):47–57. doi: 10.1016/0378-1119(86)90266-0. [DOI] [PubMed] [Google Scholar]
- Chung S., Perry R. P. Importance of introns for expression of mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2075–2082. doi: 10.1128/mcb.9.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Rotter R., Mariash C. N., Rosenberg M. E. Loading and transfer control for Northern hybridization. Biotechniques. 1992 Feb;12(2):154–158. [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Bich-Thuy L. T., Stafford J., Queen C. Synergism between immunoglobulin enhancers and promoters. Nature. 1986 Jul 24;322(6077):383–385. doi: 10.1038/322383a0. [DOI] [PubMed] [Google Scholar]
- Gasser C. S., Simonsen C. C., Schilling J. W., Schimke R. T. Expression of abbreviated mouse dihydrofolate reductase genes in cultured hamster cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6522–6526. doi: 10.1073/pnas.79.21.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Genes-in-pieces revisited. Science. 1985 May 17;228(4701):823–824. doi: 10.1126/science.4001923. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Caput D., Williams S. R., Martin D. W., Jr Cloning of human purine-nucleoside phosphorylase cDNA sequences by complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4281–4285. doi: 10.1073/pnas.80.14.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gross M. K., Kainz M. S., Merrill G. F. Introns are inconsequential to efficient formation of cellular thymidine kinase mRNA in mouse L cells. Mol Cell Biol. 1987 Dec;7(12):4576–4581. doi: 10.1128/mcb.7.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby D. B., Zilz N. D., Towle H. C. Sequences within the 5'-flanking region of the S14 gene confer responsiveness to glucose in primary hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17623–17626. [PubMed] [Google Scholar]
- Jonsson J. J., McIvor R. S. Herpes simplex virus thymidine kinase enzymatic assay in transient transfection experiments using thymidine kinase-deficient cells. Anal Biochem. 1991 Dec;199(2):232–237. doi: 10.1016/0003-2697(91)90095-b. [DOI] [PubMed] [Google Scholar]
- Jonsson J. J., Williams S. R., McIvor R. S. Sequence and functional characterization of the human purine nucleoside phosphorylase promoter. Nucleic Acids Res. 1991 Sep 25;19(18):5015–5020. doi: 10.1093/nar/19.18.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Bodine D. M., Perry L., Papayannopoulou T., Nienhuis A. W. Expression of the human beta-globin gene following retroviral-mediated transfer into multipotential hematopoietic progenitors of mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6062–6066. doi: 10.1073/pnas.85.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D. D., Sylwestrowicz T. A., Granger S., Massaia M., Franks R., Janossy G., Hoffbrand A. V. Distribution of terminal deoxynucleotidyl transferase and purine degradative and synthetic enzymes in subpopulations of human thymocytes. J Immunol. 1982 Oct;129(4):1430–1435. [PubMed] [Google Scholar]
- Madrid-Marina V., Martinez-Valdez H., Cohen A. Phorbol esters induce changes in adenosine deaminase, purine nucleoside phosphorylase, and terminal deoxynucleotidyl transferase messenger RNA levels in human leukemic cell lines. Cancer Res. 1990 May 15;50(10):2891–2894. [PubMed] [Google Scholar]
- Martinez-Valdez H., Cohen A. Coordinate regulation of mRNAs encoding adenosine deaminase, purine nucleoside phosphorylase, and terminal deoxynucleotidyltransferase by phorbol esters in human thymocytes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6900–6903. doi: 10.1073/pnas.85.18.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaia M., Ma D. D., Sylwestrowicz T. A., Tidman N., Price G., Janossy G., Hoffbrand A. V. Enzymes of purine metabolism in human peripheral lymphocyte subpopulations. Clin Exp Immunol. 1982 Oct;50(1):148–154. [PMC free article] [PubMed] [Google Scholar]
- McIvor R. S., Goddard J. M., Simonsen C. C., Martin D. W., Jr Expression of a cDNA sequence encoding human purine nucleoside phosphorylase in rodent and human cells. Mol Cell Biol. 1985 Jun;5(6):1349–1357. doi: 10.1128/mcb.5.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. M., Foresman M. D., Ronnei B. J., McIvor R. S. Isolation and expression of a murine purine nucleoside phosphorylase-encoding cDNA and sequence similarity with the human message. Gene. 1992 Apr 15;113(2):215–221. doi: 10.1016/0378-1119(92)90398-9. [DOI] [PubMed] [Google Scholar]
- Neote K., Kwan E., Snyder F. F. Purine nucleoside phosphorylase synthesis and turnover in human lymphoid cells. Proc Soc Exp Biol Med. 1985 Sep;179(4):442–447. doi: 10.3181/00379727-179-42121. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T. The intron requirement for immunoglobulin gene expression is dependent upon the promoter. Nucleic Acids Res. 1988 Jul 25;16(14B):6713–6724. doi: 10.1093/nar/16.14.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K., Green P. P., 3rd, Fowlkes D. M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987 Apr;6(2):173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- Osborne W. R., Miller A. D. Design of vectors for efficient expression of human purine nucleoside phosphorylase in skin fibroblasts from enzyme-deficient humans. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6851–6855. doi: 10.1073/pnas.85.18.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Sandgren E. P., Avarbock M. R., Allen D. D., Brinster R. L. Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):478–482. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasleau F., Leung F., Kopchick J. J. A comparison of bovine growth hormone expression directed by bGH genomic or intronless DNA in transiently transfected eukaryotic cells. Gene. 1987;57(1):47–52. doi: 10.1016/0378-1119(87)90175-2. [DOI] [PubMed] [Google Scholar]
- Reid L. H., Gregg R. G., Smithies O., Koller B. H. Regulatory elements in the introns of the human HPRT gene are necessary for its expression in embryonic stem cells. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4299–4303. doi: 10.1073/pnas.87.11.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio R., Berne R. M. Localization of purine and pyrimidine nucleoside phosphorylases in heart, kidney, and liver. Am J Physiol. 1980 Dec;239(6):H721–H730. doi: 10.1152/ajpheart.1980.239.6.H721. [DOI] [PubMed] [Google Scholar]
- Scharfmann R., Axelrod J. H., Verma I. M. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4626–4630. doi: 10.1073/pnas.88.11.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Svaren J., Chalkley R. The structure and assembly of active chromatin. Trends Genet. 1990 Feb;6(2):52–56. doi: 10.1016/0168-9525(90)90074-g. [DOI] [PubMed] [Google Scholar]
- Van Reempts J., Van Deuren B., Haseldonckx M., Van de Ven M., Thoné F., Borgers M. Purine nucleoside phosphorylase: a histochemical marker for glial cells. Brain Res. 1988 Oct 11;462(1):142–147. doi: 10.1016/0006-8993(88)90596-3. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Gibson M., Spear P. G., Scolnick E. M. Construction and isolation of a transmissible retrovirus containing the src gene of Harvey murine sarcoma virus and the thymidine kinase gene of herpes simplex virus type 1. J Virol. 1981 Sep;39(3):935–944. doi: 10.1128/jvi.39.3.935-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. R., Gekeler V., McIvor R. S., Martin D. W., Jr A human purine nucleoside phosphorylase deficiency caused by a single base change. J Biol Chem. 1987 Feb 15;262(5):2332–2338. [PubMed] [Google Scholar]
- Williams S. R., Goddard J. M., Martin D. W., Jr Human purine nucleoside phosphorylase cDNA sequence and genomic clone characterization. Nucleic Acids Res. 1984 Jul 25;12(14):5779–5787. doi: 10.1093/nar/12.14.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]