Abstract
Individuals with “metabolically benign” obesity (obesity unaccompanied by hypertension, dyslipidemia, and diabetes) are not at elevated 10-year risk of cardiovascular disease compared to normal weight individuals. It remains unclear whether these obese individuals or normal weight individuals with clustering of cardiometabolic factors display heightened immune activity. Therefore, we characterized levels of acute phase reactants (CRP, IL-6, TNF-alpha, white blood cell count), adhesion molecules (E-selectin, VCAM-1), and coagulation products (fibrinogen, PAI-1) among four body size phenotypes (normal weight with 0/1 vs. ≥2 metabolic syndrome components/diabetes and overweight/obesity with 0/1 vs. ≥2 metabolic syndrome components/diabetes) in cross-sectional analyses of 1,889 post-menopausal women from the Women’s Health Initiative Observational Study nested case-control stroke study. Higher levels of all three inflammatory marker categories were found among women with overweight/obesity or ≥2 metabolic syndrome components or diabetes. Compared to normal weight women with 0 or 1 metabolic syndrome components, normal weight women with ≥2 metabolic syndrome components or diabetes were more likely to have ≥3 inflammatory markers in the top quartile (multivariate odds ratio [OR] 2.0, 95% CI: 1.3–3.0), as were overweight/obese women with 0 or 1 metabolic syndrome components (OR 2.3; 95% CI:1.5–3.5). Overweight/obese women with ≥2 metabolic syndrome components or diabetes had the highest odds ratio (OR 4.2; 95% CI: 2.9–5.9). Despite findings that metabolically benign obese individuals are not at increased 10-year risk of cardiovascular disease compared to normal weight individuals, the current results suggest that overweight/obese women without clustering of cardiometabolic risk factors still possess abnormal levels of inflammatory markers.
Keywords: obesity, metabolic syndrome, inflammation
INTRODUCTION
We have recently shown that cardiovascular disease (CVD) risk factor burden is not uniform among all obese persons, leading to different obesity phenotypes(1). There is a subset of obese individuals, accounting for approximately 1/3rd of all obese individuals, that appears not to possess the multiple CVD risk factors which often accompany obesity such as hypertension, dyslipidemia, glucose abnormalities, or systemic inflammation(1). These individuals have been termed “metabolically benign” obese subjects. Additionally, there is a subset of normal weight individuals who possess significant clustering of these metabolic abnormalities, representing approximately 25% of all normal weight individuals(1). Recent examination of the CVD event risks associated with each of these phenotypes are conflicting, with some studies suggesting that metabolically benign obese individuals (typically defined in these publications as obese without the metabolic syndrome or diabetes) are not at increased risk of coronary heart disease or stroke compared to normal weight individuals without these conditions,(2–4) while recent studies with considerably longer follow-up find increased risk(5;6).
Obesity, in general, is thought to be associated with a chronic inflammatory state, (7;8) but it is unclear whether all obese individuals display this heightened immune activity. Published results are primarily restricted to male populations and demonstrate conflicting results(9–12). Given the absence of metabolic disturbances and a delayed risk of CVD events among those with metabolically benign obesity, it is possible that these individuals do not have enhanced levels of inflammation. Similarly, it is possible that normal weight individuals with clustering of metabolic abnormalities have enhanced levels of inflammation, despite being normal weight. However, little is known regarding the inflammatory state of these phenotypes.
The purpose of the current study was to examine the inflammatory marker profiles of each of 4 body size phenotypes (normal weight with clustering of cardiometabolic abnormalities or diabetes, normal weight without clustering of cardiometabolic abnormalities, overweight/obesity with clustering of cardiometabolic abnormalities, and overweight/obesity without clustering of cardiometabolic abnormalities or diabetes) among postmenopausal women enrolled in the Women’s Health Initiative Observational Study Hormones and Biomarkers Predicting Stroke ancillary study.
METHODS and PROCEDURES
Study Design and Population
These cross-sectional analyses utilize data obtained from the Hormones and Biomarkers Predicting Stroke (HaBPS) ancillary study to the Women’s Health Initiative Observational Study (WHI-OS). HaBPS is a case-control study nested within the WHI-OS, designed to examine the relationships between biomarkers and hormones measured at the baseline WHI-OS visit among women without a history of myocardial infarction or stroke with the subsequent development of ischemic stroke. Details of the WHI-OS and the HaBPS study have been previously published(13–16). Briefly, the HABPS includes 972 cases of ischemic stroke occurring anytime after the baseline WHI-OS examination and before July 1st, 2003 and 972 controls who did not experience a stroke, matched on baseline age, race-ethnicity, date of study enrollment, and follow-up time. Inclusion criteria for the HaBPS study were age 50–79 years at baseline, postmenopausal, absence of medical conditions with a predicted survival <3 years, absence of a history of myocardial infarction or stroke at baseline, and availability of a blood sample for biomarker assays. The extensive inflammatory biomarker profile available provided a unique opportunity for the current cross-sectional analyses utilizing the baseline data from the HaBPS to characterize the inflammation profile of body size phenotypes. Case-control status is considered as a covariate.
All participants provided written informed consent for WHI, as approved by the institutional review boards at each participating WHI-OS site.
Measurement of Physical Factors and Health Behaviors
Anthropometric measurements were obtained by trained and certified clinical center staff at the baseline clinic visit. Height was measured with a calibrated stadiometer and weight with a calibrated scale, with participants in light clothing. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. Based on their BMI, individuals were classified as being normal weight (BMI of 18.5 to 24.9 kg/m2) or overweight/obese (BMI of ≥25.0 kg/m2). Waist circumference was measured at the narrowest part of the torso and hip circumference at the maximal circumference.
