Abstract
Lang’s tripartite model posits that three main components characterize a fear response: physiological arousal, cognitive (subjective) distress, and behavioral avoidance. These components may occur in tandem with one another (concordance) or they may vary independently (discordance). The Behavioral Approach Test (BAT) has been used to simultaneously examine the three components of the fear response. In the present study, 73 clinic-referred children and adolescents with a specific phobia participated in a phobia-specific BAT. Results revealed an overall pattern of concordance: correlation analyses revealed the three indices were significantly related to one another in the predicted directions. However, considerable variation was noted such that some children were concordant across the response components while others were not. More specifically, based on levels of physiological arousal and subjective distress, two concordant groups (high arousal-high distress, low arousal-low distress) and one discordant (high arousal-low distress or low arousal-high distress) group of youth were identified. These concordant and discordant groups were then compared on the percentage of behavioral steps completed on the BAT. Analyses revealed that the low arousal-low distress group completed a significantly greater percentage of steps than the high arousal-high distress group, and a marginally greater percentage of steps than the discordant group. Potential group differences associated with age, gender, phobia severity, and phobia type were also explored and no significant differences were detected. Implications for theory and treatment are discussed.
Keywords: Tripartite Model, Behavioral Approach Test, Children, Adolescents, Phobia, Physiology
According to Lang’s tripartite model (Lang, 1967, 1979; Lang, Cuthbert, & Bradley, 1998; Lang, Levin, Miller, & Kozak, 1983), the emotion of fear is comprised of a neural network of three loosely coupled components: physiological arousal, cognitive (subjective) distress, and behavioral avoidance. Although activity in one of these components can activate the remaining components, the extent of “diffusion” is dependent upon the strength of the initiation and the level of fear. In some instances, the three components co-vary with one another and in other instances the components do not respond in concert. In fact, it is possible for any one of the components to be in ascendance while the others lay relatively dormant. Thus, although some fearful individuals experience high physiological arousal and subjective distress and avoid the phobic stimulus, others experience high physiological arousal and subjective distress but do not avoid the phobic stimulus. All combinations of the three components of the neural network are possible.
Over 35 years ago, Hodgson and Rachman (1974) referred to this phenomenon as concordance versus discordance. That is, when the three components co-vary with one another they are said to be concordant; however, when they vary independently of one another they are said to be discordant. Concordance is hypothesized to be high when there is strong emotional arousal (Hodgson & Rachman). Lang and Cuthbert (1984) also suggest that concordance among the three response components would be greater in individuals with specific phobias versus other anxiety disorders since the fear response would likely be heightened in these disorders.
The Behavioral Approach Test (BAT) is a laboratory-based behavioral measure designed to simultaneously elicit the three components of the fear response (Borkovec, Weerts, & Bernstein, 1977; Dadds, Rapee, & Barrett, 1994). The test consists of a number of increasingly difficult steps in which individuals are asked to approach a phobic object or situation, but are told they can stop the test at any time they wish to do so. During the BAT, individuals’ physiological responses are recorded and they are asked to provide “subjective units of distress” (SUDS) ratings immediately after encountering the phobic object or situation.
A number of studies have used the BAT to examine the phenomenon of concordance/discordance; however, all of these studies have been undertaken with adults. For example, Öst, Stridh, and Wolf (1998) found the behavioral and subjective components of the BAT to be significantly and inversely related in a group of spider phobic adults. Unfortunately, they did not examine whether these indices were concordant with physiological measures. In a similar investigation of blood phobic adults, Hellstrom, Fellenius, and Öst (1996) found that high levels of subjective anxiety were associated with low percentages of steps completed on the BAT; however, they too failed to assess whether physiological arousal was related to these behavioral and cognitive indices. Other studies, however, have shown positive relations between subjective distress and physiological responding (Lewis & Drewett, 2006; Sartory, Rachman, & Grey, 1977). And, in a single case study assessing all three response components, Schwartz, Houlihan, Krueger, and Simon (1997) found concordance among behavioral avoidance, subjective distress, and elevated blood pressure in a woman with a specific phobia. However, not all studies have demonstrated concordance among the three response channels. For example, Côté and Bouchard (2005) conducted BATs with spider phobic adults and reported a small and non-significant correlation between the number of steps completed and physiological arousal.
In addition to these studies, other researchers have examined individual differences that might moderate the degree of concordance among the three response systems. In an attempt to directly test Hodgson and Rachman’s (1974) hypothesis that level of emotional arousal would be one such moderator, Kaloupek and Levis (1983) used the BAT to examine fear of snakes in women who differed in level of arousal. Individuals who were unable to make physical contact with the snake were assumed to be more highly aroused than individuals who could touch the snake. Contrary to their hypothesis, they found that those women who were presumably less emotionally aroused had a greater degree of concordance among the three response components on the BAT, whereas those who were more emotionally aroused evidenced discordance. With more fearful individuals, Calvo and Miguel-Tobal (1998) found the expected effects; namely, those individuals high in arousal displayed a greater amount of concordance among the three components of the fear response during a social-evaluative BAT. Sartory, Rachman, and Grey (1977) obtained similar results in a study of adults with specific phobias.