Seated systolic and diastolic blood pressures were measured in the right arm using a conventional mercury sphygmomanometer after 5 minutes of rest, with an appropriate cuff size based on arm circumference measurement. Two blood pressure measurements were taken at least 30 seconds apart, and were averaged for the current analyses. Questionnaires were used to ascertain self-reported physical activity in metabolic equivalent tasks (METS) per week and current smoking. Women were asked to bring all of their prescription medications to the baseline visit for entry into a pharmacy database (Master Drug Database, Medi-Span), and this database was used to identify current users of anti-hypertensive, lipid-lowering, anti-diabetic, and non-steroidal anti-inflammatory drugs, and aspirin.
Laboratory Analyses
Stored blood specimens were sent to the WHI core laboratory for measurement of levels of plasma inflammatory cytokines. High-sensitivity CRP was measured by immunoturbidity initially, and then by immunonephelometry. Results from each were found to be comparable. IL-6, TNF-alpha, VCAM-1, PAI-1, and E-selectin were each measured by ELISA (R&D Systems). Fibrinogen was measured via optical clot detection (MLA 1400). Fasting plasma glucose and lipids were measured at Medical Research Laboratories, also from stored specimens. The Friedewald equation was used to calculate LDL concentration from total cholesterol, HDL cholesterol, and triglyceride concentrations. WBC was analyzed by a local laboratory at each study site at the time of collection. HOMA was used to evaluate insulin resistance using the following formula: fasting serum insulin (μU/ml) x fasting plasma glucose (mmol/l)/22.5.
Body Size Phenotype Definitions
Four body size phenotypes are examined in the current analyses based on combined consideration of BMI values, diabetes status, and the following metabolic syndrome components:1.) systolic/diastolic blood pressure ≥130/85 mmHg or antihypertensive medication use, 2.) fasting triglycerides ≥1.7 mmol/L), 3.) HDL <1.3 mmol/L or lipid-lowering medication use, and 4.) fasting glucose ≥5.6 mmol/L. Waist circumference was not considered in the body size phenotype definition due to its covariability with BMI. Body size phenotypes were defined as follows: Normal weight without metabolic syndrome components: BMI< 25 kg/m2 and 0 or 1 of the 4 metabolic syndrome components considered, but no diabetes; normal weight with metabolic syndrome components: BMI< 25 kg/m2 and ≥2 of the 4 metabolic syndrome components considered or diabetes; overweight/obese without metabolic syndrome components: BMI ≥25 kg/m2 and 0 or 1 of the 4 metabolic syndrome components considered, but no diabetes, and overweight/obese with metabolic syndrome components: BMI ≥25 kg/m2 and ≥2 of the 4 metabolic syndrome components considered, or diabetes.
Statistical Methods
Of the 1,944 women (972 matched case control pairs) in the HaBPS, 22 women were excluded for being underweight (BMI<18.5 kg/m2), 23 women did not have height and/or weight values, and in 10 women, body size phenotype could not be determined due to missing cardiometabolic syndrome component data, resulting in a sample size of 1,889 for the current analyses.
Sociodemographic characteristics, metabolic parameters, and inflammatory biomarker levels were compared between body size and metabolic groups using chi-square statistics for categorical variables, t-tests for normally distributed continuous variables and the non-parametric alternative, Mann-Whitney U for non-normally distributed continuous variables.
Odds ratios (OR) with 95% confidence intervals (CI) of being in the top quartile of each inflammatory biomarker associated with body size phenotypes (normal weight without diabetes and only 0 or 1 metabolic syndrome components as the referent category) were calculated using logistic regression adjusted for age, race/ethnicity, smoking status, income, physical activity, education, hormone therapy use, NSAID use, baseline history of CVD and stroke case/control status, and after further adjustment for waist circumference.
Four forms of sensitivity analyses were performed: 1.) the BMI criterion for body size in the definition of the phenotypes was replaced with abdominal obesity (waist ≥88 cm vs. waist <88 cm) in logistic regression analyses, 2.) analyses were restricted to the control group, only, 3.) women reporting the inflammatory conditions lupus and rheumatoid arthritis (n=113) were excluded, and 4.) a more stringent definition of metabolically benign overweight/obesity was used where-by metabolically benign overweight-obese women were not allowed to have any of the components of the metabolic syndrome or diabetes.
RESULTS
The prevalence of the four body size phenotypes was as follows: normal weight with 0 or 1 metabolic syndrome components 20.3% (n=384), normal weight with ≥2 metabolic syndrome components or diabetes 16.7% (n=315), overweight/obese with 0 or 1 metabolic syndrome components 17.5% (n=330) and overweight/obese with ≥2 metabolic syndrome components or diabetes 45.6% (n=860). Within body size groups, this corresponds to 45.1% of all normal weight women who had clustering of metabolic syndrome components or diabetes and 54.9% who did not, and 72.3% of all overweight/obese women who had clustering of metabolic syndrome components or diabetes and 27.7% who did not. Compared to normal weight women, overweight/obese women were younger, more likely to be black, had more adverse levels of blood pressure, lipids, and glucose metabolism, and reported less physical activity, regardless of whether they had the metabolic syndrome or not (Table 1). In addition, among women with 0 or 1 metabolic syndrome components, overweight/obese women had a lower income than normal weight women, while among women with ≥2 metabolic syndrome components or diabetes, overweight/obese women were more likely to be never smokers, had less education, and were less likely to be current hormone users.
Table 1.
Characteristics of Study Subjects by Body Size Phenotypes
| Risk Factor | Normal Weight, 0 or 1 Metabolic Abnormalities (Reference) (n=384) | Normal Weight, 2 Metabolic Abnormalities or Diabetes (n=315) | Overweight/Obese, 0 or 1 Metabolic Abnormalities (n=330) | Overweight/Obese, 2 Metabolic Abnormalities or Diabetes (n=860) |
|---|---|---|---|---|
| Age, years | 68.9 (6.3) | 70.2 (5.7)†† | 67.6 (6.4)** | 68.5 (6.5)*** † |
| Race-ethnicity, n (%) | ||||
| White Non-Hispanic | 357 (92.3) | 270 (85.7) † | 278 (84.2)*** | 716 (83.3)*** |
| Black | 8 (2.1) | 12 (3.8) | 39 (11.8) | 95 (11.1) |
| Other | 19 (5.0) | 33 (10.5) | 13 (3.9) | 49 (5.7) |
| Smoking Status, n (%) | ||||
| Never | 202 (53.4) | 150 (47.9) | 181 (55.7) | 465 (54.5)* |
| Former | 150 (39.7) | 136 (43.5) | 130 (40.0) | 343 (40.2) |
| Current | 26 (6.9) | 27 (8.6) | 14 (4.3) | 45 (5.3) |
| Education, n (%) | ||||
| ≤ High school | 97 (25.5) | 112 (35.7) †† | 108 (32.9) | 360 (42.1)* †† |
| Some college/post secondary training | 104 (27.3) | 84 (26.8) | 92 (28.1) | 234 (27.4) |
| College graduate or higher | 180 (47.2) | 118 (37.6) | 128 (39.0) | 261 (30.5) |
| Income, n (%) | ||||
| <$35,000 | 135 (35.2) | 149 (47.3) ††† | 153 (46.4)* | 451 (52.4) |
| $35,000–$49,999 | 69 (18.0) | 66 (21.0) | 55 (16.7) | 149 (17.3) |
| ≥$50,000 | 146 (38.0) | 81 (25.7) | 96 (29.1) | 195 (22.7) |
| Refused/Missing | 34 (8.9) | 19 (6.0) | 26 (7.9) | 65 (7.6) |
| Hormone Use, n (%) | ||||
| Never | 143 (37.2) | 122 (38.7) | 138 (41.8) | 423 (49.2)** † |
| Past | 75 (19.5) | 57 (18.1) | 54 (16.4) | 143 (16.6) |
| Current | 166 (43.2) | 136 (43.2) | 138 (41.8) | 294 (34.2) |
| Current Antihypertensive Medication Use, n (%) | 68 (17.7) | 142 (45.1) ††† | 95 (28.8)*** | 479 (55.7)*** ††† |
| Current Lipid-Lowering Medication Use, n (%) | 8 (2.1) | 45 (14.3) ††† | 7 (2.1) | 112 (13.0) ††† |
| Current NSAID Use, n (%) | 125 (32.6) | 120 (38.1) | 121 (36.7) | 356 (41.4) |
| History of CVD, n (%) | 22 (5.7) | 46 (14.6) ††† | 21 (6.4) | 128 (14.9) ††† |
| Blood Pressure ≥130/85 mmHg, n (%) | 151 (39.4) | 252 (80.0) ††† | 136 (41.5) | 732 (85.7)* ††† |
| Triglycerides ≥1.7 mmol/L, n (%) | 61 (15.9) | 234 (74.3) ††† | 52 (15.8) | 660 (76.8) ††† |
| High Density Lipoprotein Cholesterol <1.3 mmol/L, n (%) | 22 (5.7) | 124 (39.4) ††† | 17 (5.2) | 449 (52.4)*** ††† |
| Waist Circumference >88cm, n (%) | 9 (2.4) | 18 (5.7) † | 153 (46.4)*** | 563 (65.5)*** ††† |
| Fasting Glucose, n (%) | ||||
| <5.6 mmol/L | 355 (92.5) | 144 (45.7) ††† | 297 (90.0) | 321 (37.3) ††† |
| ≥5.6 mmol/L and < 7.0 mmol/L | 29 (7.6) | 131 (41.6) | 33 (10.0) | 373 (43.4) |
| ≥7.0 mmol/L | 0 (0.0) | 40 (12.7) | 0 (0.0) | 166 (19.3) |
| HOMA Insulin Resistance Index, n (%) | ||||
| Quartile 1–3 | 381 (99.2) | 270 (86.0) ††† | 300 (90.9)*** | 464 (54.0)*** ††† |
| Quartile 4 | 3 (0.8) | 44 (14.0) | 30 (9.1) | 395 (46.0) |
| Systolic Blood Pressure, mmHg | 123.9 (16.5) | 137.7 (18.5) ††† | 126.9 (17.1)* | 139.4(18.4) ††† |
| Diastolic Blood Pressure, mmHg | 71.3 (9.1) | 75.1 (10.2) ††† | 74.2 (8.8)*** | 76.5 (9.9)* ††† |
| Pulse Pressure, mmHg | 52.6 (14.1) | 62.6 (16.5) ††† | 52.7 (14.4) | 62.9 (16.5) ††† |
| Total Cholesterol, mmol/L | 220.1 (34.6) | 236.0 (38.9) ††† | 230.3 (36.0)*** | 236.4 (40.0) † |