Two possible reasons have been put forth to account for the increase in concordance as a result of heightened emotional arousal (Calvo & Miguel-Tobal, 1998). First, individuals who experience stronger emotional reactivity might be better able to perceive their physiological arousal and adjust their self-reported distress ratings to match their heightened physiological state. This possibility fits nicely with Lang and Cuthbert’s (1984) theory that the expression of fear occurs most readily when associated brain networks are accessed and processed. Such is thought to occur when a sufficient number of propositions are activated by environmental stimuli or internal associations. A second reason for these findings is that for individuals who are low in emotional arousal, there may be a bias to avoid processing threatening stimuli and to inhibit reporting distress (Calvo & Miguel-Tobal; Kaloupek & Levis, 1983). These behavioural and cognitive mechanisms consequently perturb the level of concordance that is detected.
Overall, studies of concordance and discordance in adults have yielded mixed results. One likely reason is methodological differences, particularly in the varying ways the three response components are measured, how emotional arousal is defined, and the use of clinical versus non-clinical participants (Turpin, 1991). In addition, others have suggested the mixed results may be accounted for by differences in concordance due to demographic and clinical variables such as age (Teachman & Gordon, 2009), gender (Avero & Calvo, 1999), use of divergent coping strategies activated during the BAT (Calvo & Miguel-Tobal, 1998; Kaloupek & Levis, 1983), and the type of specific phobia (Davis & Ollendick, 2006).
The present study examined concordance and discordance in a group of children and adolescents with specific phobias who completed phobia-specific BATs. To date, only a few studies have used the BAT with phobic children, and those studies have limited its use to a treatment outcome measure (Nelissen, Muris, & Merkelbach, 1995; Ollendick et al., 2009; Öst, Svensson, Hellstrom, & Lindwall, 2001). To our knowledge, concordance and discordance in the fear response have not been examined with phobic children and adolescents.
We put forth three hypotheses. First, based on the notion that specific phobias may evidence the greatest amount of concordance of all the anxiety disorders (Lang & Cuthbert, 1984), we hypothesized that concordance would be observed among the three components in our group of clinically phobic youth, such that a) higher subjective distress would be related to a lower percentage of steps completed, b) higher subjective distress would be related to increased physiological arousal, and c) increased physiological arousal would be related to a lower percentage of steps completed. Second, consistent with previous investigations with adults (Avero & Calvo, 1999; Kring & Gordon, 1998; Turner & Beidel, 1985), we formed three groups of children according to whether they were high or low on physiological arousal and subjective distress and determined how they differed in their level of behavioral avoidance (i.e., percent of steps completed on the BAT). In accord with Lang and Cuthbert (1984), we hypothesized that youth categorized as concordant on the physiological arousal and subjective distress indices would evidence co-varying behavioral responses (i.e. high arousal-high distress children would complete fewer steps on the BAT and low arousal-low distress children would complete more steps on the BAT). In addition, we expected children categorized as discordant on these two response components (i.e., high arousal-low distress or low arousal-high distress) would show intermediate behavioral responses on the BAT. This latter hypothesis was based on the notion that activation on only one of these two indices would suggest low emotional arousal and result in less activation on the third index.
Third, we examined potential group differences associated with age, gender, phobia type, and phobia severity. We reasoned that phobia severity (presumably an index of fear level) would be related to emotional arousal during the BAT; accordingly, we hypothesized there would be greater severity of phobias in the high arousal-high distress group, less severity of phobias in the low arousal-low distress group, and intermediate severity of phobias in the discordant group. We also reasoned that phobia type might be related to emotional arousal on the BAT, such that there would be more children with the natural environment type in the high arousal-high distress group, more children with the animal type in the low arousal-low distress group, and an equal number of each in the discordant group. This hypothesis was based on recent findings showing that children with natural environment phobias differ from children with animal phobias on somatic/anxious symptoms, depressive symptoms, life satisfaction, and co-occurring anxiety disorders (Ollendick, Raishevich, Davis, Sirbu, & Öst, 2010). We did not offer hypotheses about situational or blood-injury-injection phobia types because we did not have a sufficient number of youth in the former group to examine such hypotheses and the latter group was specifically excluded from the original study from which these participants were drawn (Ollendick et al., 2009). Finally, because relations among gender, age, and concordance have not yet been examined with children, we had no specific hypotheses about these potential moderators and these analyses were exploratory.
Method
Participants
Children and adolescents (ages 7–16) from Virginia were recruited for a larger treatment outcome study (Ollendick et al., 2009) through referrals from child mental health services, family medical practices, schools, and newspaper, radio and television advertisements. To be included in the study, participants had to meet criteria for a diagnosis of Specific Phobia, as defined in the DSM-IV (American Psychiatric Association, 1994). As such, it was required that the phobia receive a clinician severity rating of at least 4 on a 0 – 8 scale on a semi-structured diagnostic interview (see below) and have a duration of at least 6 months. Exclusionary criteria included presence of a pervasive developmental disorder, primary major depression but only if suicidal ideation or attempt were present, drug or alcohol abuse, or psychosis. Children with phobias of blood, injection, or injury were also excluded due to their unique physiological profile (see Sarlo, Buodo, Munafo, Palomba, & Stegagno, 2008). Participants were required to discontinue other forms of psychotherapy or anxiolytic medication for the duration of their participation in the study. See Ollendick et al. for further information about the larger study.