| Triglycerides, mmol/L‡ | 112.0 (88.0–137.0) | 179.0 (141.0–228.0) ††† | 116.5 (94.0–140.0) | 184.0 (146.0–246.0) ††† |
| High Density Lipoprotein Cholesterol, mmol/L | 69.6 (16.0) | 56.5 (15.2) ††† | 64.8 (13.2)*** | 51.7 (14.2) *** ††† |
| Low Density Lipoprotein Cholesterol, mmol/L | 126.9 (32.8) | 141.6 (37.3) ††† | 141.2 (34.9)*** | 144.6 (37.9) |
| Glucose, mmol/L‡ | 91.0 (87.0–95.0) | 100.0 (92.0–109.0) | 93.0 (88.0–97.0)** | 103.0 (95.0–118.0) *** ††† |
| Insulin, pmol/L‡ | 3.7 (2.7–5.1) | 5.6 (4.21–7.7) ††† | 5.8 (4.3–8.4)*** | 9.7(6.4–14.3)*** ††† |
| BMI, kg/m2 | 22.3 (1.6) | 23.0 (1.5) ††† | 29.0 (4.2)*** | 30.9 (5.2)*** ††† |
| Waist Circumference, cm | 74.3 (6.7) | 77.6 (6.6) ††† | 87.6 (10.0)*** | 94.2 (11.8)*** ††† |
| Waist-Hip Ratio | 0.77 (0.06) | 0.81 (0.10) ††† | 0.80 (0.07)*** | 0.85 (0.08)*** ††† |
| Physical Activity, METs per week‡ | 15.3 (6.8–26.0) | 11.1 (3.8–20.1) ††† | 8.8 (2.5–18.8)*** | 7.5 (1.5–16.5)*** † |
Cardiometabolic abnormalities considered were: 1.) systolic/diastolic blood pressure ≥130/85 mmHg or antihypertensive medication use, 2.) fasting triglycerides ≥1.7 mmol/L, 3.) HDL <1.3 mmol/L, and 4.) fasting glucose ≥5.6 mmol/L or antidiabetic med use.
Median (interquartile range);
p<0.05,
p<0.01,
p<0.001 vs. normal weight within metabolic subgroup;
p<0.05,
p<0.01,
p<0.001 vs. vs. metabolically healthy within body size subgroup.
Compared to women with 0 or 1 metabolic syndrome components, women with ≥2 metabolic syndrome components or diabetes were older, had lower levels of education, had a higher prevalence of history of CVD and each of the metabolic abnormalities considered, and had higher BMI values, larger waist circumference values, and reported lower levels of physical activity. In addition, among normal weight women, women with ≥2 metabolic syndrome components or diabetes were more likely to be non-white and had a lower income than women with 0 or 1 metabolic syndrome components, while among overweight/obese women, women with ≥2 metabolic syndrome components or diabetes were more likely to have never used hormones.
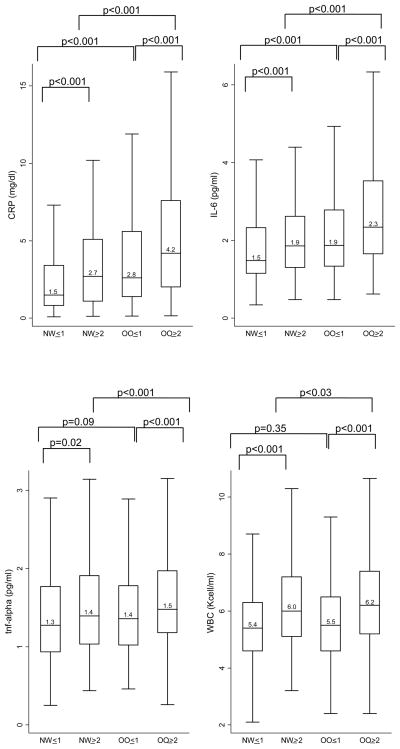
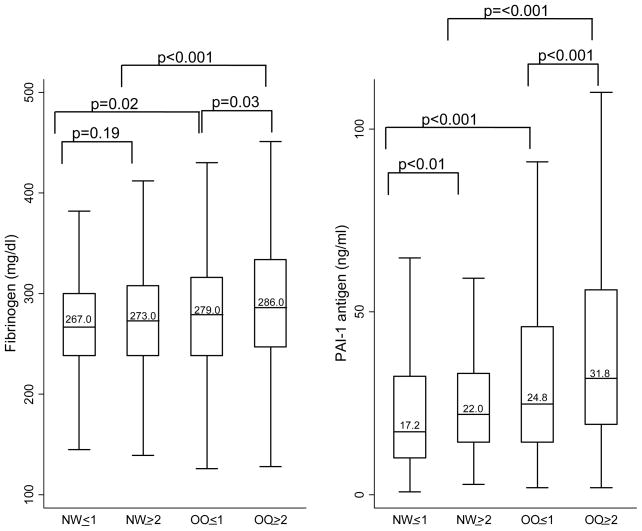
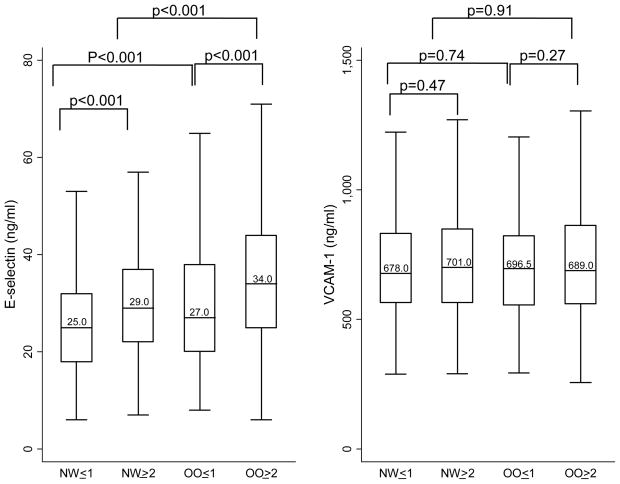
Median inflammatory marker levels for each body size phenotype are presented in Figures 1 to 3. Both overweight/obesity and clustering of cardiometabolic abnormalities were associated with higher levels of inflammatory markers. Compared to women with only 0 or 1 metabolic syndrome components, women with ≥2 metabolic syndrome components or diabetes (whether normal weight or overweight/obese) had significantly higher levels of CRP, IL-6, TNF-alpha, WBC, E-selectin, and PAI-1. In addition, among overweight/obese women, those with ≥2 metabolic syndrome components or diabetes also had higher levels of fibrinogen compared to those with 0 or 1 metabolic syndrome components. Compared to their normal weight counterparts, overweight/obese women (whether with cardiometabolic abnormality clustering or not) had significantly higher levels of CRP, IL-6, E-selectin, fibrinogen, and PAI-1. Additionally, among women with ≥2 metabolic syndrome components or diabetes, overweight/obese women had significantly higher levels of TNF-alpha and WBC compared to normal weight women.