Over the course of 5 years, 95 children participated. Of these children, 73 had a complete set of BAT information. Missing data were attributed to equipment failure, BATs conducted in the community (e.g., at the swimming pool, in a high building), or assessor error. The 73 children with complete data included 39 girls (53%) and 34 (47%) boys, with a mean age of 9.5 years (SD = 2.6). Of the children, 86% were Caucasian, 6% were African American, 1% were Hispanic, 1% were Asian, and 5% were of other ethnicities (with 1% not reporting ethnicity). Eighty-four percent of the parents were married or cohabitating, 10% were separated or divorced, and 5% were single parents (1% did not report family structure). Mean family income for the sample was approximately $77,000. Thirty-six (45%) of the children had natural environment type phobias, including fears of dark/being alone (19), thunderstorms (13), and heights (4); 29 (40%) of the children had animal phobias, including fears of dogs (14), spiders (8), bees (5), and cats (2); and 3 children had situation type phobias, comprised of fears of elevators or enclosed spaces (2) and flying (1). Finally, five children had phobias that fit into the “other” category (e.g., phobia of buttons, costumed characters, or loud noises). Approximately 95% of the youth were comorbid with at least one other psychiatric disorder, most commonly other anxiety disorders.
Procedure
Upon referral, parents completed a brief telephone screening interview with a member of the research team. If the child appeared to meet inclusion criteria, the family was scheduled for an assessment. During the initial session, assessors provided more information about the study, gained informed consent and assent, and conducted a semi-structured diagnostic interview (see below). Based on information gathered during the first session, a phobia-specific BAT was designed and administered during a second session, at which time several other measures were obtained but not reported upon here (see Ollendick et al., 2009).
Measures
Anxiety Disorders Interview Schedule for Children (ADIS-C/P; Silverman & Albano, 1996)
The ADIS-C/P is a semi-structured diagnostic interview administered separately to parents and children and designed to assess childhood anxiety disorders, as well as other childhood disorders (e.g., MDD, ADHD). For the most part, the parent and child interviews are similar. However, the parent interview contains modules for several additional disorders (e.g., Conduct Disorder, Oppositional Defiant Disorder, Enuresis), and requests supplementary information with regard to history and interference of specific problems. The child version also requests additional information regarding symptoms and phenomenology of disorders, and utilizes simpler language. The ADIS-C/P has demonstrated adequate test-retest reliability for child (ages 7 – 16, kappas of .61–80), parent (kappas of .65–1.00), and composite (kappas of .62–1.00) diagnoses (Silverman, Saavedra, & Pina, 2001).
Trained graduate clinicians conducted diagnostic interviews for this study, and all interviews were videotaped. Following the interview, the parent and child clinicians separately assigned severity ratings (CSRs) for each diagnosis on a scale from 0 to 8, where 0 = no symptoms, 2 = mild, 4 = moderate, 6 = severe, and 8 = very severe. Subsequent to this, a clinical consensus meeting was held during which final diagnoses and CSRs were assigned, based on both ADIS-P and ADIS-C clinician reports. Mean consensus CSR for the treated phobia was 5.94 (SD = 1.07) on the 0 to 8 severity scale, reflecting moderate to severe levels of disturbance.
To determine reliability of the ADIS-generated diagnoses, independent assessors randomly selected and reviewed 20% of the videotaped interviews with children and parents. Agreements on ADIS diagnoses between independent assessors and both parent and child clinicians, using Cohen’s kappa (Cohen, 1988), were .93 and .88 for primary and secondary diagnoses, respectively. Product-moment correlations were performed to determine interrater reliabilities of ADIS CSR ratings. Reliabilities were .87 and .83 for CSRs of the primary and secondary diagnoses. We did not assess reliability of the consensus diagnoses.
Behavioral Approach Test (BAT)
Based on BATs designed for children by Öst et al. (2001), specific BATs were developed for each of the specific phobias in the current study. For most phobia types, the BATs were realistic in that the child was asked to approach a live animal or actual feared object or situation. For example, a child with a phobia of dogs was asked to approach and pet a leashed dog on the head for twenty seconds and a child fearful of heights was asked to climb a 20 foot ladder to the top rung. The one exception to this was the BAT for storms in which the child was asked to view videotape segments of rain, lightning, and thunderstorms for five minutes. The BATs consisted of a series of gradually more difficult steps (ranging from 7 to 12 steps depending on the specific phobia). Due to the variability in number of steps on the BATs, percentage of steps completed was used as the primary behavioral measure of BAT performance. A subset of 30 participants completed the BAT about 1-hour after the first administration. Test-retest reliability was .92 for percent steps completed.
Prior to the start of the BAT, each child was connected to an Ambulatory Monitoring System v4.4 (AMS; Vrije Universiteit, the Netherlands) to record an electrocardiogram (ECG). Ag-AgCl electrodes were attached to the front torso region according to guidelines set forth in the user manual (Groot, de Geus, & de Vries, 1998). Following connection to the AMS system, the child was instructed to sit quietly for five minutes while watching a non-threatening cartoon program on video. This allowed for measurement of the child’s baseline cardiac activity.
The child was then escorted to a hallway outside the room in which the BAT was to be administered, and was given instructions regarding the task. All children were instructed to do their best, but were also informed they could terminate the BAT at any time if they wished to do so. BATs were terminated at the child’s request or when the terminal step was achieved. Since some children completed very few steps on the BAT or completed it very rapidly, the actual time in the BAT varied from 30 seconds to 5 minutes. Following the BAT, the children were once again asked to sit quietly for five minutes, without access to the cartoon video. This allowed for measurement of the child’s physiological recovery.
SUDS Ratings
Children were asked to provide ratings of their anxiety utilizing SUDS ranging from 0 to 8 (0 = None, 2 = A Little, 4 = Some, 6 = A Lot, and 8 = Very Much). Ratings were obtained immediately following the last step of the BAT. As noted above, a subset of participants completed the BAT twice. Test-retest reliability was .92 for the SUDS rating.
Physiological Measure
One measure of physiological activity, inter-beat interval (IBI), was derived from the ECG recording during baseline (300 sec) and the BAT (M = 145.15 sec, SD = 111.03, range 30 sec to 300 sec). IBI was chosen as the physiological index of arousal because of its demonstrated relationship with the fear response (Fowles, 1980; Rushen, Passille, & Munksgaard, 1999). The mean IBI, quantified as the average time (msec) between successive R-spikes on the ECG, was used as a cardiac chronotropic measure (Berntson, Cacioppo, & Quigley, 1995). IBI is the reciprocal of heart rate, so that longer IBIs represent slower heart rates. An age related reference (ages 7 – 16) for the range of IBI values (706 – 845 msec) may be useful when interpreting our results (Wallis, Healy, Undy, & Maconochie, 2005).
Movement
To control for increased metabolic demand caused by physical activity during the BAT (Porges et al., 2007), vertical acceleration was quantified in G’s, or gravitational force, as measured by an accelerometer located in the AMS device. Vertical movements were recorded and averaged online every 30 seconds. Movement was quantified as the average value during the BAT.
Results
Behavioral Approach Test and Cardiovascular Change
A pairwise comparison of IBI demonstrated significant physiological changes from baseline to the BAT (see Table 1). IBI decreased significantly during the BAT, as was expected. An effect size and 95% confidence intervals (CI) were computed for IBI to illustrate the magnitude of the physiological effect during the BAT. Cohen’s d (Cohen, 1988) was computed as: d = (BATmean – Baseline mean)/pooled standard deviation (SDP). SDP was computed as: SDP = [((σ2 BAT + σ2 Baseline)/2) ^ 0.5]. A moderate to large effect size was found for IBI (d = −0.72, CI = −1.05, −0.38), demonstrating physiological arousal during the BAT.
Table 1.
Means and Standard Deviations on Physiological, Subjective, and Behavioral Measures for the Whole Sample
| Measure | M | SD | l |
|---|---|---|---|
| Baseline IBI | 712.23 | 98.80 | |
| BAT IBI | 638.00** | 107.73 | |
| SUDS | 4.22 | 2.95 | |
| % Steps Completed | 69.11 | 35.37 |
Note. IBI = interbeat interval; SUDS = subjective units of distress;
signifies a significant (p < .001) decrease relative to baseline.
Avoidant Behavior, Subjective Distress, and Change in Physiology during the BAT
Subjective units of distress on the 0 – 8 scale (SUDS; M = 4.22, SD = 2.95) collected at the conclusion of the BAT and the percentage of steps completed during the BAT (M = 69.11, SD = 35.37) were correlated to examine the relationship between subjective distress and approach/avoidant behavior. As hypothesized, SUDS ratings and percentage of steps completed were significantly correlated (r = −.50, p < .001), indicating a moderate to large parallel between avoidant behavior and subjective distress during the BAT. As noted, a change score (BAT mean – Baseline mean) was computed for IBI to quantify physiological arousal during the BAT (more negative change scores are indicative of increased physiological arousal). Partial correlations (controlling for movement during the BAT) were then calculated for the change in IBI with percentage of steps completed and SUDS ratings to test the hypothesis that increased physiological arousal during the BAT would be related to the level of distress reported and amount of avoidant behavior exhibited. SUDS ratings were negatively and significantly correlated with change in IBI (r = −.30, p = .012), indicating the predicted relationship that higher SUDS ratings were related to increased physiological arousal. Although the correlation between percentage of steps and change in IBI during the BAT was not significant, a trend in the predicted direction indicated that lower percentage of steps were related to increased physiological arousal (r = .20, p = .087). Overall, both avoidant behavior and subjective distress shared a modest parallel with increased physiological arousal during the BAT.
Concordance of Subjective Distress and Change in Physiology with Behavioral Avoidance
To examine between-subjects concordance and discordance, each subject was categorized as exhibiting low or high physiological arousal and reporting low or high distress. Subjective units of distress (SUDS) were recoded into low distress (SUDS < 4) and high distress (SUDS ≥ 4), paralleling cutoff criteria used on the ADIS-C/P (a rating of 4 or above signified moderate to severe distress). Change in IBI during the BAT was recoded into low arousal (change in IBI ≥ 0.00) and high arousal (change in IBI < 0.00), based on the mean change in IBI during the BAT after statistically controlling for movement (movement was regressed onto the IBI change scores and the unstandardized residuals were then used). Mean change in IBI was used as no other meaningful physiological cutoff exists. Based on the high or low distress ratings and the high or low physiological changes in IBI on the BAT, each subject was assigned to a concordant or discordant group: low arousal-low distress (n = 21); high arousal-high distress (n = 24); and discordant (either low arousal-high distress or high arousal-low distress; n = 28). Mean SUDS, percent steps completed, and change in IBI for each of these three groups are reported in Table 2.