Figure 1.
Median Acute-Phase Marker Levels by Body Size Phenotypes
Horizontal lines represent medians, while bottom and top of boxes represent 25th and 75th percentiles, respectively. NW=Normal weight; OO=overweight/obese; ≤1 = 0 or 1 cardiometabolic abnormalities and no diabetes; ≥2= 2 or more cardiometabolic abnormalities or diabetes
Cardiometabolic abnormalities considered were: 1.) systolic/diastolic blood pressure ≥130/85 mmHg or antihypertensive medication use, 2.) fasting triglycerides ≥1.7 mmol/L, 3.) HDL <1.3 mmol/L and 4.) fasting glucose ≥7.0 mmol/L or antidiabetic med use
Figure 3.
Median Coagulation Factor Levels by Body Size Phenotypes
Horizontal lines represent medians, while bottom and top of boxes represent 25th and 75th percentiles, respectively. NW=Normal weight; OO=overweight/obese; ≤1 = 0 or 1 cardiometabolic abnormalities and no diabetes; ≥2= 2 or more cardiometabolic abnormalities or diabetes
Cardiometabolic abnormalities considered were: 1.) systolic/diastolic blood pressure ≥130/85 mmHg or antihypertensive medication use, 2.) fasting triglycerides ≥1.7 mmol/L, 3.) HDL <1.3 mmol/L and 4.) fasting glucose ≥7.0 mmol/L or antidiabetic med use
When these associations were adjusted for confounders, overweight/obese women with 0 or 1 metabolic syndrome components had approximately 2 times greater odds of having CRP, E-selectin, fibrinogen, and PAI-1 levels in the top quartile compared to normal weight women with 0 or 1 metabolic syndrome components (Table 2). Once these results were further adjusted for waist circumference (Model 2), odds ratios were attenuated, but remained borderline statistically significant for e-selectin, fibrinogen, and PAI-1. The number of inflammatory markers which were elevated and the magnitude of those odds ratios were even greater among women who were both overweight/obese and had ≥2 metabolic syndrome components or diabetes. Among these women, all inflammatory markers except for VCAM-1 were statistically significantly elevated in Model 1. Once these results were further adjusted for waist circumference (Model 2), all differences but IL-6 and TNF-alpha remained statistically significant. For overweight/obese women with ≥2 metabolic syndrome components or diabetes vs. normal weight women with 0 or 1 metabolic syndrome components, differences in CRP, WBC, and e-selectin were statistically significantly elevated, even after adjustment for waist circumference, while borderline statistically significant results were seen with fibrinogen levels.
Table 2.
Adjusted* Odds Ratios (95% CI) of the Top Quartile of Inflammatory Markers Associated with Body Size Phenotypes.
| Normal Weight, 0 or 1 Metabolic Abnormalities (Reference) (n=384) | Normal Weight, ≥2 Metabolic Abnormalities or Diabetes (n=315) | Overweight/Obese, 0 or 1 Metabolic Abnormalities (n=330) | Overweight/Obese, ≥2 Metabolic Abnormalities or Diabetes (n=860) | |
|---|---|---|---|---|
| Acute-phase Markers | ||||
| CRP | ||||
| Model 1* | 1.0 | 1.4 (0.9, 2.2) | 1.7 (1.1, 2.6) | 3.5 (2.4, 5.2) |
| Model 2 | 1.0 | 1.3 (0.8, 2.0) | 1.1 (0.7, 1.7) | 1.8 (1.2, 2.8) |
| IL-6 | ||||
| Model 1* | 1.0 | 1.0 (0.6, 1.5) | 1.3 (0.9, 1.9) | 2.0 (1.4, 2.8) |
| Model 2 | 1.0 | 0.9 (0.6, 1.3) | 0.7 (0.5, 1.1) | 0.8 (0.6, 1.2) |
| TNF-alpha | ||||
| Model 1* | 1.0 | 1.4 (0.9, 2.0) | 1.2 (0.8, 1.8) | 1.5 (1.1, 2.0) |
| Model 2 | 1.0 | 1.3 (0.9, 2.0) | 1.1 (0.7, 1.6) | 1.2 (0.8, 1.8) |
| WBC | ||||
| Model 1* | 1.0 | 2.2 (1.5, 3.4) | 1.3 (0.8, 2.1) | 3.1 (2.2, 4.5) |
| Model 2 | 1.0 | 2.1 (1.4, 3.2) | 1.0 (0.6, 1.6) | 2.1 (1.4, 3.2) |
| Adhesion Molecules | ||||
| E-Selectin | ||||
| Model 1* | 1.0 | 1.9 (1.2, 3.0) | 2.1 (1.4, 3.2) | 4.0 (2.7, 5.8) |
| Model 2 | 1.0 | 1.7 (1.1, 2.7) | 1.4 (0.9, 2.2) | 2.2 (1.4, 3.3) |
| VCAM-1 | ||||
| Model 1* | 1.0 | 1.2 (0.8, 1.7) | 1.1 (0.8, 1.6) | 1.2 (0.9, 1.7) |
| Model 2 | 1.0 | 1.1 (0.8, 1.7) | 1.0 (0.7, 1.6) | 1.1 (0.7, 1.6) |
| Coagulation Markers | ||||
| Fibrinogen | ||||
| Model 1* | 1.0 | 1.3 (0.9, 2.1) | 1.7 (1.1, 2.6) | 2.0 (1.4, 2.8) |
| Model 2 | 1.0 | 1.3 (0.9, 2.1) | 1.5 (0.9, 2.3) | 1.6 (1.0, 2.4) |