Table 2.
Group Means and Standard Deviations on Physiological, Subjective, and Behavioral Measures
| Measure | Low Distress Low Arousal | Discordant | High Distress High Arousal | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Baseline IBI | 734.76 | (100.40) | 653.21 | (64.99) | 761.37 | (98.03) |
| BAT IBI | 709.44 | (117.19) | 607.61 | (72.24) | 610.94 (108.24) | |
| SUDS | 0.90 | (1.14) | 4.68 | (2.58) | 6.58 | (1.44) |
| % Steps Completed | 90.81 | (20.05) | 68.64 | (36.87) | 50.67 | (34.43) |
Note. IBI = interbeat interval; SUDS = subjective units of distress; low arousal-low distress (n = 21); high arousal-high distress (n = 24); and discordant (n = 28).
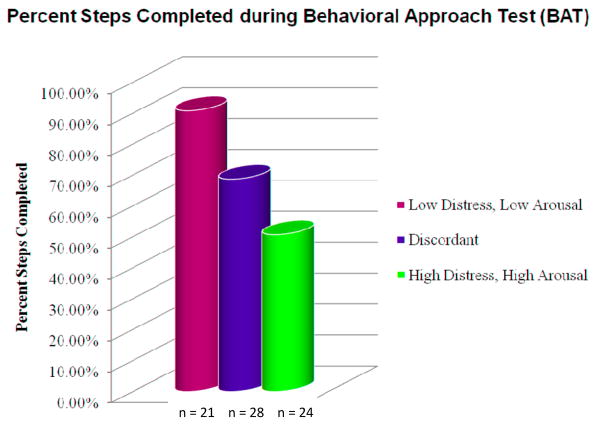
A one-way ANOVA was used to examine differences between the three groups in terms of percentage of steps completed (amount of approach behavior) during the BAT. The groups showed a significantly different percentage of steps completed, F (2, 70) = 8.78, p < .001. Tukey post-hoc comparisons indicated that the low arousal-low distress group (M = 90.81%) completed a significantly greater percentage of steps during the BAT than the high arousal-high distress group (M = 50.67%, p < .001), and a marginally greater percentage of steps than the discordant group (M = 68.64%, p = .050). The comparison between the high arousal-high distress group and the discordant group was not significant (p = .116); however, it too was in the predicted direction (see Figure 1).
Figure 1.
Percent steps completed during BAT for different combinations of high arousal (change in IBI < 0.00) or low arousal (change in IBI ≥ 0.00) and high distress (SUDS > 4) or low distress (SUDS < 4).
One-way ANOVAs were used to determine if the three groups differed in age or on phobia CSRs. There were no age differences, F (2, 69) = 1.57, p = .215, nor differences between the three groups on CSR, F (2, 63) = 0.972, p = .384. Separate Chi-square tests were then computed to determine differences between the three groups in terms of gender and phobia type (three children with a primary environment phobia were excluded from the latter analyses because they also had a comorbid animal phobia). Group differences were not found for gender, χ2 (2, N = 71) = 0.24, p = .886, or for phobia type, χ2 (2, N = 62) = 4.03, p = .133.
Discussion
On the one hand, our findings are relatively straightforward. First, and importantly, our BATs resulted in heightened physiological arousal (as indexed by decreases in IBI relative to baseline measures). The BATs also resulted in distress and behavioral avoidance in that the average child reported moderate to high levels of distress and was able to complete less than 70% of the behavioral steps. Still, on the three response measures, considerable variability was present. For example, on the index of behavioral avoidance, some children were unable to enter the room to look at the phobic object and did not complete even the first step, whereas other children were able to enter the room, approach the phobic object, and complete all of the steps.
Second, we found the three response components to co-vary with one another across this group of participants. SUDS were significantly correlated with the percent of steps completed on the BAT (large effect size) and with change in IBI (medium effect size). Change in IBI, however, was only marginally correlated with percent of steps completed (small effect size). Thus, the three response components tended to covary in the expected direction, suggesting concordance for the group of phobic youth as a whole.
Third, although evidence of concordance was observed at the aggregate level, via subgroup analyses, we were able to identify groups of youth who showed concordant and discordant patterns of covariation. Specifically, we were able to comprise groups of youth based on physiological arousal and SUDS ratings during the BAT. In doing so, we identified 24 high arousal-high distress youth, 21 low arousal-low distress youth, and 28 discordant youth (either low arousal-high distress or high arousal-low distress youth); overall, 45 of our 73 youth (61.64%) were concordant across these two response channels (high arousal-high distress, low arousal-low distress) and 28 (38.36%) were discordant (high arousal-low distress, low arousal-high distress). Thus, although the majority of youth were concordant across these two systems, a significant minority were not. In addition, the high arousal-high distress group and the low arousal-low distress group differed significantly in the percent of steps completed on the BAT (51% and 90%, respectively), whereas the discordant group was intermediate to the two concordant groups (68%).
Fourth, the high arousal-high distress and the low arousal-low distress groups tended to be concordant on all three components of the tripartite model. These findings are supportive of the tripartite model as articulated by Lang (1967, 1979) and Rachman and Hodgson (1974) inasmuch as the three response components co-varied with one another. Those individuals who were high (or low) on two of the three components (physiological arousal, subjective distress) were correspondingly high or low on the third (behavioral) component, reflecting concordance across all three components. In contrast, those individuals who were high on only one of the physiological or subjective dimensions (discordant group) were variable on the third behavioral dimension.
Fifth, we did not find differences between our concordant or discordant groups on age or gender. These findings suggest that unlike emerging research with adults (Avero & Calvo, 1999; Teachman & Gordon, 2009), concordance does not appear to vary according to age or gender in children. Reasons for these differences between adults and children are unclear and await replication and further examination.
Sixth, we did not find differences in concordance between youth with animal or natural environment type phobias as expected. This was surprising since children with the natural environment type of phobia have been shown to be more impaired than children with animal type of phobia, including the presence of more somatic and anxious symptoms (Ollendick et al., 2010). Given these differences, we reasoned that they would be more likely to become emotionally aroused during the BAT and hence show greater concordance in responding. They did not. It is possible that our failure to confirm this hypothesis is related to the way in which we administered the BAT to some of the children in the natural environment group. For the children with a phobia of storms, for example, the BAT was delivered via video presentation whereas for children in the animal phobia type (e.g., dogs) the BAT was delivered in vivo. Children in the storm BATs tended to report lower levels of distress and to complete the BAT more frequently than children exposed to the “live” BATs. These differences in methodology might have effectively obscured potential differences that might have been present between these types of phobias had they both been exposed to in vivo stimuli (e.g., real “live” thunderstorms versus videotaped ones).
Seventh and also contrary to our hypotheses, children with higher clinical severity ratings were not more often in the concordant groups than those with lower severity ratings. This suggests that phobia severity by itself might not be a reliable indicator of emotional arousal in the BAT as we anticipated. However, caution must be exercised with this conclusion since all of our youth met criteria for a diagnosis of specific phobia and all possessed severity ratings of 4 or above on the diagnostic interview. As a result, a restricted range of CSRs was present. Thus, it remains possible that a clinical group of youth with specific phobias, such as our groups, might differ on indices of concordance from clinical control or normal control groups of youth who do not possess clinical phobias. However, such a possibility remains to be investigated.
Why might the fear response be concordant among some individuals with specific phobias but not others? We offer three possible explanations. First, there may be methodological problems in the way in which we examined concordance and discordance in this study. Specifically, the BAT itself may possess significant shortcomings. Although it was designed to produce an objective and reliable behavioral measure of fear (King, Hamilton, & Ollendick, 1988), it is evident that the BAT measures the three response components under highly controlled and, perhaps, artificial conditions. In attempts to standardize the BAT for comparisons across individuals, it is possible that the fear response was not fully activated in some children, allowing for discordance. It is also evident that the BAT represents a social situation in which the expectations of the individual regarding performance in the presence of others come into play (Borkovec et al., 1977; Dadds et al., 1994). That is, expectations of performance and resulting demand characteristics might be present on the BAT such that some children boldly completed the task even though they were highly aroused physiologically (high arousal-low distress group), whereas other children refused to approach the phobic stimulus even though they experienced low levels of physiological arousal (low arousal-high distress group).
Second, in this study, we defined emotional arousal dispositionally. That is, we assumed emotional arousal during the BAT would be related to the severity of the phobic disorder – as defined by the consensus Clinician Severity Rating (CSR). Our assumption was not supported as CSRs were not related to concordance among the three response systems. In contrast to this approach, others have defined emotional arousal as a function of the situation to which the individual is exposed (e.g., Kaloupek et al., 1983; Mavissakalian, 1987). It is possible that greater concordance would be observed if emotional arousal were defined operationally through various experimental manipulations of arousal. Under such conditions and at higher levels of situational arousal, greater concordance might be expected. This is an important area of further inquiry.
Third, in addition to methodological features associated with the BAT and how it is measured, it is possible that the three components of the fear response are simply not equally activated in all individuals and that some individuals are primarily behavioral, physiological, or cognitive-affective responders. Specific phobias are known to be heterogeneous both in content and expression (Antony & Barlow, 2002; Bergman & Piacentini, 2005; Craske, 1999, 2003; Davis & Ollendick, 2005; Muris, 2005; Ollendick et al., 2010). These individual response patterns may simply reflect the notion that the three response components are loosely coupled, as suggested early on by Lang (1967) and subsequently by Hodgson and Rachman (1974), and are only “fully” coupled under conditions of high emotional arousal. Under conditions of low emotional arousal, not all systems will be activated and discordance will more likely be present. Assuming sufficient emotional arousal is enacted, it will be important to determine environmental and biological factors that moderate concordance and discordance; in all likelihood, a combination of factors will be relevant (cf, Lang, Davis, & Öhman, 2000; Ollendick, King, & Muris, 2002; 2004).