| PAI-1 | ||||
| Model 1* | 1.0 | 1.0 (0.7, 1.6) | 1.5 (1.0, 2,2) | 2.1 (1.5, 3.0) |
| Model 2 | 1.0 | 1.0 (0.7, 1.6) | 1.4 (0.9, 2.2) | 1.9 (1.3, 2.9) |
Cardiometabolic abnormalities considered were: 1) systolic/diastolic blood pressure ≥130/85 mmHg or antihypertensive medication use, 2.) fasting triglycerides ≥1.7 mmol/L, 3.) HDL <1.3 mmol/L and 4.) fasting glucose ≥5.6 mmol/L or antidiabetic med use Bolded results correspond to p<0.05.
MODEL 1 Adjusted for age, race-ethnicity, smoking, income, physical activity, education, hormone therapy use, NSAID use, baseline history of CVD, and stroke case-control status.
MODEL 2: adjusted for covariates in Model 1 plus Waist Circumference.
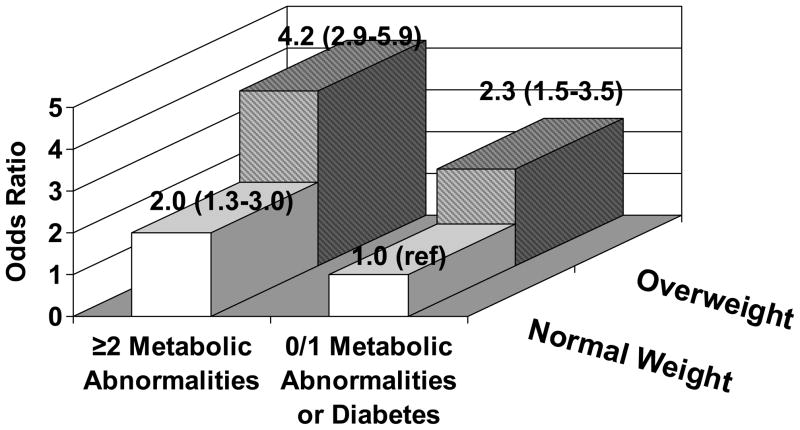
When all of the biomarkers were considered together, 30.1% of women had ≥3 inflammatory markers in the top quartile. The odds ratios associated with possessing ≥3 inflammatory markers in the top quartile are presented for each body size phenotype in Figure 4. As can be seen, the odds ratio of having ≥3 inflammatory markers in the top quartile was elevated for women who were either overweight/obese or had ≥2 metabolic syndrome components or diabetes, with the greatest odds ratio among overweight/obese women with ≥2 metabolic syndrome components or diabetes.
Figure 4.
Adjusted* Odds Ratios (95% Confidence Intervals) of Possessing 3 Inflammatory Markers in the Top Quartile associated with Body Size Phenotypes
Metabolic abnormalities considered were: 1.) systolic/diastolic blood pressure ≥130/85 mmHg or antihypertensive medication use, 2.) fasting triglycerides ≥1.7 mmol/L, 3.) HDL <1.3 mmol/L and 4.) fasting glucose ≥5.6 mmol/L or antidiabetic med use
*Adjusted for age, race-ethnicity, smoking, income, physical activity, education, hormone therapy use, NSAID use, baseline history of CVD, and stroke case-control status
Odds ratios were re-calculated using abdominal adiposity categories (waist circumference 88 cm) in place of BMI categories in the body size phenotype definition, and results were similar, where-by women with elevated waist circumference but with only 0 or 1 metabolic syndrome components had an odds ratio of ≥3 inflammatory markers in the top quartile of 3.2 (95% CI 2.1–4.9) compared to non-abdominally obese women with only 0 or 1 metabolic syndrome components. Additionally, logistic regression results were recalculated utilizing the control group, only, to ensure that underlying vascular disease related to subsequent stroke in the stroke group was not driving the results. Again, the magnitude of odds ratios was similar (odds ratio of ≥3 inflammatory markers in the top quartile of 2.4 [95% CI 1.4–4.2] and 1.6 [0.9–3.0] for overweight/obese women with 0 or 1 metabolic syndrome components and normal weight women with ≥2 metabolic syndrome components or diabetes vs. normal weight women with only 0 or 1 metabolic syndrome components, respectively). In addition, analyses were repeated excluding the 113 women reporting either lupus or rheumatoid arthritis, and results were again similar (odds ratios 2.2 [1.4–3.3] and 2.0 [1.3–3.0] for overweight/obese women with 0 or 1 metabolic syndrome components and normal weight women with ≥2 metabolic syndrome components or diabetes vs. normal weight women with 0 or 1 metabolic syndrome components, respectively). Lastly, analyses were repeated using a more stringent definition of benign overweight/obesity, requiring 0 metabolic syndrome components. The magnitude of odds ratios were similar or even stronger when comparing them to normal weight women with 0 components (odds ratios of ≥3 inflammatory markers in the top quartile 2.8 [1.0–7.2] and 3.4 [1.5–7.5] for overweight/obese women with 0 metabolic syndrome components and normal weight women with ≥1 metabolic syndrome components or diabetes vs. normal weight women with 0 metabolic syndrome components, respectively). Therefore, even without any metabolic syndrome components or diabetes, overweight/obese women had higher levels of inflammatory markers than normal weight women without any metabolic syndrome components or diabetes.