Our study is not without limitations. In addition to potential problems associated with the BAT and the absence of youth with blood-injection-injury phobias and an insufficient number of youth with situational and “other” phobia types to systematically explore specific co-variation patterns, the youth in our sample were referred to a specialty clinic and, as a result, their phobias might be qualitatively different from phobias in non-specialty clinics or community samples of youth. If so, the differences we noted in this study would be evident only in clinic-referred and treatment-seeking samples. Second, our sample was largely Caucasian, medium to high in family income, and from largely intact families; as a result, we are unsure whether such concordant and discordant response patterns would be observed in children and adolescents from other ethnicities or family backgrounds. Third, the way we defined concordance and discordance was necessarily constrained by the measures available to us. That is, we essentially undertook median split analyses to define high and low levels of distress and, high and low levels of physiological arousal, and high and low levels of behavioral approach. It is possible that different strategies would have produced different outcomes. Future studies will be essential to the validation of our findings.
These limitations notwithstanding, our findings are of import for clinic-referred and treatment-seeking youth. Our sample was well characterized and yet important differences in concordance and discordance were observed among the youth. These differences suggest that specific phobias in youth are complex and not at all “simple,” and that a fuller understanding of this complexity may assist us in both the assessment and treatment of these disorders (see Davis & Ollendick, 2005, and Mavissakalian, 1987).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Antony MM, Barlow DH. Specific phobias. In: Barlow D, editor. Anxiety and its disorders: The nature and treatment of anxiety and panic. New York: Guilford Press; 2002. pp. 380–417. [Google Scholar]
- Antony MM, Brown TA, Barlow DH. Heterogeneity among specific phobia types in DSM-IV. Behaviour Research and Therapy. 1997;35:1089–1100. [PubMed] [Google Scholar]
- Avero P, Calvo MG. Emotional reactivity to social-evaluative stress: Gender differences in response systems concordance. Personality and Individual Differences. 1999;27:155–170. [Google Scholar]
- Bergman RL, Piacentini J. Targeting discrete response channels in the treatment of childhood specific phobia. Clinical Psychology: Science and Practice. 2005;12:166–169. [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. The metrics of cardiac chronotropism: Biometric perspectives. Psychophysiology. 1995;32:162–171. doi: 10.1111/j.1469-8986.1995.tb03308.x. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Weerts TC, Bernstein DC. Assessment of anxiety. In: Ciminero AC, Calhoun KS, Adams HE, editors. Handbook of behavioral assessment. New York: John Wiley & Sons; 1977. pp. 367–428. [Google Scholar]
- Calvo MG, Miguel-Tobal JJ. The anxiety response: Concordance among components. Motivation and Emotion. 1998;22:211–230. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, New Jersey: Erlbaum; 1988. [Google Scholar]
- Côté S, Bouchard S. Documenting the efficacy of virtual reality exposure with psychophysiological and information processing measures. Applied Psychophysiology and Biofeedback. 2005;30:217–232. doi: 10.1007/s10484-005-6379-x. [DOI] [PubMed] [Google Scholar]
- Craske MG. Anxiety disorders: Psychological approaches to theory and treatment. Boulder, CO: Westview Press; 1999. [Google Scholar]
- Craske MG. Origins of phobias and anxiety disorders. Oxford: Elsevier; 2003. [Google Scholar]
- Dadds MR, Rapee RM, Barrett PM. Behavioral observations. In: Ollendick TH, King NJ, Yule W, editors. International handbook of phobic and anxiety disorders in children and adolescents. New York: Plenum Press; 1994. pp. 349–364. [Google Scholar]
- Davis TE, III, Ollendick TH. A critical review of empirically supported treatments for specific phobias in children: Do efficacious treatments address the components of a phobic response? Clinical Psychology: Science and Practice. 2005;12:144– 160. [Google Scholar]
- Fowles DC. The Three Arousal Model: Implications of Gray's Two-Factor Learning Theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Groot PFC, de Geus EJC, de Vries J. User Manual v1.2. Vrije Universiteit, Department of Psychophysiology; Amsterdam, The Netherlands: 1998. Ambulatory Monitoring System. [Google Scholar]
- Hellstrom K, Fellenius J, Öst LG. One versus five sessions of applied tension in the treatment of blood phobia. Behaviour Research and Therapy. 1996;34:101–112. doi: 10.1016/0005-7967(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Hodgson R, Rachman S. II. Desynchrony in measures of fear. Behaviour Research & Therapy. 1974;12:319–326. doi: 10.1016/0005-7967(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Kaloupek DG, Levis DJ. Issues in the assessment of fear: Response concordance and prediction of avoidance behavior. Journal of Behavioral Assessment. 1983;5:239–260. [Google Scholar]
- King NJ, Hamilton DI, Ollendick TH. Children’s phobias: A behavioural perspective. Chichester: John Wiley & Sons; 1988. [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: Expression, experience, and physiology. Journal of Personality and Social Psychology. 1998;74:686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Fear reduction and fear behavior: Problems in treating a construct. In: Shlien JM, editor. Research in psychotherapy. Washington, DC: American Psychological Association; 1967. pp. 332–368. [Google Scholar]
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Cuthbert BN. Affective information processing and the assessment of anxiety. Journal of Behavioral Assessment. 1984;6:369–395. doi: 10.1007/BF01321326. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Cuthbert BN, Bradley MM. Measuring emotions in therapy: Imagery, activation, and feeling. Behavior Therapy. 1998;29:655–674. [Google Scholar]
- Lang PJ, Davis M, Öhman A. Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response regulation. Journal of Abnormal Psychology. 1883;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Lewis LE, Drewett RF. Psychophysiological correlates of anxiety: A single case study. Journal of Anxiety Disorders. 2006;20:829–835. doi: 10.1016/j.janxdis.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotional experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mavissakalian M. Trimodal assessment in agoraphobia research: Further observations on heart rate and synchrony/desynchrony. Journal of Psychopathology and Behavioral Assessment. 1987;9:89–98. [Google Scholar]
- Muris P. Treatment of specific phobia in children: Are all the components of the phobic response of equal importance? Clinical Psychology: Science and Practice. 2005;12:170–173. [Google Scholar]
- Nelissen I, Muris P, Merkelbach H. Computerized exposure and in vivo exposure treatments for spider fear in children: Two case reports. Journal of Behavior Therapy and Experimental Psychiatry. 1995;26:153–156. doi: 10.1016/0005-7916(95)00002-h. [DOI] [PubMed] [Google Scholar]
- Ollendick TH, King NJ, Muris P. Fears and phobias in children: Phenomenology, epidemiology, and aetiology. Child and Adolescent Mental Health. 2002;7:98–106. doi: 10.1111/j.1475-3588.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Ollendick TH, King NJ, Muris P. Phobias in children and adolescents. In: Maj M, Akiskal HS, Lopez-Ibor JJ, Okasha A, editors. Phobias. London: John Wiley & Sons, Inc; 2004. pp. 245–279. [Google Scholar]
- Ollendick TH, Öst LG, Reuterskiöld L, Costa N, Cederlund R, Sirbu C, Davis TE, III, Jarrett MA. One-session treatment of specific phobias in youth: A randomized clinical trial in the USA and Sweden. Journal of Consulting and Clinical Psychology. 2009;77:504–516. doi: 10.1037/a0015158. [DOI] [PubMed] [Google Scholar]
- Ollendick TH, Raishevich N, Davis TE, III, Sirbu C, Öst LG. Specific phobia in youth: Phenomenology and psychological characteristics. Behavior Therapy. 2010;41:133–141. doi: 10.1016/j.beth.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öst LG, Stridh B-M, Wolf M. A clinical study of spider phobia: Prediction of outcome after self-help and therapist-directed treatments. Behaviour Research and Therapy. 1998;36:17–35. doi: 10.1016/s0005-7967(97)10018-3. [DOI] [PubMed] [Google Scholar]
- Öst LG, Svensson L, Hellstrom K, Lindwall R. One-session treatment of specific phobias in youths: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2001;69:814–824. [PubMed] [Google Scholar]
- Porges SW, Heildman KJ, Bazhenova OV, Bal E, Doussard-Roosevelt JA, Koledin M. Does motor activity during psychophysiological paradigms confound the quantification and interpretation of heart rate and heart rate variability measures in young children? Development Psychobiology. 2007;49:485–494. doi: 10.1002/dev.20228. [DOI] [PubMed] [Google Scholar]
- Rachman S, Hodgson R. I. Synchrony and desynchrony in fear and avoidance. Behaviour Research & Therapy. 1974;12:311–318. doi: 10.1016/0005-7967(74)90005-9. [DOI] [PubMed] [Google Scholar]
- Rushen J, Passille AMB, Munksgaard L. Fear of people by cows and effects on milk yield, behavior, and heart rate at milking. Journal of Dairy Science. 1999;82:720–727. doi: 10.3168/jds.S0022-0302(99)75289-6. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Buodo G, Munafo M, Palomba D, Stegagno L. Cardiovascular dynamics in blood phobia: Evidence for a key role of sympathetic activity in vulnerability to syncope. Psychophysiology. 2008;45:1038–1045. doi: 10.1111/j.1469-8986.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- Sartory G, Rachman S, Grey S. An investigation of the relation between reported fear and heart rate. Behaviour Research and Therapy. 1977;15:435–438. doi: 10.1016/0005-7967(77)90048-1. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Houlihan D, Krueger KF, Simon DA. The behavioral treatment of a young adult with post traumatic stress disorder and a fear of children. Child and Family Behavior Therapy. 1997;19:37–49. [Google Scholar]
- Silverman WK, Albano AM. Anxiety Disorders Interview Schedule for DSM-IV: Child and Parent Versions. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Silverman WK, Saavedra LM, Pina AA. Test-retest reliability of anxiety symptoms and diagnoses with anxiety disorders interview schedule for DSM-IV: Child and parent versions. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(8):937–944. doi: 10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- Teachman BA, Gordon T. Age differences in anxious responding: Older and calmer, unless the trigger is physical. Psychology and Aging. 2009;24:703–714. doi: 10.1037/a0016813. [DOI] [PubMed] [Google Scholar]
- Turner SM, Beidel DC. Empirically derived subtypes of social anxiety. Behavior Therapy. 1985;16:384–392. [Google Scholar]
- Turpin G. The psychophysiological assessment of anxiety disorders: Three-systems measurement and beyond. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:366–375. [Google Scholar]
- Wallis LA, Healy M, Undy MB, Maconochie I. Age related reference ranges for respiration and heart rate from 4 to 16 years. Archives of Disease in Childhood. 2005;90:1117–1121. doi: 10.1136/adc.2004.068718. [DOI] [PMC free article] [PubMed] [Google Scholar]