DISCUSSION
In this study of postmenopausal women we found that normal weight women with ≥2 metabolic syndrome components or diabetes as well as overweight/obese women both with ≥2 or more metabolic syndrome components or diabetes (at-risk phenotype), or without diabetes and with only 0 or 1 metabolic syndrome components (metabolically benign phenotype) had higher inflammatory biomarker levels compared to normal weight women without diabetes and with only 0 or 1 metabolic syndrome components. Results were similar when the metabolically benign phenotype was defined as having 0 metabolic syndrome components. Therefore, excess body size, even in the absence of elevated cardiometabolic risk factors and diabetes was associated with elevations in inflammatory biomarkers. Further, those with both excess body size and cardiometabolic risk factor clustering or diabetes had the greatest burden of inflammation.
Obesity and the metabolic syndrome have each been shown to be associated with elevations in inflammatory markers(17–22). However, the current study is among few to determine the independent associations of overweight/obesity vs. cardiometabolic abnormalities with inflammatory profiles and suggests that both excess body size and cardiometabolic abnormalities are independently associated with the inflammatory profile. Similar results were found for CRP and fibrinogen among the approximately 1,600 participants of the Insulin Resistance Atherosclerosis Study (IRAS), whereby body fat was positively associated with inflammation in both type 2 diabetics and non-diabetics(9). Additionally, our results in post-menopausal women are similar to those reported among a small sample (n=43) of pre-menopausal women, in whom IL-6 and CRP levels were higher among metabolically benign obese women (defined in this case as obese without hypertension, diabetes or other diseases) compared to healthy normal weight women(23). However, some studies have failed to find a higher burden of inflammatory markers in those with metabolically benign obesity (defined as obesity in the absence of the metabolic syndrome)(10–12). In contrast to our study and the other prior studies finding elevations in inflammatory markers among overweight/obese individuals with the metabolically benign phenotype, null studies were restricted primarily to middle-aged men. Data suggest that associations between both obesity and the metabolic syndrome with inflammatory markers may be stronger in women vs. men,(9;17;20;21) raising the possibility that the independent effects of obesity vs. metabolism may also differ between men and women. However, even our study demonstrated only weak associations with IL-6, TNF-alpha, and WBC, which were the inflammatory markers assessed in the null studies. In the current study, our results were strongest for CRP, e-selectin, and coagulation markers, especially after adjusting for waist circumference. Because IL-6 and TNF-alpha are, in part, produced within adipose tissue, it is unclear why elevations in these markers among metabolically benign, but overweight/obese individuals would be lesser for these markers, even prior to adjustment for waist circumference.
It has been suggested that abdominal adiposity may underlie differences in health risks between groups of similar body size. We and others have shown that at-risk obese individuals have more abdominal adiposity than metabolically benign obese individuals despite similar BMI values(1;24). Karelis et al. (2005) showed that lower CRP levels in metabolically benign vs. at-risk obese postmenopausal women were completely attenuated by adjustment for abdominal visceral fat(25). However, in the current analyses, statistical adjustment for waist circumference only partially attenuated elevations in inflammatory markers among obese women without metabolic syndrome components or diabetes compared to normal weight women without metabolic syndrome components or diabetes, especially when all inflammatory markers were considered together, as in Figure 4. Additionally, when waist circumference was used to defined obesity rather than BMI in the current analyses, abdominal obesity and cardiometabolic abnormalities were each independently associated with inflammation markers.
Data concerning the risk of incident cardiovascular disease in metabolically benign obese individuals is inconclusive. In multiple studies with follow-up times ranging from 3 to 11 years, metabolically benign obese individuals were not at significantly increased risk of incident cardiovascular disease or cardiovascular death compared to normal weight individuals (2–4). However, recent studies with follow-up extending to 16–30 years report an elevated risk of cardiovascular disease in metabolically benign obese(5;6), suggesting that metabolically benign obese individuals are at a lifetime increased risk of cardiovascular events compared to normal weight individuals, but experience a delay in those events compared to their at-risk obese counterparts. Although cross-sectional, the results of the current study finding intermediate levels of inflammatory markers in metabolically benign overweight/obese women between healthy normal weight women and at-risk overweight/obese women are in support of an elevated risk of cardiovascular events compared to normal weight women, but delayed in comparison to at-risk obese women. Prospective studies of inflammatory markers and CVD events are needed in metabolically benign obese individuals.
This study must be viewed within the context of its limitations. This was a cross-sectional study. Therefore, causality between body size and metabolic abnormalities with inflammatory markers could not be determined. Additionally, we did not have sufficient numbers of minority participants to examine the consistency of these associations in race-ethnic subgroups. Finally, we did not have a direct measure of visceral adipose tissue. Recent data suggest that higher abdominal visceral adipose tissue among at-risk obese may partially underlie this group’s metabolic complications(24). However, as noted above, analyses utilizing waist circumference as the measure of obesity rather than BMI produced similar results.
This study had several strengths. This study is among the first to assess the inflammatory biomarker profile of important body size phenotypes at differential risk of CVD. Previous studies have frequently been limited to assessment of benign obese individuals vs. a normal weight control group, while we were able to examine four phenotypes. Second, this study assessed these relationships in women, among whom relationships between obesity and metabolic syndrome with inflammation have been suggested to be stronger compared to men(9;17;20;21). Finally, we assessed a large panel of inflammatory biomarkers representing acute phase, adhesion, and coagulation components of the inflammatory response.
In conclusion, this is among the first studies to assess the inflammatory marker profile of body size phenotypes, finding that body size and clustering of metabolic abnormalities were independently associated with inflammatory markers in these post-menopausal women. Compared to normal weight women without the metabolic syndrome or diabetes, normal weight women with the metabolic syndrome or diabetes, as well as overweight/obese women with (at-risk phenotype) and without (metabolically benign phenotype) clustering of metabolic abnormalities or diabetes had elevated levels of inflammatory markers. These data support recent data suggesting that metabolically benign obese individuals do have elevated risk of CVD events compared to healthy normal weight individuals, but that CVD events are delayed compared to at-risk obese individuals. Prospective inflammatory marker data among body size phenotypes is needed.
Figure 2.
Median Adhesion Molecule Levels by Body Size Phenotypes
Horizontal lines represent medians, while bottom and top of boxes represent 25th and 75th percentiles, respectively. NW=Normal weight; OO=overweight/obese; ≤1 = 0 or 1 cardiometabolic abnormalities and no diabetes; ≥2= 2 or more cardiometabolic abnormalities or diabetes
Cardiometabolic abnormalities considered were: 1.) systolic/diastolic blood pressure ≥130/85 mmHg or antihypertensive medication use, 2.) fasting triglycerides ≥1.7 mmol/L, 3.) HDL <1.3 mmol/L and 4.) fasting glucose ≥7.0 mmol/L or antidiabetic med use
Acknowledgments
The research on which this publication is based was funded by Grant Numbers R01NS042618 (Wassertheil-Smoller) and R03NS061114 (Wildman) from the National Institutes of Neurological Disorders and Stroke. The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
The authors thank the following key investigators involved in the WHI:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Footnotes
DISCLOSURE STATEMENT
The authors declare no conflicts of interest.
References
- 1.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 2.Wildman RP, Lin J, Muntner P, et al. Risk of incident coronary heart disease and stroke associated with abdominal obesity versus the metabolic syndrome and diabetes. Obesity In Press. [Google Scholar]
- 3.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109(6):706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Manson JE, Meigs JB, Ridker PM, Buring JE, Liu S. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol. 2007;100(11):1654–1658. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flint AJ, Hu FB, Glynn RJ, et al. Excess weight and the risk of incident coronary heart disease among men and women. Obesity (Silver Spring) 2010;18(2):377–383. doi: 10.1038/oby.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121(2):230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Yudkin JS, Stehouwer CD, Schalkwijk CG, Pratley RE, Tataranni PA. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis. 2002;161(1):233–242. doi: 10.1016/s0021-9150(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 8.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 9.Festa A, D’Agostino R, Jr, Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25(10):1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 10.Desai MY, Dalal D, Santos RD, Carvalho JA, Nasir K, Blumenthal RS. Association of body mass index, metabolic syndrome, and leukocyte count. Am J Cardiol. 2006;97(6):835–838. doi: 10.1016/j.amjcard.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Nishida M, Moriyama T, Sugita Y, Yamauchi-Takihara K. Abdominal obesity exhibits distinct effect on inflammatory and anti-inflammatory proteins in apparently healthy Japanese men. Cardiovasc Diabetol. 2007;6:27. doi: 10.1186/1475-2840-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring) 2006;14(12):2127–2131. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 13.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 14.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan RC, McGinn AP, Baird AE, et al. Inflammation and hemostasis biomarkers for predicting stroke in postmenopausal women: the Women’s Health Initiative Observational Study. J Stroke Cerebrovasc Dis. 2008;17(6):344–355. doi: 10.1016/j.jstrokecerebrovasdis.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassertheil-Smoller S, Kooperberg C, McGinn AP, et al. Lipoprotein-associated phospholipase A2, hormone use, and the risk of ischemic stroke in postmenopausal women. Hypertension. 2008;51(4):1115–1122. doi: 10.1161/HYPERTENSIONAHA.107.103721. [DOI] [PubMed] [Google Scholar]
- 17.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183(2):308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Bluher M, Fasshauer M, Tonjes A, Kratzsch J, Schon MR, Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005;113(9):534–537. doi: 10.1055/s-2005-872851. [DOI] [PubMed] [Google Scholar]
- 19.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25(11):2016–2021. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 21.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110(4):380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES. The metabolic syndrome and C-reactive protein, fibrinogen, and leukocyte count: findings from the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2003;168(2):351–358. doi: 10.1016/s0021-9150(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 23.Malavazos AE, Corsi MM, Ermetici F, et al. Proinflammatory cytokines and cardiac abnormalities in uncomplicated obesity: relationship with abdominal fat deposition. Nutr Metab Cardiovasc Dis. 2007;17(4):294–302. doi: 10.1016/j.numecd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 25.Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90(7):4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]